Severe Cerebellar Atrophy Following Acetylsalicylate Poisoning: A Case Report
Received: 10-Jan-2024 / Manuscript No. JADP-24-124826 / Editor assigned: 12-Jan-2024 / PreQC No. JADP-24-124826 (PQ) / Reviewed: 26-Jan-2024 / QC No. JADP-24-124826 / Revised: 02-Feb-2024 / Manuscript No. JADP-24-124826 (R) / Published Date: 12-Feb-2024 DOI: 10.4172/2161-0460.1000589
Abstract
Introduction: Acetylsalicylate is a common Over-The-Counter (OTC) drug that is used for suicide attempts. Acetylsalicylate toxicity ranges from mild to death, according to the amount that is taken and the response of the patient. In particular, toxicity may occur in a delayed manner, causing serious damage to the brain as a sequela.
Case presentation: A 43-year-old woman attempted suicide by overdosing with Bufferin®. Due to frequent vomiting, she was transported to an emergency hospital in the morning. However, in the hospital, she became alert and looked well after intravenous infusions, and was transferred to a psychiatric hospital for observation. She vomited several times during the night and was transported to the emergency hospital the following morning due to obtunded consciousness. Due to hypoxemia, intra-tracheal intubation and respiratory control with a respirator were performed. Metabolic acidosis and acute rhabdomyolysis were successfully treated. However, support with a respirator and non-invasive positive pressure ventilation were necessary up to the 20th hospital day. Thereafter, she regained normal consciousness and intelligence. She subsequently presented with dysarthria and gait disturbance. Three years later, a detailed neurological examination revealed marked cerebellar ataxia and exclusive cerebellar atrophy on Magnetic Resonance Imaging (MRI) of the brain.
Conclusion: The exclusive cerebellar ataxia and atrophy on MRI associated with acetylsalicylate poisoning were probably induced by respiratory insufficiency secondary to Hypoxic-Ischemic Encephalopathy (HIE). However, salicylate toxicity and several other disorders may have contributed to the pathogenesis.
Keywords: Cerebellar atrophy; Cerebellar ataxia; Salicylate poisoning; Respiratory insufficiency; Central nervous system lesions
Introduction
OTC drugs are often used for suicide attempts, and emergency hospitals in Canada have reported that acetaminophen is the most common OTC drug used by adults for this purpose [1]. Other OTC analgesics in that report included salicylates, ibuprofen, and other drugs, and deaths were observed in cases of acetaminophen and salicylate intoxication [1]. Respiratory insufficiency and acute rhabdomyolysis can occur in patients with salicylate intoxication due to central nervous system involvement, metabolic disorders, or vomiting-induced suffocation [1]. Severe respiratory insufficiency can cause various neurological manifestations due to HIE. In these cases, the cerebellum is one of the most vulnerable sites [2,3]. However, there have been no reports of severe cerebellar atrophy exclusively associated with marked cerebellar symptoms after respiratory insufficiency, probably because patients with HIE usually show extensive brain lesions.
We observed a patient who exhibited remarkable cerebellar dysfunction associated with cerebellar atrophy on MRI three years after acetylsalicylate poisoning. We hypothesized that cerebellar atrophy was induced by the unique clinical situations of salicylate toxicity with Hypoxemia (HIE), metabolic acidosis, and acute rhabdomyolysis, although we believe that HIE is the most probable cause.
Case Presentation
The patient was a middle-aged female homemaker who had been performing housework without any issues. No motor or sensory abnormalities were observed in this patient. At 42 years of age, she attempted suicide in the face of unsolvable family problems by taking an overdose of Bufferin®, which she bought over the counter. A single Bufferin® tablet contains 330 mg of acetylsalicylate (aspirin) and 150 mg of dialuminate. She sometimes took this drug for headaches. At that time, she crushed the tablets and drank them with water, but the total amount was unclear. In the morning, her husband found her with agonizing abdominal pain and frequent vomiting, and she was immediately transported to an emergency hospital. After arrival, she was drowsy and vomited several times, and ordinary blood and urine examinations revealed only dehydration. Following the intravenous infusion of isotonic fluid, she became alert, her vital signs normalized, and she responded correctly to the staff members' questions and orders. On the evening of that day, because she looked well, she was transferred to a psychiatric hospital and admitted due to fear of repeated suicide attempts. The patient was calm in the psychiatric ward. She received intravenous infusions of isotonic fluid due to anorexia, along with mirtazapine (15 mg, oral), olanzapine (2.5 mg, oral), and brotizolam (0.25 mg, oral). During the night, she vomited several times, and in the early morning of the next day, she showed a fever of 37.6°C and dyspnea. Her oxygen saturation measured using pulse oximetry (SpO2) was 90%. Oxygen (5 L/min) was administered via nasal cannula. The patient was transferred to a former emergency hospital in the morning.
In the hospital, she was comatose with a high fever (>40°C), hypoxemia, and decreased blood pressure, indicating shock. An arterial blood examination revealed obvious hypoxemia and metabolic acidosis, with pH 7.216 (normal range 7.4 ± 0.05), pO2 20.0 Torr (80-100), pCO2 42.7 Torr (35-45), HCO¯3 16.7 mEq/L (22-26) and base excess -9.7 mmol/L (0 ± 2). Therefore, emergent endotracheal intubation was performed. Respiration was controlled using a respirator. Metabolic acidosis was corrected by the third hospital day. Radiographic findings showed no pulmonary edema or pneumonia. Her high fever (>39°C) persisted for several days, despite continuous intravenous ceftriaxone infusion. Acute rhabdomyolysis, with maximum serum creatine kinase and myoglobin concentrations of 14,218 U/L and 19,300 ng/mL, respectively, occurred on the second hospital day (Table 1).
| Category | Contents | Unit | Normal range | Hospital day* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 7th | 10th | 15th | 27th | ||||
| Serum | AST | U/L | May-35 | 27 | 28 | 245 | 528 | 284 | 88 | 45 | 18 |
| ALT | U/L | May-45 | 33 | 36 | 141 | 232 | 340 | 121 | 75 | 23 | |
| ALP | U/L | 115-360 | 267 | 268 | 271 | 200 | 290 | 173 | 234 | 230 | |
| BUN | mg/dL | 8-20 | 6.3 | 13.1 | 15 | 11.9 | 11.8 | 24.9 | 22.7 | 21.5 | |
| Creatinine | mg/dL | 0.4-1.2 | 0.72 | 1.09 | 1.14 | 1.19 | 0.73 | 0.76 | 0.59 | 0.47 | |
| Na | mmol/L | 136-148 | 145 | 149 | 142 | 146 | 143 | 148 | 144 | 138 | |
| K | mmol/L | 3.6-5.0 | 4.15 | 4.33 | 3.52 | 3.38 | 3.17 | 3.76 | 3.65 | 3.89 | |
| Cl | mmol/L | 98-108 | 104 | 107 | 108 | 115 | 100 | 112 | 106 | 100 | |
| CK | U/L | 30-165 | 63 | 245 | 6,761 | 14,218 | 5,456 | 4,849 | 879 | - | |
| Myoglobin | ng/mL | Oct-92 | - | 2,326 | 19,300 | 7,790 | 2,377 | 2,534 | 865 | - | |
| Glucose | mg/dL | 60-110 (fasting) | 104 | 107 | 108 | 115 | 100 | 134 | 107 | 103 | |
| CRP | mg/dL | 0-0.3 | 0.14 | 2.6 | 12.93 | 10.27 | 4.85 | 1.84 | 1.21 | 0.18 | |
| CBC | WBC | /μL | 3100-8800 | 12,080 | 22,850 | 14,280 | 10,830 | 10,600 | 7,330 | 15,930 | 10,460 |
| Neut | % | 40-75 | 42.9 | 85.5 | 74.1 | 78.9 | 79.4 | 44.2 | 69.4 | 71.2 | |
Note: Day of hospitalization at the emergency hospital after the second admission. CBC: Complete Blood Cell count; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; ALP: Alkaline Phosphatase; BUN: Blood Urea Nitrogen; Na: Sodium; K: Potassium; Cl: Chloride; CK: Creatine Kinase; CRP: C-Reactive Protein; WBC: White Blood Cells; Neut: Neutrophils. Serum albumin and bilirubin, and red blood cells, hemoglobin, hematocrit and platelets are omitted because the values were almost within the normal limits.
Table 1: Laboratory findings during admission.
Accordingly, continuous hemodiafiltration was performed for five days with the infusion of a large amount of intravenous fluid, which successfully avoided renal insufficiency (Table 1). The serum concentration of salicylate examined immediately after the second admission was later reported to be 845 μg/mL (toxicity level >300 μg/ mL). Extubation was attempted on the seventh day of hospitalization. However, as the patient remained hypoxemic and comatose, oxygen was supplied by a respirator via an endotracheal tube until the 13th day of hospitalization. During this time, the patient received nasogastric tube feeding without any major complications. After extubation on the 14th hospital day, she required additional non-invasive positive pressure ventilation for a week due to edematous stenosis of the upper respiratory tract. On the 20th hospital day, respiration and consciousness returned to normal and oral feeding was started. She later reported that her memory had fully recovered on the 20th hospital day. In cases of meningitis, nuchal rigidity and Kernig’s sign were not observed throughout the clinical course, and a spinal tap was not performed.
Upon readmission to the former psychiatric hospital on the 27th day of hospitalization at the emergency hospital, her mental state was stable, although her speech was inarticulate and she could only walk with the aid of a walker. Electrocardiography and chest X-ray findings, serum levels of markers reflecting liver and kidney function, and electrolyte levels were all normal. Her complete blood cell count was within the normal limits. Forty days after her suicide attempt using Bufferin®, she was transferred to a rehabilitation hospital where she received physical therapy for three months, with mild improvement in motor function. She returned home and spent her days there uneventfully with the aid of her family members. She used a walker in her room and a wheelchair outside of her home.
Three years later, at 45 years of age, she visited the neurology department of our hospital to obtain an official physical disability certificate and underwent a neurological examination. She was alert, and her cognitive level, as evaluated using the revised Hasegawa Dementia Scale (HDS-R) [4], was 30 (full score: 30). The HDS-R is a verbal cognitive test that is as useful as the Mini-Mental State Examination (MMSE) [4]. Other tests covering speech (aphasia) and visuospatial and praxic functions were normal. A moderate degree of dysarthria (definitely scanning and explosive) was observed, suggesting cerebellar dysfunction. Nystagmus was not observed. Tendon reflexes of her limbs were normal, without positive toe extensor signs. Muscle tone was normal. She exhibited dysmetric clumsiness in the coordination tests of all limbs. She could only walk with human aid or a walker and displayed a staggering and wide-based gait. Her sensory test results were normal. These findings exclusively implied cerebellar dysfunction without other obvious nervous system abnormalities.
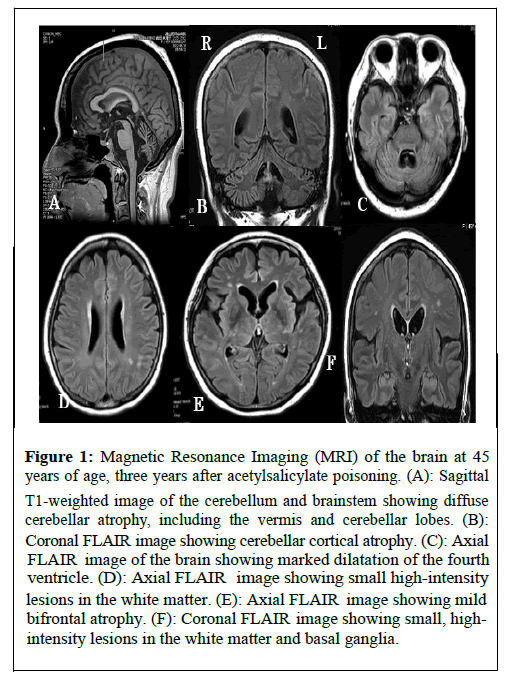
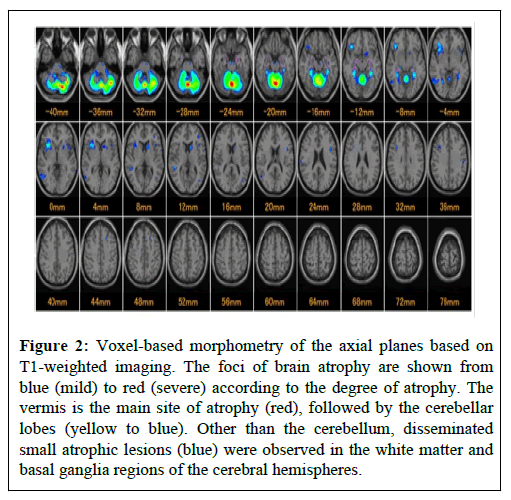
Brain MRI on the same day showed remarkable cerebellar atrophy involving the vermis and cerebellar cortex (more marked in the vermis), associated with slight bifrontal atrophy on T1-weighted and Fluid-Attenuated Inversion Recovery (FLAIR) images, without obvious lesions on diffusion-weighted or T2* images. Scattered small high-intensity lesions in the white matter and basal ganglia regions of the cerebral hemispheres were observed on the FLAIR images (Figure 1). Voxel-based morphometry based on T1-weighted imaging, which was introduced by Matsuda et al. [5], was performed to identify sites of focal brain atrophy (Figure 2).
Figure 1: Magnetic Resonance Imaging (MRI) of the brain at 45 years of age, three years after acetylsalicylate poisoning. (A): Sagittal T1-weighted image of the cerebellum and brainstem showing diffuse cerebellar atrophy, including the vermis and cerebellar lobes. (B): Coronal FLAIR i mage showing cerebellar cortical atrophy. (C): Axial FLAI R image of the brain showing marked dilatation of the fourth ventricle. (D): Axial FLAIR i mage showing small high-intensity lesions in the white matter. (E): Axial FLAIR im age showing mild bifrontal atrophy. (F): Coronal FLAIR image showing small, highintensity lesions in the white matter and basal ganglia.
Figure 2: Voxel-based morphometry of the axial planes based on T1-weighted imaging. The foci of brain atrophy are shown from blue (mild) to red (severe) according to the degree of atrophy. The vermis is the main site of atrophy (red), followed by the cerebellar lobes (yellow to blue). Other than the cerebellum, disseminated small atrophic lesions (blue) were observed in the white matter and basal ganglia regions of the cerebral hemispheres.
This software program, which exhibits the sites and degrees of focal brain atrophy based on a comparison with averaged brain images of healthy controls, was introduced for MRI analysis [5]. In this map, the sites of brain atrophy are indicated by blue (mild), green, and yellow to red (severe) according to the severity of atrophy. The patient exhibited cerebellar atrophy, which was most marked in the vermis (red), followed by the cerebellar cortex (yellow to blue). Outside the cerebellum, scattered small focal atrophy was observed in the frontotemporal lobes and basal ganglion regions (Figure 2).
The functional neurological deficits of this patient remained unchanged during the two years of follow-up, and she had been spending her daily life at home.
Results
Acetylsalicylate (aspirin) toxicity usually occurs within a short time after oral ingestion because it is readily absorbed from the gastrointestinal tract with peak serum concentrations achieved in 1 h [1]. However, in special conditions, the onset of systemic effects can be delayed by 8-12 hours, which is consistent with our case, although the cause was unclear in this patient [1].
She had not suffered from any brain disorders, including spinocerebellar degeneration, before attempting suicide with Bufferin® (acetylsalicylate). First, it must be determined whether a large amount of acetylsalicylate could introduce cerebellar destruction as a sequela. It has been reported that an acute dose of salicylate exceeding 150 mg/ kg or a total of 6.5 g may induce toxicity, including hematemesis, tachypnea, hyperpnea, dyspnea, tinnitus, deafness, lethargy, and seizures, and severe toxicity is expected at doses of 300-500 mg/kg [1]. The serum concentration in our patient at the second admission was 845 μg/mL, and salicylate toxicity occurred with a serum concentration of >300 μg/mL. The correlation between salicylate concentration and the severity of toxicity has not been established [6]. Specific brain lesions associated with salicylate poisoning have not been documented. Exceptionally, it was reported that damage to the peripheral acoustic nerves and auditory cortex may occur [7].
In another case with a previous cerebral infarction, left hemiparesis recurred following chronic salicylate ingestion without fresh cerebral infarction on MRI [8].
Salicylate toxicity may occur via multiple pathways, including metabolic disorders and respiratory insufficiency. If direct stimulation of the respiratory center causes an increase in minute ventilation, respiratory alkalosis occurs [9]. On the other hand, metabolic acidosis is induced if a low intracellular adenosine triphosphate concentration causes lactic and ketogenic acidosis [9]. Our patient showed obvious hypoxemia and metabolic acidosis, as demonstrated by an arterial blood gas analysis, which were corrected within two days. However, rhabdomyolysis unexpectedly occurred. Although salicylate-induced rhabdomyolysis has been well documented, it is rare and has been reported to occur in 1.9% of 258 salicylate-related admissions [9]. It is associated with increased morbidity and mortality, but its pathogenesis has not been established [9]. Rare cases of rhabdomyolysis during ceftriaxone treatment have been reported [10]. This is not applicable to our patient because her rhabdomyolysis improved during the time of ceftriaxone treatment (Table 1).
Discussion
The cause of the prolonged impaired consciousness that persisted until the 20th day of hospitalization, even after hypoxemia and metabolic acidosis were corrected, remains unclear. It may be reasonable to assume that HIE occurred during this period, finally causing remarkable cerebellar atrophy in this patient. However, severe cerebellar atrophy on MRI as a sequela of HIE is rare. In the relevant literature, severe HIE in older children and adults has been reported to extensively affect the gray matter structures (basal ganglia, thalami, and cerebral cortex), cerebellum, and hippocampi [2,3]. Therefore, the sequelae of HIE are usually expressed as severe incapacity in activities of daily living, as opposed to severe focal clinical manifestations [11]. It has been reported that the cerebellum is more vulnerable to hypoxicischemic events in postnatal and older patients than in the perinatal period because the Purkinje cells become mature in the postnatal period [2,3]. However, no exclusive extensive cerebellar lesions on MRI have been described as sequelae of HIE in postnatal or older patients [2,3]. As an exception, a 36-year-old woman presented with typical Lance-Adams syndrome associated with right cerebellar infarcts and a concomitant decrease in blood perfusion at the same site three months after cardiac arrest [12]. In conclusion, the features of the present case have not been reported before.
Voxel-based morphometry was first introduced by Matsuda, et al., [5]. It can reveal the focal atrophy of the brain. Significant atrophy of the hippocampus and entorhinal cortex was demonstrated in 116 patients with Alzheimer’s disease based on a comparison with 40 agematched healthy controls [5]. This software is now widely used in clinical settings in Japan. White matter volume reduction has been demonstrated in patients with Corticobasal Syndrome (CBS) and Richardson syndrome, with a significantly greater white matter volume reduction in CBS [13]. Atrophy of the thalamus in Gerstmann- Sträussler-Scheinker syndrome with the P102L mutation was observed using this method [14]. In our patient, marked cerebellar atrophy was confirmed with this method in addition to ordinary brain MRI.
Conclusion
This is a rare case of severe cerebellar atrophy which occurred in a 42-year-old woman. She exhibited respiratory insufficiency and metabolic disorders following a suicide attempt using acetylsalicylate. At the first admission to an emergency hospital, she looked well after intravenous infusion of isotonic fluid, and then she was transferred to a psychiatric hospital. However, at the second admission to the emergency hospital about 12 hours later, she became seriously ill with impaired consciousness, respiratory insufficiency and metabolic acidosis, followed by acute rhabdomyolysis. Approximately 20 days later her respiration and consciousness returned to normal, although she showed motor disturbance suggesting remarkable cerebellar ataxia, not associated with disorders of other central nervous system. Three years later, marked cerebellar atrophy was demonstrated on MRI. In this case toxicity of salicylate appeared in a relatively delayed manner. Its toxicity usually occurs within a short time after oral ingestion because it is readily absorbed from the gastrointestinal tract. HIE probably played a major role in the pathogenesis of her brain lesions, although there is a possibility that other disorders may have contributed to it.
Acknowledgement
We gratefully thank the patient and her family for their cooperation, and Brian Quinn (Japan Medical Communication; http://www.japanmc.co.jp) for performing the English language review.
Ethics Statement
Informed consent was obtained from the patient’s family to publish this case report and accompanying images.
Author Contribution
MM conducted the neurological studies and drafted the manuscript. TT conducted the study as a primary care physician. TN conducted the study as a psychiatrist. KS conducted the study as an emergency physician.
References
- Chyka PA, Erdman AR, Christianson G, Wax PM, Booze LL, et al. (2007) Salicylate poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol 45:95-131.
[Crossref] [Google Scholar] [PubMed]
- White ML, Zhang Y, Helvey JT, Omojola MF (2013) Anatomical patterns and correlated MRI findings of non-perinatal hypoxic–ischaemic encephalopathy. Br J Radiol 86:20120464.
[Crossref] [Google Scholar] [PubMed]
- Huang BY, Castillo M (2008) Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics 28:417-439.
[Crossref] [Google Scholar] [PubMed]
- Jeong JW, Kim KW, Lee DY, Lee SB, Park JH, et al (2007). A normative study of the Revised Hasegawa Dementia Scale: comparison of demographic influences between the Revised Hasegawa Dementia Scale and the Mini-Mental Status Examination. Dement Geriatr Cogn Disord 24:288-293.
[Crossref] [Google Scholar] [PubMed]
- Matsuda H, Mizumura S, Nemoto K, Yamashita F, Imabayashi E, et al. (2012) Automatic voxel-based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer Disease. AJNR Am J Neuroradiol 33:1109-1114.
[Crossref] [Google Scholar] [PubMed]
- O’Keefe M, Stanton M, Feldman R, Theobald J (2023) Incidence of rebound salicylate toxicity following cessation of urine alkalinization. Clin Toxicol 61:536-542.
[Crossref] [Google Scholar] [PubMed]
- Sheppard A, Hayes SH, Chen GD, Ralli M, Salvi R (2014) Review of salicylate-induced hearing loss, neurotoxicity, tinnitus and neuropathophysiology. Acta Otorhinolaryngol Ital 34:79-93.
[Google Scholar] [PubMed]
- Delaney TM, Helvey JT, Shiffermiller JF (2020) A case of salicylate toxicity presenting with acute focal neurologic deficit in a 61-year-old woman with a history of stroke. The American Journal of Case Reports 21:e920016-e920021.
[Crossref] [Google Scholar] [PubMed]
- Kaewput W, Thongprayoon C, Petnak T, Cheungpasitporn W, Qureshi F, et al. (2021) Rhabdomyolysis among hospitalized patients for salicylate intoxication in the United States: Nationwide inpatient sample 2003–2014. Plos one 16:e0248242.
[Crossref] [Google Scholar] [PubMed]
- Al-aqeedi RF, Kamha A, Al-aani FK, Al-ani AA (2009) Salmonella myocarditis in a young adult patient presenting with acute pulmonary edema, rhabdomyolysis, and multi-organ failure. J Cardiol 54:475-479.
[Crossref] [Google Scholar] [PubMed]
- Sandroni C, Cronberg T, Sekhon M (2021) Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med 47:1-22.
[Crossref] [Google Scholar] [PubMed]
- Waddell A, Dirweesh A, Ordonez F, Kososky C, Reddy Peddareddygari L, et al. (2017) Lance-Adams syndrome associated with cerebellar pathology. J Community Hosp Intern Med Perspect 7:182-184.
[Crossref] [Google Scholar] [PubMed]
- Sakurai K, Imabayashi E, Tokumaru AM, Hasebe S, Murayama S, et al (2015) The feasibility of white matter volume reduction analysis using SPM8 plus DARTEL for the diagnosis of patients with clinically diagnosed corticobasal syndrome and Richardson’s syndrome. NeuroImage Clin 7:605-610.
[Crossref] [Google Scholar] [PubMed]
- Sugiyama A, Sato N, Kimura Y, Maekawa T, Wakasugi N, et al. (2017) Thalamic involvement determined using VSRAD advance on MRI and easy Z-score analysis of 99mTc-ECD-SPECT in Gerstmann-Sträussler-Scheinker syndrome with P102L mutation. J Neurol Sci 373:27-30.
[Crossref] [Google Scholar] [PubMed]
Citation: Morimatsu M, Yamashita T, Nabeyama T, Shimizu K (2024) Severe Cerebellar Atrophy Following Acetylsalicylate Poisoning: A Case Report. J Alzheimers Dis Parkinsonism 14:589. DOI: 10.4172/2161-0460.1000589
Copyright: © 2024 Morimatsu M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2542
- [From(publication date): 0-2024 - Nov 14, 2025]
- Breakdown by view type
- HTML page views: 2162
- PDF downloads: 380