Research Article Open Access
Serum MSM Concentrations Following One Month of MSM Treatment in Healthy Men
| Richard J. Bloomer1*, Daniel A. Melcher1 and Rodney L. Benjamin2 | |
| 1Cardiorespiratory/Metabolic Laboratory, Department of Health and Sport Sciences, University of Memphis, Memphis, TN, USA | |
| 2Bergstrom Nutrition, Vancouver, WA, USA | |
| Corresponding Author : | Richard J. Bloomer Department of Health and Sport Sciences 106 Roane Fieldhouse, The University of Memphis Memphis, TN 38152,USA Tel: 901-678-5638 Fax: 901-678-3591 E-mail: rbloomer@memphis.edu |
| Received February 13, 2015; Accepted March 31, 2015; Published April 06, 2015 | |
| Citation: Bloomer RJ, Melcher DA, Benjamin RL (2015) Serum MSM Concentrations Following One Month of MSM Treatment in Healthy Men. Clin Pharmacol Biopharm 4:135. doi:10.4172/2167-065X.1000135 | |
| Copyright: © 2015 Bloomer RJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Introduction: Methylsulfonylmethane (MSM) is a commonly used dietary supplement for the alleviation of joint and muscle pain. It is known primarily for its anti-inflammatory and antioxidant properties. While it is believed to have excellent bioavailability, little is known about its serum concentrations following chronic ingestion. Methods: 20 healthy men were supplemented with 3 grams of MSM daily for four weeks. Blood was collected at baseline and after two and four weeks of supplementation. Serum was analyzed for MSM concentration using Nuclear Magnetic Resonance (NMR) spectroscopy. Results: All baseline samples but one (0.028 mM) was below the limit of quantification for the NMR assay (0.002 mM). Serum MSM values increased across time (p<0.0001) to a mean (± SD) of 1.68 ± 0.60 mM at week 2 and 1.91 ± 0.81 mM at week 4. Values at week 2 and week 4 were greater than at baseline (p<0.05), but not different from one another (p>0.05). A total of 13 of the 20 men demonstrated higher serum MSM values at week 4 as compared to week 2. and eight of these men demonstrated an increase at week 4 of at least 25% above what was observed at week 2. Conclusions: Serum MSM concentrations increase following oral MSM supplementation, in somewhat of a timedependent manner in selected subjects. The pattern of increase varies somewhat from subject to subject, although all individuals experience an increase of approximately 1-3 mM after 2-4 weeks of supplementation.
| Keywords |
| Blood; Dietary supplements; Methylsulfonylmethane |
| Introduction |
| Methylsulfonylmethane (MSM) is a naturally occurring compound that is composed of sulfur, oxygen, and methyl groups [1]. It is found in small quantities in a variety of foods, [2] such as milk, fruits and vegetables (e.g. tomatoes, corn), coffee, and tea. While being a very stable compound, MSM does sublimate and may be removed during the processing and/or preparation of foods. An early MSM researcher, Robert Herschler, suggested this may lead to lower than optimum amounts of MSM in the average diet. As a result, supplementation with MSM has grown in popularity in recent years for its health-enhancing properties. For example, multiple health-related benefits have been reported in association with MSM use, primarily related to its antioxidative and anti-inflammatory activity [3], which may be specific to both skeletal muscles during exercise recovery and reductions in joint pain during normal ambulation. |
| MSM is Generally Recognized as Safe (GRAS) as determined by a panel of experts and the United States Food and Drug Administration- Center for Food Safety and Applied Nutrition has issued a letter of no questions [4]. Human clinical trials have commonly included dosages of MSM between 1.5 [5] and 6 [6,7] grams daily, for periods of several weeks to months with no major adverse events reported from the MSM treatment. Anecdotally, benefits are often obtained within just a few days following the initiation of supplementation, suggesting that serum concentrations may be elevated rapidly following intake. While MSM is believed to have excellent bioavailability [8] and a single 3 gram dosage has been shown to increase serum MSM concentrations to approximately 0.15 - 0.20 mM just 90 minutes after ingestion, [9] little is known about its serum concentrations following chronic ingestion. Specifically, there are currently no data detailing the change in plasma MSM concentrations following chronic supplementation with this ingredient; something of interest to both investigators studying MSM and formulators who use MSM within dietary supplements for its health-enhancing properties. Hence, the purpose of the present study was to determine the impact of daily MSM supplementation over the course of a four week period on serum MSM concentrations in healthy men. It was our objective through this study to provide a base data set for potential future dose and time ranging studies that may assist in optimizing the suggested dosing of MSM, in particular as its popularity continues to grow. |
| Materials and Methods |
| Subjects |
| A total of 20 men were assigned to consume three grams of MSM daily for a period of four weeks. It should be noted that an additional 20 men were assigned to consume an identical- appearing placebo for the same period of time. Assignment was performed in a randomized, double-blind manner. The characteristics of subjects assigned to MSM are presented in Table 1. |
| Men were recruited to participate via informal word of mouth conversations, email communications, and recruitment flyers posted on campus. All subject recruitment was performed under the direction of the investigators. Women were not enrolled in this study in an attempt to maintain a more homogenous sample. Subjects were not current smokers and were considered to be in good overall health, without a history of cardiovascular or metabolic disorders. All subjects were engaged in a regular program of structured exercise, including resistance training. Health history, medication and dietary supplement usage, and physical activity questionnaires were completed by all subjects and reviewed by an investigator to determine eligibility. Subjects were informed of all procedures, potential risks, and benefits associated with the study through both verbal and written form. The study procedures were approved by the University Institutional Review Board (IRB) for Human Subjects Research (protocol #3006). |
| Supplementation and Testing |
| This study involved a randomized, double-blind, placebocontrolled design. Subjects were assigned to MSM (n=20) or placebo (n=20) capsules to ingest every day for a period of four weeks. Subjects ingested 3 capsules, once daily for a total daily dosage of 3 grams of MSM. Due to scheduling conflicts, one subject supplemented with MSM for one additional week (for a total of five weeks). The MSM (OptiMSM®; Bergstrom Nutrition, Vancouver, WA) and placebo (rice flour) capsules were produced according to Good Manufacturing Practice (GMP). Capsules were provided to subjects in unlabeled bottles every two weeks. Subjects returned their capsule bottles to investigators at the end of each two week period and the remaining capsules were counted to determine compliance to intake. All subjects were instructed to maintain their normal diet and physical activity patterns throughout the four week study period. Food intake was standardized during the days before pre and post intervention testing (e.g. baseline and week 4). Specifically, subjects received pre-packaged meal replacement drinks and bars, along with fruit to consume each day. The daily ration included 4 “ready-to-drink” meal replacement shakes (~250 kcal each), 4 meal replacement bars (~200 kcal each), one package of nuts (~200 kcal), and 4 pieces of fresh fruit (apples and bananas). This provided subjects with approximately 2500 kcal per day. Subjects were instructed not to consume any other food or beverages, aside from water. |
| Subjects reported to the lab following an overnight fast and provided a blood sample for analysis of serum MSM on three occasions: baseline (prior to beginning supplementation), following two weeks of supplementation, and following four weeks of supplementation. No MSM was ingested on the morning of each blood draw. Prior to obtaining the blood sample, subjects voided and then rested quietly in a chair for a period of 20 minutes. Venous blood samples were taken from subjects via needle and Vacutainer®. Blood was allowed to clot at room temperature for 30 minutes and then spun at 2000×g in a refrigerated centrifuge (4°C) in order to obtain serum. Serum samples were removed and stored at −70ºC until analyzed using Nuclear Magnetic Resonance (NMR) spectroscopy. All sample analysis was performed by Bruker BioSpin Corp (Billerica, MA). |
| Specifically, samples were prepared using buffer (75 mM NaH2PO4, 0.04% NaN3, 4.6 mM TSP in 20% D2O); 200 microliters of serum was combined with 400 microliters of buffer in a 5 mm NMR tube and vortexed. Samples were prepared fresh each morning and stored at 4ºC until they were analyzed. NMR spectra were acquired at 25ºC with an equilibration time of 5 minutes on a Bruker AVANCE III HD spectrometer operating at 500 MHz. The time domain data were apodized with a line broadening of 1.0 Hz. Apodized data were Fourier transformed, phased, and referenced to TSP as 0.00 ppm. An automatic script was used to extract the dataset name, the 90 degree pulse calibration, and half-width at half-height of TSP signal. This information was used for quality analysis. The data quality was judged from the CPMG spectra. |
| The halfwidth of TSP in serum samples was used as a shimming quality monitor. All samples had a half width of less than 10 Hz. The Limit of Quantification (LOQ) for a resolved peak was estimated to be 0.002 mM. |
| Statistical Analysis |
| Data were analyzed using a one way analysis of variance (ANOVA). Tukey post-hoc tests were used to compare means at different times. In an attempt to determine if capsule compliance, body mass, or age was associated with serum MSM concentrations, a correlation analysis was performed using these variables. The data are presented as mean ± SD. All analyses were performed using JMP statistical software. Statistical significance was set at p ≤ 0.05. |
| Results |
| Blood samples were obtained from all 40 subjects at each of the three time points (baseline, week 2, week 4). For all 60 samples obtained from subjects assigned to the placebo condition, MSM levels were below the LOQ (0.002 mM). Hence, there are no data to present for the placebo condition and the remaining focus of this paper is on serum MSM levels obtained from subjects assigned to the MSM condition. |
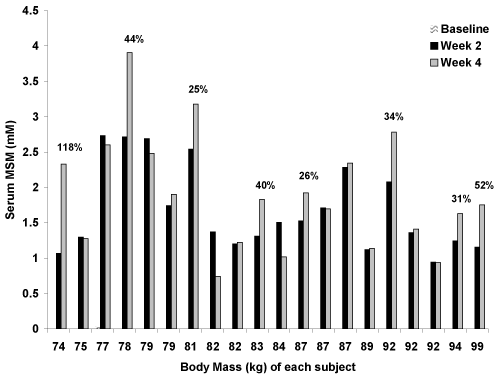
| All but one baseline sample (0.028 mM) for subjects assigned to MSM were below the limit of quantification for the NMR assay. A main effect for time was noted (p<0.0001), with MSM values increasing from ~0 mM at baseline to 1.68 ± 0.60 mM at week 2 and 1.91 ± 0.81 mM at week 4. Based on Tukey post-hoc testing, values at week 2 and week 4 were greater than at baseline (p<0.05), but values at week 2 and week 4 were not different from one another (p>0.05). That said, 13 of the 20 men demonstrated higher serum MSM values at week 4 as compared to week 2. Of these 13 men, 8 men demonstrated an increase at week 4 of at least 25% above what was observed at week 2 (Figure 1). |
| Pairwise correlations using subjects’ capsule compliance (both total and 2 week) indicated significant but weak/moderate correlations as follows: total capsule compliance over the four week period (r=0.40; p=0.01); capsule compliance at each two week period (r=0.32; p=0.04). The body mass of subjects was also weakly/moderately and negatively correlated to MSM concentrations (r= −0.34; p=0.03). The age of subjects was not correlated to serum MSM concentrations (r=0.12; p=0.45). |
| Discussion/Conclusion |
| The main findings of the present study are as follows: 1. MSM is not detectible in serum samples of healthy men using NMR unless they are supplemented with a daily dosage of the nutrient, corroborating existing data indicating that circulating MSM levels in human plasma are between 0 mM and 0.025 mM [10] ; 2) Daily supplementation with MSM at a dosage of 3 grams reliably increases serum MSM concentrations (all subjects experience an increase in serum MSM concentrations between approximately 1-3 mM); 3) serum MSM increases in somewhat of a time-dependent manner following chronic supplementation with MSM (as demonstrated in 13 of the 20 men); and 4) body mass is weakly/moderately and negatively related to serum MSM concentrations, as serum values increased to a slightly greater extent in individuals with lower body mass. |
| As can be seen in Figure 1, serum MSM values increased in all 20 subjects who received MSM supplementation. While the total accumulation varied from one subject to the next, it was generally consistent and within a fairly narrow range of 1-3 mM. In 13 of the 20 men, values were higher at week 4 as compared to week 2. This increase was 25% or more for eight of the men (as shown in Figure 1). Prior work has shown that a single 3 gram dosage of MSM results in a serum MSM concentration of approximately 0.15 - 0.20 mM just 90 minutes after ingestion [9]. It is possible that daily ingestion of MSM leads to an accumulation that can be detected in serum in significant quantities in the days following the initiation of supplementation. |
| As has been suggested for many dietary supplements, we hypothesized that the body mass of individuals may have impacted the overall serum MSM concentrations. A cursory review of the data presented in Figure 1 suggests this to some extent. For example, of the 20 subjects receiving MSM, those who experienced the highest serum MSM concentrations at week 4 had a body mass of: 78 kg, 81 kg, 92 kg, 77 kg, and 79 kg. That said, other subjects with a relatively low body mass (e.g. 75 kg, 82 kg) did not exhibit very high serum MSM levels. A correlation analysis indicated that body mass was weakly/moderately and negatively correlated to serum MSM (r= −0.34; p=0.03), indicating that the body mass of subjects does impact the serum MSM concentrations to some extent following supplementation. Aside from body mass, it is likely that metabolic factors involved in the uptake, distribution, and storage of MSM in humans also contribute to serum MSM concentrations. This is supported by the somewhat inconsistent findings for serum MSM concentrations across our subject pool, which is composed of different body masses. Perhaps a larger sample of subjects that is inclusive of a wider range of body mass might reveal more robust findings, as body mass in the present study only ranged from 74 kg to 99 kg. Moreover, an analysis based on lean body mass might provide additional insight as compared to our analysis based solely on total body mass. This may be considered in future studies. |
| Aside from body mass, capsule compliance influenced serum MSM concentrations. We noted a significant but weak/moderate correlation between compliance and serum MSM: total capsule compliance over the four week period (r=0.40; p=0.01); capsule compliance at each two week period (r=0.32; p=0.04). Only two of the 20 subjects had a total compliance of less than 90% (84% and 89%). Of the 20 men tested, these two men displayed the lowest (0.941 mM) and third lowest (1.130 mM) mean serum MSM concentration; however, they had a body mass of 92 kg and 89 kg, respectively. This relatively high body mass may have impacted the serum MSM concentration, as indicated above. One additional subject (82 kg) had a compliance of 91% and experienced the second lowest mean serum MSM concentration (1.056 mM). |
| When focusing on the compliance numbers at week 2 (97.6 ± 6.6%; range: 72-100%) and week 4 (94.9 ± 7.8%; range: 74-100%), as well as the corresponding serum MSM values at these times, it was evident that compliance was of importance. For example, when viewing Figure 1, the third from last subject shown (92 kg) failed to experience a further increase in serum MSM levels at week 4; however, this subject had a capsule compliance of 100% at the end of week 2 and only 72% at the end of week 4. This reduced compliance may have been the reason why this individual failed to experience a further increase beyond the week 2 value. Moreover, the 74 kg subject who displayed an increase of 118% in serum MSM from week 2 to week 4 had a capsule compliance of 74% at the end of week 2 and 100% at the end of week 4. As noted in the Methods section, this was the one subject who supplemented with MSM for one additional week-lending some evidence to the idea that longer-term treatment yields higher serum MSM values. In opposition to this observation, the eighth subject shown (82 kg) experienced a 46% decrease in serum MSM concentration from week 2 to week 4 yet had an increase in capsule compliance from 79% to 100% during the same period. Only one other subject experienced a major decrease in serum MSM (33%) from week 2 to week 4 (eleventh subject shown; 84 kg); however, compliance to capsule intake was 100% at both measurement times. Other subjects had similar capsule compliance between week 2 and week 4, while most subjects increased serum MSM concentrations during this time. Considering the above, our findings support the idea that capsule compliance to intake influences serum MSM concentrations in individuals who chronically supplement MSM. That said, other factors must be involved in the uptake and distribution of supplemental MSM, as compliance does not account for 100% of the variability in serum MSM concentrations. |
| In conclusion, we report for the first time that chronic treatment with MSM results in a reliable increase in serum MSM concentrations, in the range of 1-3 mM following 2-4 weeks of supplementation. The increase in serum MSM appears to be somewhat time-dependent, with the majority of subjects experiencing a continued rise following the initial two-week treatment period. Future studies may seek to determine if serum MSM values continue to rise with longer-term supplementation, to determine the time course of decline in serum MSM concentrations following cessation of MSM use, to determine the influence of different MSM dosing patterns on serum accumulation, and to determine if the pattern of increase is the same for both men and women. |
| Acknowledgements |
| Funding for this work was provided in part by Bergstrom Nutrition. Appreciation is extended to Drs. Kimberly Colson, Amy Freund, and Michelle Markus of Bruker BioSpin Corp. for assistance with MSM sample analysis and reporting of data. |
| Author’s contributions |
| RJB was responsible for the study design, statistical analyses, and manuscript preparation. DAM was responsible for management of the study protocol, all data collection, and review of the final manuscript. RLB assisted with the study design and manuscript preparation. All authors read and approved of the final manuscript. |
| Conflict of Interest Statement |
| RJB has been a Consultant for and/or Principal Investigator on research studies funded by various dietary supplement and ingredient companies. RLB is the Director of Research and Development and Technical Support for Bergstrom Nutrition. DAM declares no competing interests. |
References
- Parcell S (2002) Sulfur in human nutrition and applications in medicine. Altern Med Rev 7:22-44.
- Pearson TW, Dawson HJ, Lackey HB (1981) Natural occurring levels of dimethyl sulfoxide in selected fruits, vegetables, grains, and beverages. J Agric Food Chem 29:1089-1091.
- Kim YH, Kim DH, Lim H, Baek DY, Shin HK et al. (2009) The anti-inflammatory effects of methylsulfonylmethane on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biol Pharm Bull 32:651-656.
- http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm153891.htm
- Usha PR, Naidu MUR (2004) Randomised, Double-Blind, Parallel, Placebo-Controlled Study of Oral Glucosamine, Methylsulfonylmethane, and Their Combination in Osteoarthritis. Clin Drug Invest 24: 353-363
- Kim L, Axelrod ND, Howard P, Buratovich ND, Waters RF (2008) Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: a pilot clinical trial. Osteo and Cart 14: 286-294.
- Pagonis T, Givissis A, Aristidis KC, Anastasios CC (2014) The Effect of Methylsulfonylmethane on Oseoarthritic Large Joints and Mobility. Int Journal of Orhtopaedics 1: 19-24.
- Wilcox L (2005) Pharmacokinetic Study in Rats on OptiMSMmicroprill with 0.5% silicon dioxide.Northview Pacific Laboratory Report X4K167.
- Krieger DR, Schwartz HI, Feldman R, Pino I, Vanzant(2009)A Pharmacokinetic Dose-Escalating Evaluation of MSM in Healthy Male Volunteers. Final Report - Miami Research Associates for Bergstrom Nutrition.
- Engelke UFH, Tangerman A, Willemsen MAAP, Moskau D,Moskau D et al. (2005) Dimethyl Sulfone in Human Cerebrospinal Fluid and Blood Plasma Confirmed by One-Dimensional 1H and Two dimensional 1H-13C NMR. NMR in Biomedicine 18: 331-336.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 19276
- [From(publication date):
February-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 14645
- PDF downloads : 4631
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
