Research Article Open Access
Separation of Phenol-Containing Pyrolysis Products Using Comprehensive Two-Dimensional Chromatography with Columns Based on Pyridinium Ionic Liquids
Mikhail V Shashkov1* and Vladimir N Sidelnikov21Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russian Federation
2Novosibirsk State University, Novosibirsk, Russian Federation
- *Corresponding Author:
- Mikhail V. Shashkov
Boreskov Institute of Catalysis SB RAS
Novosibirsk, Russian Federation
Tel: +73833308269
Fax: +73833308056
E-mail: shashkov11@gmail.com
Received date: March 09, 2016; Accepted date: March 28, 2016; Published date: April 04, 2016
Citation: Shashkov MV, Sidelnikov VN (2016) Separation of Phenol-Containing Pyrolysis Products Using Comprehensive Two-Dimensional Chromatography with Columns Based on Pyridinium Ionic Liquids. J Anal Bioanal Tech 7:313. doi:10.4172/2155-9872.1000313
Copyright: © 2016 Shashkov MV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The work describes the application of columns with high-polar stationary liquid phases based on pyridinium ionic liquids for GC × GC separation of bio-oil and product of coal pyrolysis. By using inverse combination columns- the first ionic liquid column, the second is HP-5 the good separation results have been obtained. In the analysis of coal pyrolysis products, the suggested approach provides a much better resolution between components in comparison with a less polar first-dimension column (ZB-WAX). The good selectivity for peaks of phenols is observed, and the group of phenols is well detached and separated from the group of diaromatics. For bio-oil also good separation picture was obtained, the groups of phenols and guaiacol derivatives are distinguished with good resolution of substances within each group.
Keywords
Comprehensive two-dimensional chromatography; Ionic liquids; High polar stationary phases; Bio-oil
Introduction
Although world reserves of conventional hydrocarbon feedstock are big, they are not inexhaustible and are being rapidly depleted [1]. This is why technologies for the production of fuels and valuable chemicals from alternative and renewable sources are under development. Among them are fossil coals, in particular large reserves of low-grade coals, and biomass of different origin. An efficient method for processing of such resources is pyrolysis [2,3]. This process yields a complex mixture of organic compounds that can further be used to produce motor fuels or valuable chemicals.
To choose the optimal conditions of pyrolysis and further processing of pyrolysis liquids, it is necessary to determine their exact chemical composition. Comprehensive two-dimensional chromatography (GC × GC) is the advanced and most informative method for the analysis of multicomponent mixtures of various classes of chemical compounds, specifically those with strongly different polarities [4]. The main advantages of GC × GC are its higher separation capacity in comparison with conventional chromatography, and the possibility to perform group analysis of complex mixtures [4]. However, the best results can be achieved only with the properly chosen separation system (a combination of columns) [5] and separation conditions [6].
It is known that the liquid part of coal pyrolysis products includes various types of aromatic hydrocarbons and substantial amounts of phenols [7]. Another complex mixture is the product of biomass pyrolysis called bio-oil (BO). BO is characterized by a high content of different classes of phenolic compounds, mostly the guaiacol derivatives [8]. In addition, BO contains a wide spectrum of other oxygen-containing compounds, particularly carbohydrates, carbonyl compounds and phenols [8].
At present, publications devoted to GC × GC analysis of the liquid products of pyrolysis of various coals [3,7] can be found in the literature. Due to increased interest in renewable fuel resources, the GC × GC analysis of BO is reported in a greater number of works [9-13]. These works deal with GC × GC analysis of components in the initial BO [9-11] and its fractions [10,13].
However, virtually in all the indicated works, separations of pyrolysis products were carried out on a pair of columns with non-polar (HP- 5 and its analogs) and medium-polar (HP-17 and its analogs) liquid phases. Only in some works, columns with phases having a slightly higher polarity, HP-1701 [3] and WAX [12], were employed. Although such a combination of columns gives satisfactory separation results, the separation pattern could be further improved. In some cases, columns with strongly different polarities give better GC × GC separations, which make it possible to find an additional number of components and obtain a more exact boundary between groups of components on separation pictures of complex mixtures [5,14]. In this connection, it seems interesting to perform GC × GC with combinations of non-polar and high-polar columns.
To this end, high-polar columns can be represented by those with stationary liquid phases based on ionic liquids (ILs). ILs form a relatively new class of stationary liquid phases that have some advantages, in particular, high thermal stability in comparison with other types of high-polar phases, polarity, and the possibility to control phase selectivity in a wide range [15,16]. Earlier it was shown that the use of columns with ILs provides good separations of various objects: fatty acids, oil products, objects of plant origin, and pesticides [16,17].
As for the GC × GC analysis of coal and bio-oil pyrolysis products with the use of IL columns, we have found only one work devoted to this issue [18]. However, its authors did not manage to obtain a satisfactory separation on the SP-IL100 column. Also we can found the work devoted GC × GC analysis of sulfur polyaromatics in coal tar on different IL columns [19].
Our work describes the application of columns with high-polar stationary liquid phases based on pyridinium ILs for GC × GC separation of pyrolysis products. A high contribution of specific interactions is typical of such ILs [16]. Thus, phases with such ILs are highly selective for polar compounds, particularly for phenols [16], which make columns with these phases attractive for separation of objects considered in this work.
Experimental
Objects of separation: Sample preparation
A sample of bio-oil, typical flash pyrolysis oil was purchased from Biomass Technology Group BV (Netherlands). The initial bio-oil was extracted with toluene (5 mL of toluene per 1 g of the initial bio-oil). This procedure is required to remove non-volatile components, such as carbohydrates and dicarboxylic acids, which cannot be analyzed by GC [20]. A pyrolizate of bituminous coal from Kuzbass region deposit was obtained by heating at a temperature of 600°C for 3 hours without air access. The liquid fraction of the pyrolizate was analyzed without any preparation steps.
Columns used in the study
The study was carried out using columns with the nonpolar phase HP-5 (Agilent), 30 m × 0.25 mm × 0.25 μm and 5 m × 0.25 mm × 0.15 μm, and a column with the polar phase ZB-WAX (Phenomenex), 30 m × 0.25 mm × 0.25 μm.
Homemade columns prepared by a static high pressure method using the techniques reported in Ref. [21] were employed as highpolar IL columns. ILs were synthesized according to Ref. [15], characterization and purity info also in Ref. [15]. This procedure was used to prepare the (30 m × 0.25 mm × 0.25 μm) columns with pyridine ILs (2MPyC9) and (4MPy), and the (5 m × 0.25 mm × 0.1 μm) column with IL (2MPyC9). Structures of the ILs used in the study are displayed in Figure 1. The efficiency of the prepared columns was ca. 2400 plates per meter.
GC × GC analysis
All the experiments were carried out using an Agilent 7890А chromatograph and G4513A autosampler. An Agilent flow modulator and ZOEX software (for visualization of 2D samples) were employed in GC × GC experiments.
Separation conditions
The temperature program of the column oven was as follows: in the case of coal pyrolysis products, 80°C for 3 min followed by programming at a rate of 7°C/min up to a final temperature of 270°C; in the case of bio-oil, 70°C for 3 min followed by programming at 8°C/min up to a final temperature of 270°C. Temperature of inlet and flame-ionization detector was set equal to the final temperature of the analysis. Carrier gas flow rate: for the first column (helium), 0.65 mL/min; for the second column (hydrogen), 23 mL/min. In all cases, modulation period was 1.5 s.
In the experiment with ZB-WAX column, final temperature was 260°C, and flow rate through the first column, 0.9 mL/min. The other conditions were similar to those used for IL columns.
Some components were identified by GC × GC analysis of the mixtures with the test components: naphthalene, phenol, methyland dimethylphenols. In other cases, comparative GC-MS analysis of the mixtures was carried out on an Agilent 7000B instrument using a 30 m × 0.25 mm × 0.25 μm column with the liquid phase 2MPyC9 under the conditions similar to those used for the first column in GC × GC.
The retention time of components, which is required to calculate resolution coefficients, was found by means of ZOEX software.
Discussion
Analysis of coal pyrolysis products on HP-5/2MPyC9 columns
Let us consider the separation of pyrolysis products using the conventional scheme of columns connection. The commonly accepted sequence of columns (non-polar/polar) is convenient because along the abscissa axis a sample is separated according to the boiling points, and then the axis of ordinates shows the separation that corresponds to increasing polarity. Two-dimensional separation gives the patterns of mixtures (for example, of oil products) that are convenient for interpretation.
The first-dimension column was represented by the most universal HP-5. The second-dimension IL column was 2MPyC9. As was shown in our earlier study, this IL has high polarity and provides good separation of phenols [15].
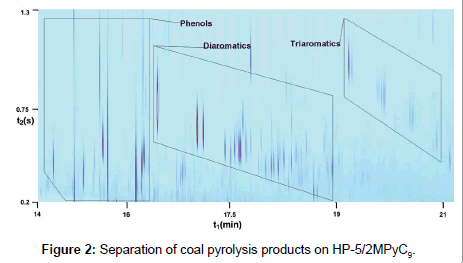
The optimal separation conditions were chosen for the analysis, which gave the following separation pattern (Figure 2).
It is seen that separation cannot be considered as satisfactory. The peaks corresponding to phenols are strongly broadened on the second column. Although individual zones corresponding to the groups of aromatic hydrocarbons can be distinguished in Figure 1, the pattern is difficult to interpret. As was shown earlier [15], good separations of phenols can be obtained on columns with 2MPyC9 in the case of onedimensional chromatography, and their peaks have good shape at good efficiency. Nevertheless, good separations are not observed under the conditions of GC × GC. This effect can be explained as follows. It is known that the second column in two-dimensional chromatography operates at the flow rates strongly exceeding the optimal value. Earlier it was shown that in the case of IL columns the efficiency decreases much faster with increasing the flow rate as compared to the case of columns with polysiloxane stationary liquid phases and phases based on polyethylene glycol [15].
So, further separations were performed with IL column in the first dimension to provide conditions of its operation close to optimal. In some cases, such scheme of columns connection in GC × GC provides a better separation of the groups of polar components [22].
Analysis of coal pyrolysis products with IL as the firstdimension column
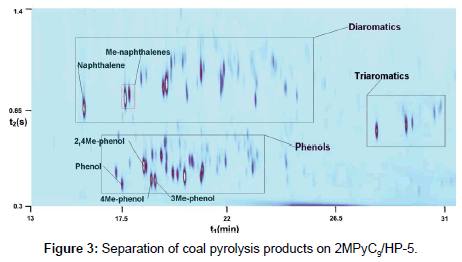
Thus, the conventional scheme of columns connection (nonpolar/ polar) does not provide a satisfactory separation of a mixture of pyrolysis products. Therefore, the inverse connection of columns (high-polar/non-polar) was employed, and separation conditions (flows through the columns and modulation time) were chosen so as to obtain the best separation of the mixture. An example of such separation is illustrated on Figure 3.
Figure 3 shows that, on the one hand, the group of phenols is well detached and separated from the group of diaromatics. On the other hand, a distinct separation can be observed also between the peaks of phenols in this group. The peaks have good symmetry and are not broadened upon separation on the second column, in distinction to the pattern that is observed on Figure 1. For this reason, the number of the observed peaks of phenols is greater due to a better separation and an increased intensity of minor peaks.
Now let us examine the separation of the same mixture on columns with the phase based on another pyridinium IL, 4MPy. This IL has a somewhat lower McReynolds polarity as compared to 2MPyC9 (Table 1); so, the separation pattern should also be different. However, it was found that separation picture in case of 4Mpy very similar to 2MPyC9, a good resolution between the peaks of phenols within the group is observed, and the group of phenols itself is well separated from diaromatics (due to the similarity the picture is not shown).
| Diaromatics/Phenol | 2,4Me-phenol/ 4Me-phenol |
4Me-phenol/ 3Me-phenol |
McReynolds polarity [15] | |
|---|---|---|---|---|
| 2MPyC9 | 6.18 | 3.80 | 1.55 | 92 |
| 4MPy | 4.78 | 3.58 | 1.58 | 84 |
| ZB-WAX | 3.18 | 0.33 | 1.95 | 51 |
Table 1: Resolution of pairs of substances in GC × GC separation using the first column with IL or ZB-WAX and the second HP-5 column.
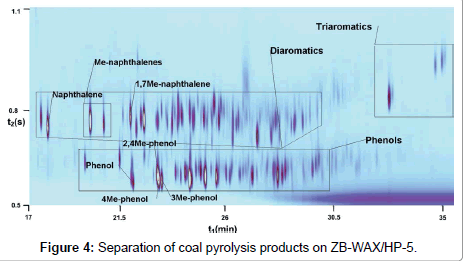
Now let us compare the separation results for this mixture with those obtained on a pair of columns where the first column was less polar than ionic liquids. Figure 4 shows the separation obtained on a pair of columns ZB-WAX/HP-5. All separation conditions were similar to those on other columns, except for the carrier flow through the column with ZB-WAX: its value was 0.9 ml/min, which corresponds to the optimal He flow through the column.
One can see that the general separation pattern is worse than in the case of first columns with the IL phase. The group of phenols has a considerably smaller number of peaks in comparison with chromatograms obtained on IL columns. On Figure 4 the group of phenols is visually more close to the group of diaromatic hydrocarbons than on Figure 3. Such differences in separation patterns in the case of system with the first WAX column can be attributed to its lower polarity as compared to IL. The causes of these differences are considered.
2D resolution
To estimate differences in the separation capacity of column systems ZB-WAX/HP-5, 2MPyC9/HP-5 and 4MPy/HP-5, resolution was measured for a series of selected peaks corresponding to this mixture. It is known that the resolution between peaks in GC × GC, Rs2D (2D resolution), is calculated by formula (1) [23]:
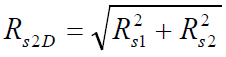 (1)
(1)
where Rs1 and Rs2 are the resolutions along first and second columns, respectively.
For the mixture under analysis, the pairs of peaks were chosen so as to estimate separations: 1) between groups of phenols and diaromatics, and 2) between peaks within the group of phenols. The peak of phenol and the nearest peak from diaromatics group were chosen as a pair to estimate separation between groups of aromatics and phenols. In the case of 2MPyC9, resolution of the Naphthalene/Phenol pair was analyzed; for 4MPy, 1Me-naphthalene/Phenol; and for ZB-WAX, 1,7Me-naphthalene/Phenol.
To estimate peak resolution within the group of phenols, for all columns the pairs of the most close and most intense peaks were chosen: 2,4Me-phenol/4Me-phenol and 4Me-phenol/3Me-phenol. The results obtained are listed in Table 1.
One can see from Table 1 that in all cases except for the 4Mephenol/ 3Me-phenol pair, systems with IL columns show better results of separation than the system with the first column based on polyethylene glycol.
The better separation between groups of diaromatics and phenols (column 1 in Table 1) can have the following explanation. Due to high polarity of ILs, and low contribution of dispersive interactions comparing with ZB-WAX [15] compounds retained considerably less, and leaving the column faster in case of ILs. As the result, separation on the second non polar column occurs at a relatively lower temperature, and resolution increases in comparison with ZB-WAX.
A much better separation for the 2,4Me-phenol/4Me-phenol pair on IL can be attributed to a higher polarity of phases with IL (last column in Table 1). Separation for the 4Me-phenol/3Me-phenol pair is slightly better in the case of ZB-WAX column. This may be related to a higher efficiency of this column, because its selectivity for polar compounds was shown to be lower as compared to IL columns [15].
Separation of the toluene extract of bio-oil on 2MPyC9 column in the first dimension
Now it is clear that the GC × GC separation of a mixture of aromatic and phenolic compounds should be performed using a combination of polar/non-polar columns. Let us consider the separation of bio-oil products.
Bio-oil is a mixture of liquid products that are produced by thermolysis of hemicellulose, cellulose and lignin as the components of biomass. Thermal decomposition products of hemicellulose and cellulose are carbohydrates and carbonyl compounds. Lignin decomposes mostly into phenolic compounds, particularly guaiacol and its derivatives [11]. Among various products constituting BO, only the phenolic compounds can be subjected to GC analysis, because the other compounds are thermally unstable or non-volatile.
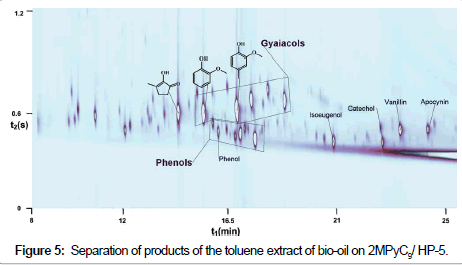
Components of the extract were separated using 2MPyC9/HP-5 columns, which showed the best results in separation of coal pyrolysis products. The resulting separation pattern is displayed on Figure 5.
One can see that separation of bio-oil is more difficult as compared to separation of coal pyrolysis products. The reason is that the main substances in the mixture to be separated have close chemical features. Nevertheless, a combination of 2MPyC9/HP-5 columns makes it possible to perform a successful separation. It is seen that the group of phenols is close to the group of guaiacol derivatives. However, phenols are well separated within their group because the IL phase is highly selective for phenols.
Conclusion
It was shown that in comprehensive two-dimensional chromatography the use of columns with pyridinium ILs in the first dimension ensures good separations for the analysis of pyrolysis products of coal and biomass. In the analysis of coal pyrolysis products, the suggested approach provides a much better resolution between components in comparison with a less polar first-dimension column (ZB-WAX). Therewith, group analysis can be performed: the regions corresponding to phenolic compounds and diaromatics can be distinguished on a chromatogram. A good selectivity is observed within the group of phenols. In the analysis of bio-oil sample, the groups of phenols and guaiacol derivatives are distinguished with good resolution of substances within each group.
References
- Bentley RW (2002) Global oil & gas depletion: an overview. Energy Policy 30: 189-205.
- Onorevoli B, Machadoa ME, Dariva C, Franceschi E, Krause LC, et al. (2014) A one-dimensional and comprehensive two-dimensional gas chromatography study of the oil and the bio-oil of the residual cakes from the seeds of Crambe abyssinica. Ind Crop Prod 52: 8-16.
- Rathsack P, Otto M (2014) Classification of chemical compound classes in slow pyrolysis liquids from brown coal using comprehensive gas-chromatography mass-spectrometry. Fuel 116: 841-849.
- Hantao LW, Najafi A, Zhang C, Augusto F, Anderson JL (2014) Tuning the selectivity of ionic liquid stationary phases for enhanced separation of nonpolar analytes in kerosene using multidimensional gas chromatography. Anal Chem 86: 3717-3721.
- Omais B, Courtiade M, Charon N, Ponthus J, Thiébaut D (2011) Considerations on orthogonality duality in comprehensive two-dimensional gas chromatography. Anal Chem 83: 7550-7554.
- Májek P, Krupčík J, Gorovenko R, Špánik I, Sandra P, et al. (2014) Computerized optimization of flows and temperature gradient in flow modulated comprehensive two-dimensional gas chromatography. J Chromatogr A 1349: 135-138.
- Stihle J, Uzio D, Lorentz C, Charon N, Ponthus J, et al. (2012) Detailed characterization of coal-derived liquids from direct coal liquefaction on supported catalysts. Fuel 95: 79-87.
- Song Q, Nie JQ, Ren M-G, Guo Q-X (2009) Effective phase separation of biomass pyrolysis oils by adding aqueous salt solutions. Energy Fuels 23: 3307-3312.
- Marsman JH, Wildschut J, Evers P, de Koning S, Heeres HJ (2008) Identification and classification of components in flash pyrolysis oil and hydrodeoxygenated oils by two-dimensional gas chromatography and time-of-flight mass spectrometry. J Chromatogr A 1188: 17-25.
- Schneider JK, da Cunha ME, dos Santos AL, Maciel GP, Brasil MC, et al. (2014) Comprehensive two dimensional gas chromatography with fast-quadrupole mass spectrometry detector analysis of polar compounds extracted from the bio-oil from the pyrolysis of sawdust. J Chromatogr A 1356: 236-240.
- Michailof C, Sfetsas T, Stefanidis S, Kalogiannis K, Theodoridis G, et al. (2014) Quantitative and qualitative analysis of hemicellulose, cellulose and lignin bio-oils by comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry. J Chromatogr A 1369: 147-160.
- Hatch LE, Luo W, Pankow JF, Yokelson RJ, Stockwell CE, et al. (2015) Identification and quantification of gaseous organic compounds emitted from biomass burning using two-dimensional gas chromatography–time-of-flight mass spectrometry. Atmos Chem Phys 15: 1865-1899.
- Cunha ME, Schneider JK, Brasil MC, Cardoso CA, Monteiro LR, et al. (2013) Analysis of fractions and bio-oil of sugar cane straw by one-dimensional and two-dimensional gas chromatography with quadrupole mass spectrometry (GC × GC/qMS). Microchem J 110: 113-119.
- Cordero C, Rubiolo P, Sgorbini B, Galli M, Bicchi C (2006) Comprehensive two-dimensional gas chromatography in the analysis of volatile samples of natural origin: a multidisciplinary approach to evaluate the influence of second dimension column coated with mixed stationary phases on system orthogonality. J Chromatogr A 1132: 268-279.
- Shashkov MV, Sidelnikov VN (2013) Properties of columns with several pyridinium and imidazolium ionic liquid stationary phases. J Chromatogr A 1309: 56-63.
- Qi M, Armstrong DW (2007) Dicationic ionic liquid stationary phase for GC-MS analysis of volatile compounds in herbal plants. Anal Bioanal Chem 388: 889-899.
- Vidal L, Riekkola ML, Canals A (2012) Ionic liquid-modified materials for solid-phase extraction and separation: a review. Anal Chim Acta 715: 19-41.
- Omais B, Courtiade M, Charon N, Thiébaut D, Quignard A, et al. (2011) Investigating comprehensive two-dimensional gas chromatography conditions to optimize the separation of oxygenated compounds in a direct coal liquefaction middle distillate. J Chromatogr A 1218: 3233-3240.
- Antle P, Zeigler C, Robbat A Jr (2014) Retention behavior of alkylated polycyclic aromatic sulfur heterocycles on immobilized ionic liquid stationary phases. J Chromatogr A 1361: 255-264.
- Oasmaa A, Kuoppala E, Solantausta Y (2003) Fast pyrolysis of forestry residue. 2. Physicochemical composition of product liquid. Energy Fuels 17: 433-443.
- Shashkov MV, Sidel’nikov VN (2012) Single cation ionic liquids as high polarity thermostable stationary liquid phases for capillary chromatography. Russ J Phys Chem A 86: 138-141.
- Seeley JV, Seeley SK, Libby EK, Breitbach ZS, Armstrong DW (2008) Comprehensive two-dimensional gas chromatography using a high-temperature phosphonium ionic liquid column. Anal Bioanal Chem 390: 323-332.
- Dutriez T, Courtiade M, Thibaut D (2009) High-temperature two-dimensional gas chromatography of hydrocarbons up to nC60 for analysis of vacuum gas oils. J Chromatogr A 1216: 2905-2912.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11751
- [From(publication date):
June-2016 - Jul 03, 2025] - Breakdown by view type
- HTML page views : 10787
- PDF downloads : 964