Seroprevalence of Hepatitis C Virus and Human Immunodeficiency Virus among HBsAg Positive Patients-A Cross-Sectional Study
Received: 26-Jan-2022 / Manuscript No. jcmhe-22-52549 / Editor assigned: 28-Jan-2022 / PreQC No. jcmhe-22-52549 (PQ) / Reviewed: 11-Feb-2022 / QC No. jcmhe-22-52549 / Revised: 16-Feb-2022 / Manuscript No. jcmhe-22-52549 (R) / Published Date: 23-Feb-2022 DOI: 10.4172/2168- 9717.1000738
Abstract
Background and objectives: Human immunodeficiency virus (HIV), Hepatitis C virus (HCV) and Hepatitis B virus (HBV) co-infections are common due to their overlapping modes of transmission. Since, there has been a rapid increase in the HBV cases and sparse literature regarding the viral co-infections in Belagavi, the current study aimed to assess the prevalence and factors influencing the co-infection of HCV and HIV among Hepatitis B surface antigen (HBsAg) positive patients in Belagavi.
Methodology: Serum samples were collected from 3278 subject and screened for HBsAg by Rapid Test and later positive samples were confirmed by HBsAg ELISA. The samples were further screened for HCV ELISA (Enzyme-linked immunosorbent assay) and HIV rapid test and ELISA for confirmation. Data analysis was done using Chi- square test and categorical outcomes were studied using percentage. p<0.05 was considered as statistically significant.
Results: Of the 75 HBsAg positive patients, prevalence peaked in the age range of 33-47 years. Sexual contact (18.67%) was the common mode of transmission. HBsAg+HIV co-infection was 5%, while HBsAg+HCV and HBsAg+HCV+HIV coinfection was found to be 0%.
Conclusion: The study observed a high prevalence of HBsAg and HIV co-infections among the Belagavi population, emphasizing the need for a thorough screening for all the HBV patients.
Keywords: Coinfection; Enzyme-linked immunosorbent assay; Hepatitis B surface antigens; Hepatitis C; HIV
Keywords
Coinfection; Enzyme-linked immunosorbent assay; Hepatitis B surface antigens; Hepatitis C; HIV
Introduction
Human Immunodeficiency Virus (HIV), Hepatitis C Virus (HCV) and Hepatitis B Virus (HBV) are Retrovirus, RNA virus and DNA virus, respectively. Besides the variations in their genetic makeup, the mechanism of target organ systems, host cell integration and life cycles are also different [1]. But these viruses have similar routes of transmission, such as through blood and blood products, sharing of needles to inject drugs and sexual activity [2]. World-wide these infections are documented as the most prevalent chronic viral infections [3]. According to the survey in 2015, worldwide 36.7 million people were infected with HIV, 257 million people with chronic HBV infection, and 71 million people with HCV infection [4].
Based on the literature, rates of co-infection between HIV, HCV and HBV vary from region to region, study population and risk factors for virus acquisition. It is reported that co-infections rates of HIV-HBV and HCV-HBV are about 2-4 million and 4-5 million, respectively [4,5]. It is observed that the presence of a single virus influenced the life cycle of the other viruses. Co-infections of HBV, HIV and HCV viruses have a negative impact on the liver and often complicate the management of the disease. HCV and HBV co-infected patients are at high risk of developing cirrhosis while HIV and HBV co-infections also increase the toxicity to antiretroviral medications [6,7]. Although the pathogenesis of liver diseases in co-infected patients is controversial, immunologic alterations such as immune activation, apoptosis (Fas/FasL pathway accelerated apoptosis of the hepatocytes) and direct viral effects could be considered [8]. Co-infections not only complicate the disease management but it also leads to casualties due to hepatic pathologies [9].
The prevalence of co-infection of HBV and HCV in countries like Italy, Spain, Taiwan, Japan and China was reported to be 7%-14%, 10-13%, 3.4-18%, 13%-22% and 11%-15% respectively. Study reports from Iran showed that 7.8% were co-infected by HIV, HBV and HCV. In India, the prevalence of HBV and HCV co-infection among patients has been estimated up to 3%, while the co-infection of HBV and HIV is estimated upto 13.7 % [7,10].
Hepatitis B surface antigen (HBsAg), a HBV viral coat antigen produced in large quantities in the infected cell cytoplasm and is produced in patients with chronic, active HBV infection. Sensitivities and specificities of current testing methods for HBsAg are high [10,11]. The sensitivity and specificity for HBsAg in detecting HBV is 90% and 99.5% respectively [12].
Diagnosis of these viruses is through Enzyme Immunoassays (EIAs) and Electrochemiluminescence (ECLIA) with serum or plasma samples. However these assays mandate infrastructure and skilled man power. As an alternative, rapid test offers several benefits such as they are easy to perform, requires minimal training and provides conclusive and rapid results. Additionally, these tests may be performed on a case-by-case basis and eliminating the need for require laboratory infrastructure [13]. Rapid tests and Enzyme-linked immunosorbent assay (ELISA) are efficacious tools to assess the co-infection because Rapid test devices have a sensitivity >99% and specificity >98% while ELISA can detect different variables [14].
Recent literature review suggested a scarcity of studies among the geographical region of North Karnataka in this regard. Therefore, the current study was undertaken to assess the prevalence of co-infection of HCV and HIV among HBsAg positive patients in Belagavi.
Materials and Methods
This cross-sectional study was carried out in the Department of Microbiology, Belagavi (Northern Karnataka, India) from January 2017 to December 2017. The study was approved by the Institutional Ethics Committee and an informed consent was obtained from all the patients included in the study and strict confidentiality was followed for all the investigations.
Sampling method
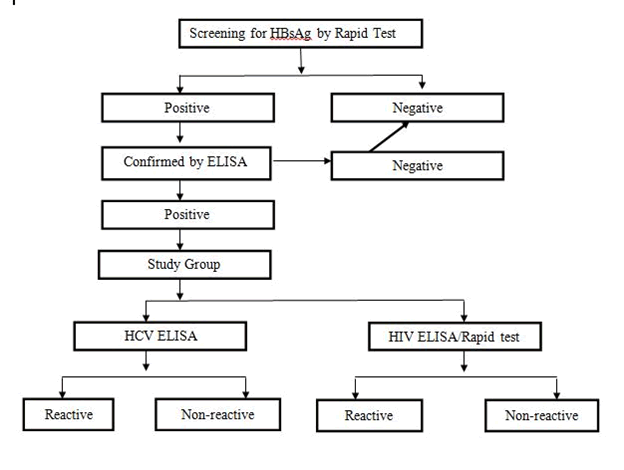
Universal Sampling Technique was used for collection of sample. Out of total 3278 subjects, 75 subjects included in the study based on screening on HBsAg positive patients (Figure 1).
The serum samples were tested with Hepacard (Reckon Diagnostics P limited, Gorwa, and Baroda, India) a rapid immunochromatographic assay, following the manufacturer’s instructions. Two drops (70 μl) of human serum/plasma specimen was added into the sample well and the results were read after 20 minutes of the reaction. The appearance of pink coloured line, one each in test region “T” and control region “C” was taken as the sample is “REACTIVE” for HBsAg and appearance of one distinct pink line in the control region “C” was taken as that the sample is “NON REACTIVE” for HBsAg. When neither control line nor the test line appeared on the membrane the test was treated as invalid.
The positive samples from Rapid test were confirmed with HEPALISA- Microwell ELISA Test (J. Mitra and Co. Pvt Ltd., New Delhi, India) for the detection of HBsAg in Human Serum/Plasma samples following the manufacturer’s instructions. Fresh human serum or plasma samples were used, and the specimens were stored at 2°C-8°C for one week, or frozen at -20°C or lower. The absorbance (O.D.-optical density) was measured at 450 nm within 30 minutes in the Enzyme-linked immune sorbent assay (ELISA reader). Test specimens with O.D. less than cut-off value were non-reactive and were considered as negative for HBsAg. Test specimens with O.D. values greater than or equal to cut-off value was positive for HBsAg by HEPALISA. Test specimens with absorbance value within 10% below the cut-off was suspected of the presence of HBsAg.
HIV rapid whole blood finger prick test was performed using MERISCREEN HIV 1-2 WB (Meril Diagnostics) following the manufacturer’s instructions. Two drops (70 μl) of human serum/plasma specimen was added into the sample well. After 20 minutes, the results were read, and the card was discarded appropriately. Appearance of only the control band, corresponding to control region ‘C’ indicated negative results. In addition to the control band ‘C,’ appearance of reactive band in the test region ‘1’ indicated positive results for antibodies to HIV-1. In addition to the control band ‘C,’ appearance of reactive band in the test region ‘2’ indicated positive result for antibodies to HIV-2. In addition to the control band ‘C,’ appearance of reactive bands in the test region ‘1’ and test region ‘2’ indicated positive result for antibodies to HIV-1 and HIV-2. If neither, the test band nor the control band appeared, the test was treated as invalid and repeated.
Microwell ELISA test was performed for the detection of Antibodies to HIV-1 (Including Group O & Subtype C) and HIV-2 in Human Serum/Plasma using Microlisa-HIV (J. Mitra and Co. Pvt Ltd, New Delhi, India) following the provided instruction’s manual. After reading the absorbance at 450 nm within 30 minutes, test specimens with absorbance value less than the cut-off value was considered as negative for anti-HIV. Test specimens with absorbance value greater than or equal to the cut-off value was considered reactive for anti-HIV by Microlisa-HIV. Specimens with absorbance value equal to or greater than the cut-off values were considered initially reactive by the criteria of Microlisa-HIV and retested.
Microwell ELISA Test for the detection of antibodies to Hepatitis C virus in human serum/plasma was carried out using HCV Microlisa (J. Mitra & Co. Pvt. Ltd., New Delhi, India) following the manufacturer’s instructions. After reading the absorbance at 450 nm within 30 minutes, test specimens with absorbance value less than the cut-off value were non-reactive for Anti-HCV. Test specimens with the absorbance value greater than or equal to the cut-off value were reactive for anti-HCV.
Test specimens with absorbance values within 10% below the cut-off were suspected of the presence of antibodies and was retested in duplicate. If both duplicate retest sample absorbance value is less than cutoff value, the specimen was considered nonreactive. If any one of the duplicate retest sample absorbance value is equal to or greater than the cutoff or both duplicate retest value are equal to or greater than the cutoff, the specimen was considered reactive by the criteria of HCV Microlisa.
Standard operating protocol for handling the infectious materials were followed for all the tests and precautions were taken while labeling the test sample with the patient’s name or identification number.
Data was analyzed using R software version 3.6.1 and Excel. Categorical variables were recorded given in the form of frequency tables. Continuous variables were represented as mean ± Standard deviation (SD). p<0.05 was considered as statistically significant.
Results
Of the 3278 patients screened for HBsAg, 75(2%) were positive for HBsAg via the card test which was later confirmed by ELISA. The mean age of the study subjects was 46.87 ± 14.45 years. Baseline features of the study population are tabulated in (Table 1).
Table 1: Baseline characteristics of the cohort.
| Factors | Count (%) | |
|---|---|---|
| HBsAg positive | 75 (2%) | |
| Age groups (years) |
18-32 | 12 (16%) |
| 33-47 | 29 (38.67%) | |
| 48-62 | 19 (25.33%) | |
| 63-78 | 15 (20%) | |
| Sex | Male | 56 (74.67%) |
| Female | 19 (25.33%) | |
| Occupation | Farmer | 33 (44%) |
| Driver | 10 (13.33%) | |
| Business | 2 (2.67%) | |
| Student | 4 (5.33%) | |
| Housewife | 16 (21.33%) | |
| Retailer | 5 (6.67%) | |
| Tailor | 2 (2.67%) | |
| Peon | 1 (1.33%) | |
| Priest | 1 (1.33%) | |
| Road Construction worker | 1 (1.33%) | |
| Educational qualification | Illiterate | 42 (56%) |
| Up to 10th | 27 (36%) | |
| Pre-University and above | 6 (8%) | |
Among these 75 participants, fever (n=40) was the most common compliant among the HbsAg positive subjects. Fourteen subjects had a history of high risk of sexual activity (Table 2). Sexual transmission was the most common mode of transmission 75(100%).
Table 2: Case history details of the subjects.
| Factors | Sub-category | Count (%) |
|---|---|---|
| Complaints | Fever | 40 (53.33%) |
| Loss of appetite | 18 (24%) | |
| Nausea and vomiting | 15 (20%) | |
| Abdominal pain | 10 (13.33%) | |
| Jaundice | 5 (6.67%) | |
| Weight loss | 7 (9.33%) | |
| Cough | 4 (5.33%) | |
| History | Sexual activity | 14 (18.67%) |
| TB | 3 (4%) | |
| Blood transfusion | 2 (2.67%) | |
| Any STD | 1 (1.33%) | |
| DM | 0 (0%) | |
| Injectable drug abuse | 0 (0%) | |
| Habits | Smoking | 35 (46.67%) |
| Alcohol | 33 (44%) |
Of the 56 male patients, HIV co-infection was seen in 4 (7%) subjects. This co-infection was absent among females. Co-infection of HBsAg+HCV and HBsAg+HCV+HIV was absent (Table 3).
Table 3: Distribution of patients with HBsAg, HIV and HCV Co-Infection.
| Co-Infection | Count (%) |
|---|---|
| HBsAg + HIV | 4 (5%) |
| HBsAg + HCV | 0 |
| HBsAg + HCV + HIV | 0 |
Using Chi-square test with simulation, loss of appetite (p=0.04) and loss of weight (p=0.002) were significantly associated with HIV co-infection in HbsAg subjects. History of similar complications (p=0.007), high risk of sexual activity (p=0.02) and consumption of alcohol (p=0.04) were significantly associated with HIV infection (Table 4).
Table 4: Comparison of HBsAg infected subjects.
| HBsAg infected subjects | |||||
| Positive | Negative | p-value | |||
| Demographic details | Age (years)(mean ± SD) | 44.75 ± 8.21 | 46.99 ± 14.74 | 0.8 | |
| Sex | Male | 4 | 52 | 0.3 | |
| Female | 0 | 19 | |||
| Educational level | Illiterate | 2 | 40 | 0.5 | |
| Up to 10th | 1 | 26 | |||
| Pre-University and above | 1 | 5 | |||
| Chief Complaints |
Fever | Y | 4 | 36 | 0.1 |
| N | 0 | 35 | |||
| Nausea or vomiting | Y | 1 | 14 | 1 | |
| N | 3 | 57 | |||
| Abdominal pain | Y | 1 | 9 | 1 | |
| N | 3 | 62 | |||
| Jaundice | Y | 0 | 5 | 1 | |
| N | 4 | 66 | |||
| Loss of appetite | Y | 3 | 15 | 0.04* | |
| N | 1 | 56 | |||
| Loss of weight | Y | 3 | 4 | 0.002* | |
| N | 1 | 67 | |||
| N | 2 | 70 | |||
| Sexual Activity | Y | 3 | 11 | 0.02* | |
| N | 1 | 60 | |||
| TB | Y | 1 | 2 | 0.2 | |
| N | 3 | 69 | |||
| Blood Transfusion | Y | 0 | 2 | 1 | |
| N | 4 | 69 | |||
| Habits | Smoking | Y | 3 | 32 | 0.3 |
| N | 1 | 39 | |||
| Alcohol | Y | 4 | 29 | 0.04* | |
| N | 0 | 42 | |||
Discussion
HIV and HCV co-infections among HBV infected patients have been reported infrequently from region to region [8]. There by the current study evaluated the prevalence of co-infection of HCV and HIV among HBsAg positive patients in Belagavi through ELISA and Rapid tests. The study also probed the factors affecting HBsAg positive patients.
The prevalence of HbsAg positive was quite low i.e. 2%. Similar results were observed by Malhotra et al., (1.5%), Negero et al., (4.5%-6.8%) and Arun et al., (4.94%) [15-17]. Higher prevalence of HbsAg positives (26%) were reported in a study conducted in Punjab [18]. The present study reported positive findings exclusively among the male subjects. High prevalence among males could be attributed promiscuity [19].
In the study, the age group between 33-47 years was frequently affected and this correlates with a study conducted by Yakasiri et al [20]. In the current study, the maximum HBsAg positive patients were farmers (44%) and analphabetic (56%) which is in agreement with a study conducted by Yang et al, who reported with 35.3% farmers and 44% illiteracy. Education is a major influencing factor for HBV infection. The awareness about the infectious diseases along with the acceptance of vaccination is found to be more marked among the educated community [21].
Mode of transmission through sexual route was common in this study (100%) which was in agreement with (71%) Saravanan et al. [3] But the study conducted by Grewal et al., in Punjab inferred that alcohol/drug addiction (42.2%) and blood transfusion (35.2%) were common routes of transmission [18]. This could be due tothe prevalence of drug abuse, usage of contaminated needles and syringes and improper screening before blood transfusion in that geographical region [18,22].
In the current study the prevalence of HBsAg-HIV co-infection observed was 5% which was similar to reports by Sarkar et al. (8.3%), Tiewsoh et al. (6.6%), and Ionita et al (4.4%) [23-25]. On the contrary, regions like Zimbabwe, South Africa, Malawi showed a higher prevalence of HBsAg-HIV co-infection 21%, 15%, 21% respectively [26]. Co-infection of HBV-HCV in the current study was 0%. High prevalence of HBV-HCV co-infection was reported by Mahajan et al. (1.41%) and Anwar et al (3.83%). The inconsistency in these results may be due to the variation in endemicity of these viruses according to geographical distribution [27,28]. HIV is co-infected with HCV and HBV because they have common routes of transmission.
The discovery of effective and well tolerated nucleoside/nucleotides analogues against HBV has greatly improved the views for HBV–HIV co-infections. But drug resistance is probably going to develop against all these compounds. HIV–HCV co-infected patients should be assessed for anti-viral therapy and liver biopsy should be considered. Further efforts should be focused on developing effective and well resistant combination therapies [29].
A small sample size and unequal distribution of sexes were a few limitations of the study. Future studies could validate these findings among a larger cohort. Molecular analysis methods could aid in the genetic identification of strains and delineate treatment strategies.
Conclusion
The present study validated the distinctions of HBV co-infections in this population. Public education programs could integrate with Government policies to establish prudent health care strategies to improve the quality of life among the co-infected subjects.
Acknowledgement
We are grateful to the Department of Microbiology JNMC, KAHER, Nehru Nagar, Belagavi, Karnataka for providing an opportunity to work and successfully complete this research.
Conflict of Interest
There is no conflict of interest.
References
- Leoni C, Ustianowski A, Farooq H, ArendsE (2018) HIV, HCV, and HBV: A Review of Parallels and Differences. Infect Dis Ther 7(4):407–419.
[Cross Ref] [Google Scholar] [PubMed]
- Ahuja S, Malhotra S, Chauhan A, Hans C (2013) Seroprevalence of hepatitis B and C Co-infection in HIV positive patients from a tertiary care hospital. JIMSA 26(2): 91-92.
[Google Scholar] [PubMed]
- Saravanan S, Velu V, Kumarasamy N, Nandakumar S, Murugavel KG, et al. (2007) Coinfection of hepatitis B and hepatitis C virus in HIV-infected patients in south India. World J Gastroenterol 13(37): 5015–5020.
[Cross Ref] [Google Scholar] [PubMed]
- Silva CMD, Peder LDD, Guelere AM, Horvath JD, Silva ES, et al. (2018) Seroprevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) among human immunodeficiency virus (HIV)-infected patients in an HBV endemic area in Brazil. PLoS ONE. 13(9): e0203272.
[Cross Ref] [Google Scholar] [PubMed]
- Chandra N, Joshi N, Raju YS, Kumar A, Teja VD (2013) Hepatitis B and/or C co-infection in HIV infected patients: a study in a tertiary care center from South India. Indian J Med Res 138(6):950–954. [Cross Ref]
[Google Scholar] [PubMed]
- Chu C, Lee S (2008) Hepatitis B virus/hepatitis C virus coinfection: Epidemiology, clinical features, viral interactions, and treatment. J Gastroenterol Hepatol 23(4): 512-520.
[Cross Ref] [Google Scholar] [PubMed]
- Adewole OO, Anteyi E, Ajuwon Z, Wada I, Elegba F, et al. (2009) Hepatitis B and C virus coinfection in Nigerian patients with HIV. J Infect Dev Ctries 3(5): 369-375.
[Cross Ref] [Google Scholar] [PubMed]
- Operskalski EA, Kovacs A (2011) HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep 8(1): 12-22.
[Cross Ref] [Google Scholar] [PubMed]
- Lincoln D, Petoumenos K, Dore GJ (2003) Australian HIV Observational Database. HIV/HBV and HIV/HCV coinfection, and outcomes following highly active antiretroviral therapy. HIV Med 4(3):241-249.
[Cross Ref] [Google Scholar] [PubMed]
- Askari A, Hakimi H, NasiriAhmadabadi B, Hassanshahi G, KazemiArababadi M (2014) Prevalence of Hepatitis B Co-Infection among HIV Positive Patients: Narrative Review Article. Iran J Public Health 43(6):705‐712.
[Google Scholar] [PubMed]
- Kessler DA, Jimenez A (2019) Hepatitis B Virus Screening. Transfus Med Hemother. 15: 73–76.
[Google Scholar] [PubMed]
- Amini A, Kelly H, Boeras D, Chen W, Falconer J, et al. (2015) Annex 5.3 PICO 1-How to test (HBV) Diagnostic accuracy of tests to detect hepatitis B surface antigen: a meta-analysis and review of the literature 189-265.
[Cross Ref] [Google Scholar] [PubMed]
- Cruz HM, de Paula SL, de Paula VS, da Silva EF, Milagres FA et al. (2015) Evaluating HBsAg rapid test performance for different biological samples from low and high infection rate settings & populations. BMC infect dis 15(1): 548.
- WHO (2010) Recommendations on the Diagnosis of HIV Infection in Infants and Children. Geneva: World Health Organization.
- Malhotra R, Soin D, Grover P, Galhotra S, Khutan H, et al. Hepatitis B virus and hepatitis C virus co-infection in hemodialysis patients: A retrospective study from a tertiary care hospital of North India. J Nat SciBiol Med 7(1): 72–74.
[Cross Ref] [Google Scholar] [PubMed]
- Negero A, Sisay Z, Medhin G (2011) Prevalence of Hepatitis B surface antigen (HBsAg) among visitors of Shashemene General Hospital voluntary counselling and testing center. BMC Res Notes 4 (35): 1-5.
- Arun G (2014) Prevalence of Hepatitis B Infection in a Tertiary Care Hospital, North East Region of India. J Evolut Med Dent Sci 3(41): 10413-10418.
- Grewal US, Walia G, Bakshi R, Chopra S (2018) Hepatitis B and C Viruses, Their Coinfection and Correlations in Chronic Liver Disease Patients: A Tertiary Care Hospital Study. Int J Appl Basic Med Res 8(4): 204–209.
[Cross Ref] [Google Scholar] [PubMed]
- Balogun TM, Emmanuel S, Ojerinde EF (2012) HIV, Hepatitis B and C viruses' coinfection among patients in a Nigerian tertiary hospital. Pan Afr Med J 12:100.
[Cross Ref] [Google Scholar] [PubMed]
- Yakasiri HP, Suguneswari (2016) Seroprevalence of hepatitis B and hepatitis C infections in patients with liver diseases. J Evolution Med Dent Sci 5(99): 7241-7243.
[Google Scholar] [PubMed]
- Yang S, Ding C, Cui Y, Wu J, Yu C, et al. (2017) Prevalence and influencing factors of hepatitis B among a rural residential population in Zhejiang Province, China: A cross-sectional study. BMJ Open 7(4): e014947.
[Cross Ref] [Google Scholar] [PubMed]
- Ray G (2017) Current Scenario of Hepatitis B and Its Treatment in India. J Clin Transl Hepatol 5(3): 277–296.
[Cross Ref] [Google Scholar] [PubMed]
- Sarkar J, Bandyopadhyay B, Chakrabarty R, Bhattacharya N, Adhikari S, et al. (2013) HIV-HBV Coinfection among Individuals Attending the ICTC of a Tertiary Care Hospital in West Bengal, India. ISRN Virol 9: 1-3.
- Tiewsoh JB, Boloor R, Antony B (2017) Seroprevalence of hepatitis B virus/hepatitis C virus among human immunodeficiency virus-infected patients at a tertiary care teaching hospital in Mangalore, South India. Ann Trop Med Public Health 10:1443-1447.
- Ionita G, Malviya A, Rajbhandari R, Schluter WW, Sharma G, et al. (2017) Seroprevalence of hepatitis B virus and hepatitis C virus co-infection among people living with HIV/AIDS visiting antiretroviral therapy centres in Nepal: a first nationally representative study. Int J Infect Dis 60: 64-69.
[Cross Ref] [Google Scholar] [PubMed]
- Thio CL, Smeaton L, Saulynas M, Hwang H, Saravanan S, et al. (2013) Characterization of HIV-HBV coinfection in a multinational HIV-infected cohort. AIDS 27(2): 191–201.
[Cross Ref] [Google Scholar] [PubMed]
- Mahajan S, Chauhan M, Manish S, Abrol Rk (2015) Preliminary Screening for Prevalence of Hepatitis B, C Viruses, and their co-infection: A Hospital Based Study of North India. Indian J Microbiol Res 2(3): 189-191.
- Anwar MS, Nafees M, Nabi UZ (2011) Sero-prevalence of HCV and associated infections with HIV and HBV among prisoners in Lahore. Biomedica 27(2): 119-122.
- Thimme R, Spangenberg HC, Blum HE (2005) Hepatitis B or hepatitis C and human immunodeficiency virus infection. J hepatol 42(1): 37-44.
Citation: Patil D, Nagamoti MB (2022) Seroprevalence of Hepatitis C Virus and Human Immunodeficiency Virus among HBsAg Positive Patients-A Cross-Sectional Study J Comm Med Health Educ 12:738. DOI: 10.4172/2168- 9717.1000738
Copyright: © 2022 Patil D, et al,. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2741
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 2203
- PDF downloads: 538