Research Article Open Access
Seroprevalence of Influenza A (H1N1) pdm09 Infection and Risk Factors Associated in Pikine, Dakar Region, Senegal
Sylvain Paquet1, Anta Tal-Dia2, Mbayame Ndiaye Niang3, Some Aymar Narodar1, Ndongo Dia3, Debora Goudiaby3, Mayassine Diongue2 and Jean-Yves Le Hesran1*1Mother and Child with Tropical Infections (UMR216), Research Institute for Development Faculty of Pharmacy, Paris Descartes University, Sorbonne Paris Cite, France
2Institute of Health and Development (ISED), Cheikh Anta Diop University, Dakar, Senegal
3National Reference Center for Influenza and Other Respiratory Viruses, Pasteur Institute of Dakar, Dakar, Senegal
- *Corresponding Author:
- Jean-Yves les Hesran
UMR 216, Faculty of Pharmacy, 4 avenue de l’Observatoire
75270 Paris cedex – 6, France
Tel: (33) 1 53 73 15 07
Fax: (33) 1 53 73 96 17
E-mail: yves.lehesran@ird.fr
Received date: November 02, 2016; Accepted date: December 16, 2016; Published date: December 27, 2016
Citation: Paquet S, Tal-Dia A, Niang MN, Narodar SA, Dia N, et al. (2016) Seroprevalence of Influenza A (H1N1) pdm09 Infection and Risk Factors Associated in Pikine, Dakar Region, Senegal. J Emerg Infect Dis 1:119. doi: 10.4172/2472-4998.1000119
Copyright: © 2016 Paquet S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Disease and Pathology
Abstract
There is lack of data describing the A (H1N1) pdm09 virus pandemic in Africa. In 2011, we carried out a crosssectional study that aimed to estimate the prevalence of A (H1N1) pdm09 serology in the general population of Pikine, in the region of Dakar, Senegal. 2669 persons from 347 households were tested for A (H1N1) pdm09 using a hemagglutination inhibition test (threshold of 1/80). The general seroprevalence was of 49,0%. Our result showed a pronounced heterogeneity according to neighborhood (16,7%-71%). Multilevel analysis showed that “covering one’s mouth while coughing” was the only variable related to a reduction of A (H1N1) pdm09 infection (OR=0.50 (0.25- 0.98)). Additionally, variance analysis showed significant effects of neighborhood and household. In conclusion, this study showed intense circulation of the A (H1N1) pdm09 virus among the general population in Pikine. The «Neighborhood» and «household» effects identified require elucidation in order to understand the epidemiology of this epidemic.
Keywords
Senegal; Seroprevalence; Influenza A virus subtype H1N1; Risk factors
Introduction
In June 2009, the WHO declared the influenza A (H1N1) pdm09 virus outbreak as the first pandemic of the 21st century. It reached more than 200 countries and was responsible for over 18,000 deaths worldwide [1]. In Africa, the A (H1N1) pdm09 virus was detected for the first time in Cape Verde in June 2009 [2]. In May 2010, the WHO announced more than 18,000 confirmed cases identified by local monitoring networks [3].
In Senegal, A (H1N1) pdm09 virus was detected by the influenza sentinel surveillance network entrusted to the Pasteur Institute of Dakar (IPD) [4] for the first time in January 2010 with a single epidemic peak in February 2010 [2]. They collected blood samples taken from symptomatic individuals who had consulted a sentinel health center spontaneously. They identified 345 cases of A (H1N1) pdm09/1328 samples (25.9%). This limited recruitment failed to properly assess the incidence of the epidemic A (H1N1) pdm09 virus in the general population [2]. Moreover, self-medication is high in Africa and this recruitment did not include symptomatic people who had not consulted a health center. Furthermore, they did not identify asymptomatic subjects. More generally, general population data for pandemic influenza A (H1N1pdm09) are very rare in subSaharan African countries, consequently limiting the understanding of the dynamics of diffusion of this pandemic in this part of the world.
To overcome this problem, the program “CoPanFlu Senegal” from the CoPanFlu international research program [5] was carried out through a collaboration between the Institute for Health and Development (ISED) in Cheikh Anta Diop University (Dakar), the Institute of Research for Development (IRD, France) and the Pasteur Institute of Dakar (IPD). Similar programs were also developed in Djibouti [6] and Mali [7]. The main objective of CoPanFlu Senegal was to assess the extent of the A (H1N1) pdm09 influenza pandemic in the general population, in Pikine, a dense urban city on the outskirts of Dakar. Other objectives were to determine socio-demographical factors possibly associated with influenza infection A (H1N1) pdm09 and to estimate the effectiveness of protective measures which could have been used by the population in that area.
Methodology
CoPanFlu Senegal is a cross-sectional observational study, which took place between December 2010 and March 2011. The study population comes from 8 neighborhoods of Pikine, a city of about one million inhabitants. Pikine was chosen for two main reasons: 1) In this district, the sentinel surveillance network found 5 cases out of the 14 first cases of A (H1N1) pdm09 detected in this area. This suggested this district as the starting point of the epidemic and offered the opportunity of measuring the expansion of the epidemic in general population. 2) Socio-demographical data from families living in this area were available. These families were visited in 2008 by an IRD team during a study, which investigated the treatment of malaria in the general population (ACTU- PALU-ANR-2007) [8]. For that program, the Dakar region was divided into 2000 Census Districts (CD). 50 CD were then randomly selected. According to the method [8], 60 households were included in each CD. Out of these 50 CD, 21 were located in the area of Pikine. For the current study, we chose the 8 CD closest to areas where the A (H1N1) pdm09 cases were identified
In visited households, we included all people present during the investigators’ visit, respecting three criteria of inclusion: living in the household for more than 6 months, being over 2 years old and having signed (or the tutor) the consent card.
Data collection
For each subject, two types of data were collected: 1) A venous blood sample was taken into a dry tube that was kept in a cooler until delivery to the Pasteur Institute in Dakar (IPD). Influenza serology tests were performed, including A (H1N1) pdm09. 2) A standardized CoPanFlu international questionnaire was completed. Three types of data were collected: 1) Socio-demographical data: area of residence, sex, age, ethnic group, number of inhabitants in the household, level of education. For adults, we collected information on the core business: employees or laborers, self-employed (craftsmen-traders), unemployed; 2) Health data: Antecedent of influenza-like illness, smoking habit, and 3) Information on preventive measures taken against flu.
From the data “core business” and “the school attended”, we defined a variable “main contact type” in order to study the risk of contamination of the subject through the type of contact he has with his social environment. It was defined in 6 classes: (0) children not in school (1) primary school children, (2) children enrolled in secondary school (3) adult carrying on business as an independent (mostly small artisans, merchants), (4) workers (employees or daily activities) and (5) unemployed.
Household level variables were also created from individual data such as “household adopting a measure of anti-cough prevention” coded YES/NO. We calculated for each household the proportion of people reporting “to cover their mouth and nose when coughing or sneezing”. When this ratio was below 0.25, we considered that the subject lived in a home not adopting anti-cough prevention measure. Similarly, the variable “household adopting hand-washing” was coded “NO” if no member had declared washing his hands the day before our visit. The variable “presence of a smoker in household “was coded” NO “if no one smoked and “YES” in the opposite case.
Serology
Sera were collected and sent to the National Reference Center for influenza in a refrigerated cooler (2°C to 8°C) and stored at -20°C until use. Serum samples were tested for specific pandemic A (H1N1) 2009 antibodies by hemagglutination inhibition (HAI) according to the Handbook of the World Health Organization for laboratory diagnosis and virological surveillance of influenza [9]. Sera were treated with RDE enzyme (Receptor Destroying Enzyme; Denka Seiken, Tokyo, Japan) overnight in a water bath at 37°C to remove non-specific inhibitors of hemagglutination and residual RDE was destroyed by inactivation at 56°C for 45 min. The IHA tests were performed in 96 well plates with V bottom with 0.75% to 1% of guinea pig red blood cells. A/California/07/2009 (H1N1) antigen obtained from the Centers for Diseases Control and Prevention (CDC, Atlanta) was used. Sera were tested in duplicate from the 1/10 dilution. The result is rendered as the HI, which corresponds to the reciprocal of the last dilution in which a hemagglutination inhibition is observed. Antibody titers ≥ 1:80 were considered positive for A/H1N1pdm09 because of the high specificity of this threshold for infection [10].
Analysis strategy
A descriptive analysis of characteristics of households and individuals was performed. Descriptive analysis of results of serology at different dilution levels is presented and at the threshold of 1/80 for each neighborhood of the study area. A bivariate analysis crossing serological results at the threshold of 1/80 and different socio-demographical and prevention variables were then performed. Variables with “p” less than or equal to 0.20 were included in a multivariate analysis. Due to hierarchical data contained in “neighborhoods”, “homes” and “individuals”, a multilevel logistic model was used. The share of the total variance (σ2total) assigned to a specific level (σ2neighborhoods σ2homes) was first estimated for the empty model, then for each explanatory variable in bivariate analysis and then for the final model. Additional, random effect was tested for each household-level variable in the final model. Analyses were conducted with a significance level of 5%. We used Stata version 13.0 software.
The National Ethics Committee for Scientific Research of Senegal gave its approval for this study.
Results
Study population
Among 480 households registered in ACTU-PALU base and identified for inclusion in the Senegal-CoPanflu study, 409 households were found (50 had moved and 21 were not found). At the first visit, 394 of them have agreed to participate in the study. At the second visit, 47 heads of household refused the blood test. In total, 347 households were surveyed and 2669 persons were tested. The average proportion of people sampled per household was 58.9%.
Household description
The average number of people per household was 13.1, median=11.5 (1-61). Over 90% of households were made up of at least 6 people. Only 7 families were childless.
Socio-demographical characteristics
Our sample included 1622 women (61%) and 1038 men (39%), giving a sex ratio of 0.64. Within subjects over 15 years, the proportions were 34% men versus 66% women. Amongst those under 15 years, 48.4% were male versus 51.6% female (Table 1).
| Variables | n | % |
|---|---|---|
| Gender | ||
| Men | 1038 | 39% |
| Women | 1622 | 61% |
| Age | ||
| [2-6] | 220 | 8.3% |
| [6-15] | 706 | 26.5% |
| [15-25] | 626 | 23.5% |
| [25-45] | 711 | 26,7% |
| [45-65] | 294 | 11% |
| ≥65 | 103 | 3.87% |
| Ethnicity | ||
| Wolof | 1270 | 47.7% |
| Sérere | 307 | 11.5% |
| Bambara-Sonike- mandingue | 205 | 7.7% |
| Pular | 690 | 25.9% |
| Other | 188 | 7% |
| School Children (6 ans -15ans) | 599 | 85.8% |
| Adults (≥15 ans) | ||
| Women | 544 | 51.6% |
| Men | 363 | 71.7% |
| Work (15-49 years) | ||
| Working women | 332 | 38,8% |
| Working men | 289 | 69.2% |
| Type of job | ||
| Employees or daily activities | 613 | 85.6% |
| Independent worker | 103 | 14.4% |
| Individual with chronic disease | ||
| All ages | 680 | 25.6% |
| <15 years | 141 | 15.2% |
| ≥ 15 years | 539 | 31.1% |
| Smokers (≥15ans) | ||
| Men | 90 | 15.35% |
| Women | 4 | 0.34% |
| Anti-cough prevention method | ||
| Yes | 1373 | 51.6% |
| Number of hand washing the day before the visit | ||
| 0 | 1994 | 74.9% |
| 1-2 | 491 | 18.4% |
| ≥ 3 | 175 | 6.5% |
Table 1: Population characteristics, Pikine, Senegal, 2010 (n=2660).
The average age of participants was 24.9 years, with a median age of 21 (2-92). The ethnicity of the participants as well as their age distribution was consistent with the Demographic Health data (EDS-MICS 2010) which is representative of the Senegalese population [11].
Individual health data
223 people (8.4%) reported flu over the past 3 years. Nine individuals reported receiving an anti-influenza vaccine in the past 3 years; they were excluded from the analysis.
Measures reported to prevent flu
1287 people (48.3%) answered “no” to the question “Do you cover your mouth when you cough or sneeze?” Regarding the question, “How many times have you washed your hands with soap yesterday?” 1994 people (74.9%) respond “0”, 288 people (10.3%) “1 time” 203 people (7.7%) “2 times” and 175 people (6.5%) “3 times or more” in ninety households (25.9%), all subjects reported not having washed their hands the day before our visit.
Seroprevalence of A (H1N1) pdm09
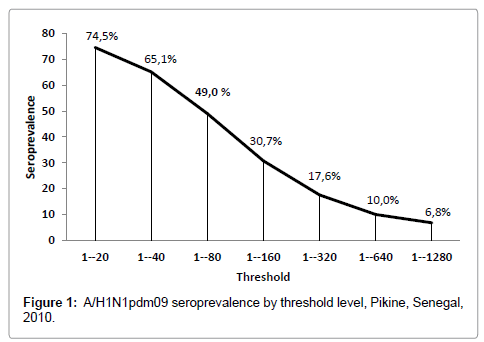
At 1/80th dilution threshold, the overall prevalence of A (H1N1) pdm09 in the study area was 49.0% (47.2-51.0). From 1/20th to 1/640th threshold, the rate of positive A (H1N1) pdm9 decreases slowly, from 74.5% to 10.0% (Figure 1).
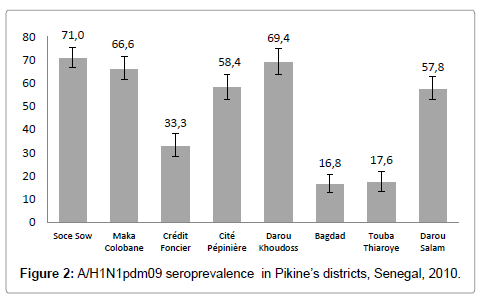
A (H1N1) pdm09 seroprevalence differed significantly between neighborhoods (p<0.001). The lowest prevalence was in Baghdad neighborhood, 16.8% (12.8-20.7) and the highest was in Soce Sow neighborhood, 71.0% (66. 7-75.3) (Figure 2).
Individual socio-demographic variables and health data reported
There was no significant association between either sex or ethnicity and A (H1N1) pdm09 serology. Age class was globally significantly associated with A (H1N1) pdm09 positivity (p global=0.03). Only the 2-5 years age group had a significantly higher positivity than the reference class (25-44 years) (OR=1.83 (1.25-2.69)). The link between A (H1N1) pdm09 serology and variable “main contact type” was close to significance (p=0.056). Among people who reported a history of flu over the past three years, only 39.9% were positive for A (H1N1) pdm09.
Variables related to household composition
There was no significant association between A (H1N1) pdm09 serology and (i) the number of people living in the household (p=0.27), (ii) the number of children in the household (p=0.46), (iii) the number of school attending children living in the household (p=0.91).
Protective measures against A (H1N1) pdm09 flu
The variable “how many times did you wash your hands with soap yesterday” was coded into three classes (0 times, 1-2 times or ≥ 3 times per day) and was significantly associated with a reduction in A (H1N1) pdm09 infection (p global=0.03).
Regarding household variables, “living in household having adopted hand washing” was not associated with decrease of A (H1N1) pdm09 infection (OR=0.72 (0.48-1.07); p global=0.11). However, “living in a household having adopted anti-cough prevention method” was significantly associated with a decrease of A (H1N1) pdm09 infection (OR=0.44 (0.24-0.84), p=0.01).
Multivariate analysis
We included in the multivariate model variables with p ≤ 0.20 in the bivariate analysis: “type of main contact”, “hand washing the day before”, “household having adopted home hand washing “,” household having adopted anti-cough prevention method”, “presence of a smoker at home” (Table 2). We chose not to include age in the model because of its high co-linearity with the variable “type of main contact”. Only “home have adopted a method of anti-cough prevention” was significantly associated with a reduced risk of individual A (H1N1) pdm09 infection (OR=0.50 (0.25-0.98); p=0.045).
| VARIABLES | OR | IC 95% | p |
|---|---|---|---|
| Gender (W/M) | 1.14 | 0.93-1.41 | 0.192 |
| Type of main contact | |||
| Non-schooled Children | 1 | ref | |
| Primary school children | 1.04 | 0.72-1.50 | |
| Secondary school children | 1.30 | 0.82-2.06 | |
| Independent worker | 0.52 | 0.27-0.96 | 0.077 |
| Employees or daily activities | 0.96 | 0.67-1.39 | |
| Unemployed | 0.86 | 0.61-1.22 | |
| Number of hand washing the day before the visit | |||
| 0 | 1 | ref | |
| 1 ou 2 per day | 0.92 | 0.70-1.22 | 0.058 |
| ≥ 3 fois per day | 0.58 | 0.37-0.90 | |
| Households having adopted home hand washing | |||
| Yes | 0.88 | 0.56-1.38 | 0.58 |
| Household having adopted anti-cough prevention method | |||
| Yes | 0.50 | 0.25-0.98 | 0.045 |
| Smoker in household | |||
| Yes | 0.81 | 0.54-1.22 | 0.33 |
Table 2: Determinants of H1N1 seroprevalence- multivariate analysis, pikine, Senegal, 2010 (n=2644).
Analysis of variance
In the empty model, the total variance was 6.14. Individual level explained 53.6% of the variance while characteristics of households and neighborhoods explained respectively 21.8% and 24.6% of the variance.
In the final model, the results were similar: the total variance was 6.12. Individual level represents 53.8% of the variance while households and neighborhoods levels explained respectively 21.9% and 24.3% of the variance.
Additionally, a random effect variable at household level was studied. No model was significantly better than the simple random intercept model, which was the one retained.
Discussion
Our study shows that 49% of subjects tested positive to A (H1N1) pdm09 which is very high when compared with studies already published in sub-Saharan African countries [6,7,11,12]. This information within the general population is essential since it takes into account symptomatic individuals who did not use health centers as well as asymptomatic subjects. However, can we attribute these antibodies to the last A (H1N1) pdm09 influenza pandemic? We did not know the pre-epidemic status of individuals. Therefore, we chose a positivity threshold of 1/80th to favor the specificity of the test compared to the threshold of 1/40th (sp>96% versus 72%) applied in other studies [13,14]. This threshold allows us to consider as positive those subjects with a high level of A (H1N1) pdm09 neutralizing antibodies, equating to a seroconversion and confirming a recent infection [7,15,16]. However, the sensitivity of the test is lower than with a 1/40th threshold (68% vs. 90%), creating potential false negative tests. This could lead to an underestimation of the prevalence of A (H1N1) pdm09 positivity.
Conversely, vaccination against flu could make us overestimate the prevalence of A (H1N1) pdm09 flu. Only 9 subjects reported having received flu vaccine in the past three years. In the same way, it is important to note that the main flu virus circulating in Senegal during the two years preceding our study [17] suggested no cross reactivity with A (H1N1) pdm09.
47 heads of household refused to participate in the study during the second visit. This could constitute a selection bias. Our data shows that a huge majority of A (H1N1) pdm09 infections remained asymptomatic and there was nothing to suggest that households would have agreed to participate differently depending on whether there was an A (H1N1) pdm09 infection or not in the household. The main reason for their rejection was the blood sample. In the same sense, the relative lack of men in our sample is simply due to migration or to working away from home.
The study area was chosen because of the initial detection of 5/14 of the first cases of pandemic influenza activity. The choice of this area in particular could partly explain the observed high prevalence. However, the prevalence was very heterogeneous between neighborhoods (min 16.7% to max 71%) and indicated highly variable spread of the virus depending on the area. We therefore believe that our data represent a good reflection of the epidemic diffusion phenomenon in urban areas such as Pikine city. These results suggest specific factors linked to environmental, economic and/or social situations. In the Djibouti urban area, the general prevalence was 29.1% at 1/80 threshold. That study also observed a very heterogeneous seroprevalence between neighboring districts [6]. The authors did not find any satisfactory hypotheses to explain this observation except for one district for which they evoked the presence of a more precarious population (refugees) in the area.
Thus, as suggested by our variance analysis, socio-demographical characteristics or the cultural specificity of neighborhoods could contain factors related to dissemination of the disease. Several studies have found that urban areas could be a favorable place for the spread of A (H1N1) pdm09 [18,19]. Conversely, in Mali, out of a sample of 202 people from Dioro district in a rural area, they found a lower prevalence, 16.3% (11.5-22.2) [7], which corroborates this hypothesis.
In France, in a representative sample of the population, the rate of prevalence was of 18.8% [11]. The average age of the study population was greater than in our study (43.1 vs. 25 years). Young age is described as a risk factor for infection [6,12,13]. However, this difference in age is not sufficient to explain the difference of prevalence observed between the 2 studies.
In a meta-analysis, Van Kerkhove estimated the average prevalence of 32% (26-39%) at 1/40 threshold [12]. However, they clearly also illustrate the heterogeneity of the results (Appendix 1). They also highlighted the diversity of methodologies used in these studies and the lack of standardized protocols. Indeed, techniques, thresholds and delay between sampling collection and epidemic peak are different from one study to another. That is the reason why, since 2011, an international working group on sero-epidemiological studies of influenza infections (Concise) has been trying to harmonize research protocols, but a guide for influenza A (H1N1) pdm09 [20] is not yet available.
A (H1N1) pdm09 determinants of infection
The level of “external contact” is regularly used in the literature to investigate potential determinants of influenza infection [21-24]. Therefore, we created a “type of main contact” variable. This variable is highly colinear with age. The variable “type of main contact” was finally retained. It provides more information than age because of the potential existence of several types of “primary contact” in the same age group. Reference class chosen for this variable, “lack of education”, represents the slightest contact with the outside world, and concerns mostly very young children. Our result is similar to CoPanFlu Djibouti study where outdoor working conditions were significantly associated with a lower risk of infection A (H1N1) pdm09 [6]. For the group “primary or secondary school”, there was no association with A (H1N1) pdm09 when compared with children not attending school. However, other studies have revealed quite different results [6,11,16] which could be related to different school characteristics (level of education, food, school life). In conclusion, no contact is exclusive and all other contacts could dilute the effect of contact linked to business or school activities. Moreover, if the first contamination occurred outside the home, there would be secondary intra-household contamination, from 3 to 38% according to the meta-analysis of Lincoin [25]. These secondary intra-household contaminations complicate the highlighting of “primary contact” as a potential factor of A (H1N1) pdm09 infection.
Individual and collective protection measures
In the final multivariate model, the association between “individual washing hands” and A (H1N1) pdm09 infection was of borderline significance (p=0.058): frequent hand-washing (at least three times daily) was linked with a reduction of influenza infection as previously described in other studies [26]. On the contrary, this variable considered at the household level “household having adopted hand-washing” was not associated with a reduction of individual infection. This result has already been described by Warren-Gash [27]. Thus, individuals washing their hands would protect themselves against influenza infection, but would have no impact on the protection against infection of other household members.
On the other hand, the variable “household having adopted anti-cough prevention method” is significantly associated with a reduction of A (H1N1) pdm09 infection. A similar result was highlighted by Delabre [16]. However, in case of responses being given by the mother for all the children, her home could amplify any error in estimating the strength of association [28]. In addition, the threshold chosen for this variable is arbitrary and could be subject to discussion.
Moreover, the questionnaire was planned by international CoPanFlu for a European population. It should therefore be used with caution particularly for questions about protection measures used since they could be not enough adapted to the subjects living in Pikine.
Analysis of Variance
There is no difference between total variance among the empty model and the final model. This is consistent with the fact that we have only one significantly associated variable with the variable of interest in multivariate analysis. The “household” effect, as well as the “neighborhood” effect, represents nearly a quarter of the total variance in the final model, close to that recorded in the empty model. Individual and household variables had no effect on the “household “or “neighborhood” effects which remain to be fully explained.
Our data showed wide variability from one neighborhood to another. Neighborhood factors are definitely the key to understanding the dynamics of the spread of A (H1N1) pdm09 in the general population. One should study the areas in terms of socio-economic characteristics, population density, health service, etc., to understand these differences.
Limits of the Study
We discussed above the limits concerning the threshold of positivity. Another limit is the extension of our sample prevalence to the general population. Indeed, we collected samples in different neighborhoods. H however, as we had no precise demographic information for 2010 in these areas, we have not applied a weighting coefficient based on the population density in each neighborhood. However, Pikine is a very popular district near Dakar city, with a homogeneously high population density across the whole zone.
Conclusion
This study provides new information about the spread of A (H1N1) pdm09 flu in southern countries. The seroprevalence observed in Pikine (49%) is one of the highest seroprevalences published. This result, obtained from assessment of 2660 individuals, shows that pandemic influenza A (H1N1) pdm09 circulated widely in Pikine. However, the results also show a heterogeneous distribution between neighborhoods and it would be important to identify the risk factors associated with neighborhood to understand the dynamics of the spread of this highly contagious influenza disease in southern countries.
Acknowledgments
Acknowledgements to the investigators from ISED and IRD for their hard work in the field and thanks to the population of Pikine for their welcome. Acknowledgements to Richard Lalou and Stéphanie Dos Santos (IRD researchers) for the collection of demographic data in 2008. Thanks to Adrian Luty and William Sackey for their English re-reading of this article. This study was support by grants from IRD and EHESP.
References
- World Health Organisation (WHO)(2010) Pandemic H1N1.
- Dia N, Niang MN, Monteiro M, Koivogui L, Ould Bara M, et al. (2013) A Subregional Analysis of Epidemiologic and Genetic Characteristics of Influenza A(H1N1)pdm09 in Africa: Senegal, Cape Verde, Mauritania, and Guinea, 2009–2010. Am J Trop Med Hyg 88: 946-953.
- WHO(2009) Pandemic (H1N1) 2009 in the African Region. Geneva.
- Niang MN, Dosseh A, Ndiaye K, Sagna M, Gregory V, et al. (2012) Sentinel Surveillance for Influenza in Senegal, 1996-2009. J Infect Dis 1:129-135.
- Lapidus N, De Lamballerie X, Salez N, Setbon M, Ferrari P, et al.(2012) Integrative study of pandemic A/H1N1 influenza infections: design and methods of the CoPanFlu-France cohort. BMC Public Health 2:417.
- Andayi F, Crepey P, Kieffer A, Salez N, Abdo AA, et al. (2014) Determinants of individuals’ risks to 2009 pandemic influenza virus infection at household level amongst Djibouti city residents - A CoPanFlu cross-sectional study. Virol J11:13.
- KoitaOA, Sangare L, Poudiougou B, Aboubacar B, Samake Y, et al. (2012) A seroepidemiological study of pandemic A/H1N1(2009) influenza in a rural population of Mali. ClinMicrobiol Infect 18:976-981.
- Lalou R (2008) ACTU-PALU 2008.
- WHO(2011) Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza.
- Delangue J, Salez N, Ninove L, Kieffer A, Zandotti C, et al. (2012) Serological study of the 2009 pandemic due to influenza A/H1N1 in the metropolitan French population. ClinMicrobiol Infect18:177-183.
- Lapidus N, de Lamballerie X, Salez N, Setbon M, Delabre RM, et al. (2013) Factors Associated with Post-Seasonal Serological Titer and Risk Factors for Infection with the Pandemic A/H1N1 Virus in the French General Population. PlosONE8: e60127.
- Van Kerkhove MD, Hirve S, Koukounari A, Mounts AW (2013) Estimating age-specific cumulative incidence for the 2009 influenza pandemic: a meta-analysis of A(H1N1) pdm09 serological studies from 19 countries. InfluenzaRespir Virus 7: 872-886.
- Kieffer A, Paboriboune P, Crepey P, Flaissier B, Souvong V, et al. (2009) A(H1N1) Seroconversion Rates and Risk Factors among the General Population in Vientiane Capital, Laos (2013). PLoSONE 8: e61909.
- NicolasS (2013) Contribution to the sero-epidemiological study of the flu.Thesis: specialty communicable diseases and tropical parhologies.University of Marseille138.
- Demographic and Health Survey of Multiple Indicators in Senegal (EDS-MICS) 2010-2011. National Agency of Statistics and Demography (ANSD), Senegal 2012.
- DelabreRM, Lapidus N, Salez N, Mansiaux Y, De Lamballerie X, et al. (2015) Risk factors of pandemic influenza A/H1N1 in a prospective household cohort in the general population: results from the CoPanFlu-France cohort.Influenza Respir Virus9:43-50.
- Niang MN, Dosseh A, Ndiaye K, Sagna M, Gregory V, et al. (2012) Sentinel Surveillance for Influenza in Senegal, 1996-2009. J Infect Dis 1:129-135.
- Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, et al. (2009) Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 375: 1100-1108.
- Broor S, Krishnan A, Roy DS, Dhakad S, Kaushik S, et al. (2012) Dynamic Patterns of Circulating Seasonal and Pandemic A(H1N1)pdm09 Influenza Viruses From 2007–2010 in and around Delhi, India. PLoSONE 7:e29129.
- WHO (2010) Weekly epidemiological record (REH)85: 229-236.
- Van Kerkhove MD, Broberg E, EngelhardtOG, Wood J, Nicoll A (2013) The consortium for the standardization of influenza seroepidemiology (CONSISE): a global partnership to standardize influenza seroepidemiology and develop influenza investigation protocols to inform public health policy. Influenza Respir Virus 7: 231-234.
- Ajelli M, Poletti P, Melegaro A, Merler S (2014) The role of different social contexts in shaping influenza transmission during the 2009 pandemic. Sci Rep4- 7218.
- Cauchemeza S, Bhattaraib A, Marchbanksc TL, Fagan RP, Ostroff S (2011) Role of social networks in shaping disease transmission during a community outbreak of 2009 H1N1 pandemic influenza. Proc Nat AcadSci USA 108:2825-2830.
- Glatman-Freedman A, Portelli I, Jacobs SK, Mathew JI, Slutzman JE, et al. (2012) Attack Rates Assessment of the 2009 Pandemic H1N1 Influenza A in Children and Their Contacts: A Systematic Review and Meta-Analysis. PLoS ONE 2012 7: e50228.
- Lau L, Nishiura H, Heath K, Dennis KM, Leung G, et al. (2012) Household transmission of 2009 pandemic influenza A(H1N1): a systematic review and meta-analysis. Epidemiology 23: 531-542.
- LiuW, YangP, DuanW, WangX, ZhangY , et al.(2010) Factors Associated with seropositivity of 2009 H1N1 Influenza in Beijing, China.Clin Infect Dis 51:251-252.
- Warren-Gash C, Fragaszy E, Hayward AC (2012) Hand hygiene to reduce community transmission of influenza and acute respiratory tract infection: a systematic review. Influenza and Other Respiratory Viruses 7: 738-749.
- Mansiaux Y, Salez N, Lapidus N, Setbon M, Andreoletti L, et al. (2015) Causal analysis of H1N1pdm09 influenza infection risk in a household cohort. J Epidemiol Community Health 69:272-277.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 3515
- [From(publication date):
December-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 2561
- PDF downloads : 954