Research Article Open Access
Separation and Quantitative Determination of Carbohydrates in Microbial Submerged Cultures Using Different Planar Chromatography Techniques (HPTLC, AMD, OPLC)
Tatiana Bernardi1, Paola Pedrini2, Maria Gabriella Marchetti2 and Elena Tamburini2*
1Department of Chemical and Pharmaceutical Sciences, University of Ferrara, Ferrara, Italy
2Department of Life Sciences and Biotechnology, University of Ferrara, Ferrara, Italy
- *Corresponding Author:
- Elena Tamburini
Department of Life Sciences and BBiotechnology
University of Ferrara, Via L. Borsari 46
44121, Ferrara, Italy
Tel: +390532455103
Fax: +390532240709
E-mail: elena.tamburini@unife.it
Received date: June 05, 2015; Accepted date: June 16, 2015; Published date: June 23, 2015
Citation: Bernardi T, Pedrini P, Marchetti MG, Tamburini E (2015) Separation and Quantitative Determination of Carbohydrates in Microbial Submerged Cultures Using Different Planar Chromatography Techniques (HPTLC, AMD, OPLC). J Anal Bioanal Tech 6:250. doi: 10.4172/2155-9872.1000250
Copyright: © 2015 Bernardi T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Carbohydrates are the principal sources of nutrient and energy in large-scale submerged fermentation processes. A method for detection and quantification of sugar levels are very advantageous because they can be considered as key indicators in determining the yields and the productivity of the process. For this reason, the objective of this study is to develop and validate a simple and relatively economical analytical method for detecting complex sugar mixtures in fermentation broth based on High-Performance Thin-Layer Chromatography (HPTLC). HPTLC is a widely used, fast and accurate method of separating complex mixtures. The proposed method involved the chromatographic separations of xilo-, galacto-, fructo-oligosaccharides mixtures at different molecular weights, tri- and disaccharides (raffinose, sucrose, lactose), and the corresponding monosaccharides (xylose, fructose, galactose) on HPTLC plates, using different eluent mixtures and elution conditions. The documentation of plates was performed using TLC visualization device and the images of plates were processed using a digital processor. HPTLC methods development using instrumental techniques as OPLC (Over Pressure Liquid Chromatography) and AMD (Automated Multiple Development) has been also described, as to simultaneously monitor several samples in the same elution, with significant time and solvent savings. Four different carbohydrates complex mixtures were analyzed using HPTLC techniques, as to optimize the quality of the separation among components. The methods set up were then applied for quantitative determination of sugars. As a model of submerged fermentations, a strain of Bifidobacterium spp. was used, a saccharolytic bacterium with probiotic activities in the human gut, able to anaerobically ferment complex sugar mixtures. Results could be easily extended to other fermentation processes.
Keywords
HPTLC; AMD; OPLC; Chromatography; Submerged cultures; Carbohydrates analysis; Complex matrix
Introduction
Biotechnology industries are based on exploiting the metabolic activities of microbes, plants and animal cells to produce a wide variety of different compounds, which are used by other industries such as chemical, food, pharmaceutical and health care [1]. With advances in biochemistry, engineering and genetic techniques, submerged fermentation industry has been often becoming a concrete alternative to traditional chemical production for several products, thanks to microbial yields improvements, optimizations of reactor design and advancements in process control and product recovery [2]. Carbohydrates are excellent sources of carbon, oxygen, hydrogen, and metabolic energy, so they have usually served as the principal nutrient source for the large-scale cultivations of microorganisms [3]. The utilizable carbohydrates can be monosaccharides (e.g. glucose, galactose, fructose, xylose), disaccharides (e.g. sucrose, maltose, lactose), oligosaccharides (e.g. kestose, raffinose, maltotriose), and polysaccharides (e.g. starch, dextrins, cellulose, inulin, xylan), commonly supplied as complex raw materials derived from industry or agriculture [4]. These raw materials can also provide a nitrogen source, salts, trace elements, and vitamins, required by the microorganisms as nutrients for growth, maintenance, reproduction, and production. The resulting fermentation broth is a complex mixture, consisting of living microbial cells, nutrients, cell debris, and other products/by-products of the fermentation process [5]. This broth must be monitored to optimize the quality and quantity of the cells and/or metabolites produced. In addition, during fermentation, as the composition of the broth changes, so does the chemistry. It is known that product concentration is approximately inversely proportional to carbon source concentration, therefore, sugar levels serves as a key indicator in determining the yields and the productivity of the process. Understanding microbial kinetics of single sugar depletion in a complex media also assures to reach the desired level of process efficiency and yield, but needs of a rapid, precise and accurate method of identification and quantification in order to avoid the not-complete substrate utilization, which always represents a money waste in the economic balance of the overall process [6]. Moreover, to establish a hierarchy of sugars utilization in microbial metabolism, the concentrations and the depletion kinetics of single carbohydrates should be accurately monitored during the processes [7]. Nowadays, sugars can be determined by different analytical methods, such as flow injection analysis [8], electrochemical analysis [9], enzymatic methods [10], gas chromatography [11], high-performance liquid chromatography [12], anion-exchange liquid chromatography [13], and thin-layer chromatography [14].
In particular, thin-layer chromatography (TLC) is a simple separation technique for both qualitative and quantitative analysis, enabling simultaneous analysis of many substances with minimal time requirement [15]. The increasing commercial availability of pre-coated TLC plates has significantly improved the achievable reproducibility of separation. Additionally, the availability of many different absorbent materials including high-performing silica, bonded phases and impregnated layers have increased the versatility of the technique for numerous and quick separations particularly in the sugars field [16]. Thus, methods of TLC and its refined version high-performance thinlayer chromatography (HPTLC) are even nowadays indispensable tools of modern analytical chemistry [17]. The most important differences between TLC and HPTLC is (a) the different particle sizes of the stationary phases and (b) the employment of state-of-the-art instrumentation for all steps in the procedure, sample application, standardized chromatogram development and software-controlled evaluation [18].
Although there were (and still are) some potential concerns against the wider application of TLC/HPTLC (e.g. the potential oxidation of the analyte caused by exposition to atmospheric oxygen), there are many advantages that make these techniques clearly competitive to other analytical approaches [19]. In particular, HPTLC is rapid and cost effective as a method of separating complex mixtures [20], and it has been widely used at industrial level for routine analysis of several substances, even in combination with automated instrumental techniques (e.g., Automated Multiple Development (AMD) and Over Pressure Layer Chromatography (OPLC)).
The aim of this study was to develop modern, rapid and simple analytical HPTLC/AMD/OPLC methods for detection and quantification of different carbohydrates mixtures of xylooligosaccharides (XOS), galacto-oligosaccharides (GOS), fructooligosaccharides (FOS) and inulin, and their corresponding mono- and di-saccharides, used as carbon substrates for submerged fermentation processes. As a case study, a strain of Bifidobacterium (B. adolescentis MB 239) able to growth on carbohydrates mixtures of different grade of composition complexity has been used.
Materials and Methods
Fermentation and strain
Bifidobacterium adolescentis MB 239 was obtained from the Collection of the Department of Agroenvironmental Sciences of the University of Bologna. The microrganism was subcultured anaerobically at 37°C for 24 h in MRS broth (Difco Laboratories, Sparks, Maryland, USA). Controlled-pH batch cultures were carried out in a BM-PPS3 bioreactor (Solaris Biotech, Porto Mantovano, and Italy) with a working volume of 2 liters. The temperature was kept at 37°C, and constant stirring (300 rpm) was applied. The fermentor was inoculated with 5% vol/vol precultures grown in the same medium. The culture pH was continuously measured (Mettler Toledo InPro 3030/325) and regulated by automatic addition of 4M NaOH. Microbial growth was monitored by following changes in biomass dry weight [21]. Samples were periodically collected for analysis of carbohydrates. For each carbohydrates mixture, fermentations were carried out in triplicate. Others details about fermentations can be found in Amaretti et al. [22].
Chemicals and media
Glucose, galactose, and lactose (Sigma-Aldrich, Steinheim, Germany) and GOS syrup (Borculo Domo, Zwolle, The Netherlands) mixture was assembled as carbon sources mixture for B. adolescentis MB239 fermentation. The GOS mixture contained 18.8% glucose, 3.5% galactose, 19.4% lactose, and 58.3% GOS with DP ranging between 3 and 9. A DP 3 oligomer accounted for 37% of total carbohydrates, and it was the most highly represented oligosaccharide. The concentration of the oligomers decreased with the increase in DP .
XOS and xylose were purchased as commercial mixtures from Co. Farmaceutica Milanese, CFM, Milano, and Italia. Glucose, fructose, sucrose, raffinose (99.9%, J.T.Baker, Deventer, The Netherlands), pure FOS (1-kestose, 99%; nystose, 99%; and fructosylnystose, 98%; Wako Pure Chemical Industries, Osaka, Japan) mixtures were also prepared as fermentation media. Inulin from natural origin (as topinambur roots extract) was also added. In all cases, carbon sources mixtures were integrated with nitrogen and mineral sources to sustain microbial growth.
Solvents, standards and samples
Acetonitrile and acetone were of LiChrosolv quality (E. Merck, Darmstadt, Germany), water was ultrapure. Glucose, fructose, galactose, xylose, lactose, and raffinose (Carlo Erba Reagenti, Milano, and Italia) were used ad pure standard sugars solutions of 1000 ppm. GOS, XOS, FOS and inulin standard solutions were prepared in acetone-water to give 1000 ppm stock solutions, then successive diluted with acetonewater (2:1) to give solutions at different concentrations depending on the linear range of the calibration plots. Samples were collected from fermentations every 2-3 h, centrifuged, filtered (cellulose acetate syringe filter, 0.22 μm; Albet Filalbet, Barcelona, Spain) to avoid timedependent changes in analyte concentration resulting from continued metabolism, and immediately chilled at 4°C. Carbohydrates were analyzed in the supernatant.
Chromatography
Standards (1-20 μL) and samples (1 μL) were applied to the plates using an automated sample applicator Linomat V (Camag, Muttenz, Switzerland) as 3 mm-wide bands 10 mm from the bottom of the plate and 6 mm apart with a delivery rate of 20 s μL-1. Five standards (in duplicate) were applied to the plate alternatively with 14 samples at different concentration. Fermentation samples were indeed deposited at different dilutions to falling into the right concentration range for calibration curves.
Glucose, galactose, lactose and GOS: Glucose, galactose, lactose and GOS concentration in fermentation media were analyzed with HPTLC/AMD method. 20 × 10 cm silica Kieselgel 60 F254s thin layer plates (Merck, Darmstadt, Germany) previously treated with sodium acetate 0.2M solution (immersion time 20 sec, drying time 20 minutes to air) were used. Isocratic development was performed at 20-22°C and 55-65% relative humidity in 20 × 10 cm Camag twin-trough chamber with 20 mL of mobile phase. Automated multiple development was performed by using Camag AMD-1 equipment with linear gradient in 15 steps of elution. Gradient was built by automatic mixing four basic eluent solutions of acetonitrile/water with 32%, 30%, 26% and 24% of acetonitrile content, respectively.
Glucose, fructose, sucrose, FOS and inulin: Chromatography was performed on 20 × 10 cm diol HPTLC plates (Merck, Darmstadt, Germany). Isocratic development was performed at 20-22°C and 55- 65% relative humidity in 20 × 10 cm Camag twin-trough chamber with 20 mL of mobile phase. Automated multiple development was performed by using Camag AMD-1 equipment with linear gradient in 20 steps of elution. Gradient was built by automatic mixing four basic eluent solutions of acetonitrile:acetone (1:1)/water with amounts of water ranging from 25 to 15%.
Xylose and XOS: Xylose and XOS mixtures were separated and quantified using thin layer 20 × 10 cm silica plate Kieselgel 60 F254s (Merck, Darmstadt, Germany), using butanol/ethanol/water (5/3/2) as eluent. Two consecutive isocratic elutions in twin-through chamber were carried out. The elution chamber was saturated with eluent 30 minutes before introducing the plate. After each step, the plates were dried for 15 minutes.
Glucose, fructose, lactose, raffinose and FOS: OPLC analysis was performed on 20 × 20 cm aluminium foil-backed silica gel HPTLC plates sealed on four sides (OPLC-NIT, Budapest, Hungary). Plates were developed with acetonitrile-water 85:15 (vol/vol) as mobile phase, with overrun development, by means of a Bionisis BS-OPLC 50 instrument (Budapest, Hungary). The conditions used for OPLC were: external pressure 50 bar, mobile phase flow rate 300 μL min-1, mobile phase volume 10000 μL, and rapid volume 300 μL; under these conditions the elution time was 2010 sec.
Derivatization: After developing and drying, plates were derivatized by immersion in appropriate solvents using Camag dipping apparatus. In particular, GOS standard and samples plates were immersed for 9 sec. in a toluene solution containing 2% (w/v) basic acetate of lead(IV) in glacial acetic acid and 0.2% (w/v) 2,7-dichlorofluorescein in pure ethanol, subsequently heated to 105°C for three minutes for color development. XOS standard and samples plates were derivatized for 5 sec. in pure acetone solution containing 0.9 mL of aniline and 1.66 g of phthalic acid, then dried at room temperature for 10 minutes and then for other 20 minutes at 120°C in an oven. Finally, FOS standard and samples plates were derivatized for 5 sec. with 4-aminobenzoic acid reagent (glacial acetic acid (36 mL), water (40 mL), 85% phosphoric acid (2 mL) and acetone (120 mL) added to 4-aminobenzoic acid (2 mL), then heated at 115°C for 15 minutes in an oven. The immersion condition must be standardized by maintaining a defined immersion time and a uniform speed to avoid tidemarks, which could interfere with densitometric evaluation.
After derivatization, the plates were air dried for 5 min and sample and standard zone areas were measured by linear scanning under fluorescence illumination at λ=366 nm with a PC-controlled Camag TLC-Scanner III (mercury lamp, filter cut-off 400 nm) (Camag, Germany). The cutoff filter is positioned between the sample and the photosensor for eliminating diffusely reflected light of the excitation wavelength. Accordingly, the measured light is directly proportional to the amount of fluorescingThe scanning was performed vertically over the plate over a distance of 5 cm and at a rate of 5 cm/min, measuring 200 points/scanning. Densitograms were generated from transmission measurements of the samples and the blank. The respective absorbance values were calculated, and peak areas were quantitated with the aid of the CATS4® software supplied with the instrument.
Principles of AMD Technique
AMD technique provides the automatic multi-development of plates in a suitable chamber. In each succeeding development, the mobile phase is permitted to advance farther by a constant distance, while enhancing the separation of each component of the sample and standard solutions. First, the chamber is flushed with purified nitrogen and, with the addition of solvent, the first elution is started. For each step, a mixer is completely filled with the solvent from reservoirs before entering in the chamber. Six reservoirs are available on the AMD apparatus to produce different eluent compositions.
A few second later, the step is terminated on the basis of the programmed gradient, and the solvent removed. Then the next step started, including drying, ventilating, and a longer elution, followed by removal of the eluting solvent. This is continued until the whole programme is completed. For each step, new mobile phase is introduced into the chambers. Because of its composition differs from the preceeding one, this will result in a gradient elution. Usually a gradient for silica gel begins strongly polar (strongly eluted) and ends less polar (weakly eluted). Gradient trend needs to be defined by carrying out a series of isocratic elution and then optimized for each application, as to obtain the best separation among substances. Theory and up-to-date principal applications of AMD technique are reported in [23].
Principles of OPLC Technique
OPLC is a forced-flow technique developed by Tyihak et al. [24] in which the vapor phase is eliminated by completely covering the sorbent layer with an elastic membrane under external pressure. The separation is thereby carried out in a closed system with parameters that may be controlled. The chromatographic plate becomes a planar column by sealing the plate all around and by draining off the solvent. Eluent could be overrun in order to increase the separation efficiency. After sample application, the plate is placed into the chamber and after closing, an oil cushion is set under pressure. Before initiating separation with a suitable mobile phase, the solvent inlet valve is closed, and the eluent pump is started so as to reach an appropriate solvent pressure. The separation is then initiated by opening the inlet valve, producing a quick solvent distribution into the channel resulting in a linear migration of the mobile phase. The eluent is forced through the silica layer by the pressured cushion and then collected in the outlet channel.
Results and Discussion
AMD method development for glucose, lactose and GOS mixtures
The combination of high-performance thin-layer chromatography (HPTLC) with automated multiple development (AMD) allows full automation of the separation step. This provides both a separation efficiency that is considerably better than that in conventional HPTLC and reproducible gradient elution on the thin layer [25].
The success of an AMD separation depends principally on the choice of the solvent components, on the stepness and shape of the gradient. Whereas the former has been deeply investigated for separating carbohydrates [26], the latter required specific approach. Isocratic elutions with binary mixtures of acetonitrile and water has demonstrated to be selective and of suitable polarity range for the compounds to be separated. On these bases, the AMD gradient was started with the study of the isocratic retention behavior of relative headmonosaccharide glucose, the first dimer lactose (glucose+galactose) and higher-molecular weight GOS, as to understand which range of eluent strength could warrant the best separation of all the analytes of interest. Increasing from time to time the amount of strongest components of the eluent mixture (water, in this case), retention time vary depending on the interaction between oligomers and eluent.
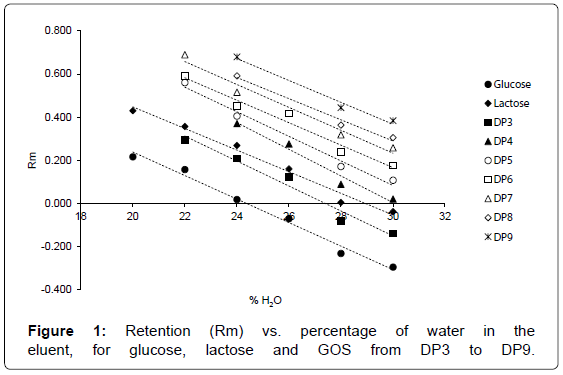
Plot of retention, expressed as
Rm=log [(1-Rf)/Rf] [27]
against the volume fraction of water (Figure 1) shows a very good linear regression (R2 from 0.9771 for glucose to 0.984 for DP 9). It is also evident that separation was achieved by a normal-phase mechanism, because increasing the water content of the solvent mixture reduced GOS retention and the order of migration followed their polarity [28]. Moreover, it is worthwhile noting that below 24% water, lines corresponding to DP4 and DP5 and DP6 and DP7 converged, indicating that for further decreasing of eluent polarity a worsening of separation quality has to be expected, due to peaks overlapping.
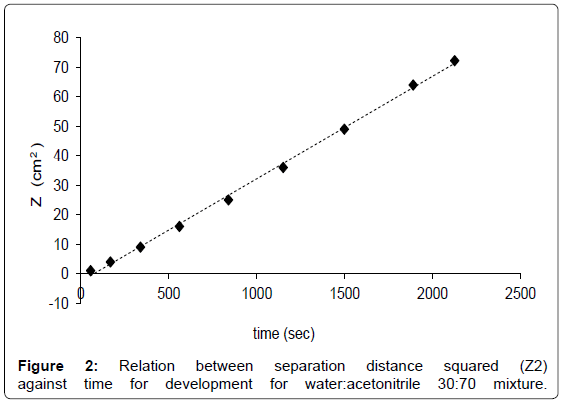
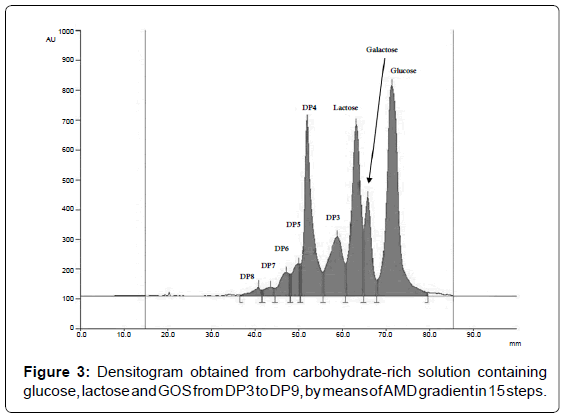
To optimize the shape of AMD gradient, the number and the duration of the steps, for each eluent composition the linear relationship between separation distance squared, expressed ad Z2, for various mobile phases against the time for development has to be proved [29]. An example for the water:acetonitrile 30:70 mixture is reported in Figure 2 as a results of three replicates. The best AMD separation for glucose, lactose and GOS was therefore performed by use of 15-steps gradient in which the amount of water was linearly reduced from 32 to 24% (Table 1). The resulting chromatogram is shown in Figure 3.
| Step | Mixture (68:32) | Mixture (70:30) | Mixture (74:24) | Mixture (76:24) | H2O (tot) |
|---|---|---|---|---|---|
| 1 | 100 | 32.00 | |||
| 2 | 81.38 | 18.62 | 31.63 | ||
| 3 | 52.59 | 47.41 | 31.05 | ||
| 4 | 33.98 | 66.02 | 30.68 | ||
| 5 | 21.96 | 78.04 | 30.44 | ||
| 6 | 14.19 | 67.19 | 18.62 | 29.54 | |
| 7 | 9.17 | 43.42 | 47.41 | 28.29 | |
| 8 | 5.92 | 28.06 | 66.02 | 27.48 | |
| 9 | 3.83 | 18.13 | 78.04 | 26.96 | |
| 10 | 2.47 | 11.71 | 85.81 | 26.61 | |
| 11 | 1.6 | 7.57 | 72.21 | 18.62 | 26.03 |
| 12 | 1.03 | 4.89 | 46.66 | 47.41 | 25.31 |
| 13 | 0.67 | 3.16 | 30.15 | 66.02 | 24.85 |
| 14 | 0.43 | 2.04 | 19.48 | 78.04 | 24.54 |
| 15 | 0.28 | 1.32 | 12.59 | 85.81 | 24.35 |
Table 1: Linear gradient for 15 steps based on mixture of acetonitrile with water for glucose, lactose and GOS separation.
AMD method development for glucose, fructose, sucrose, FOS and inulin
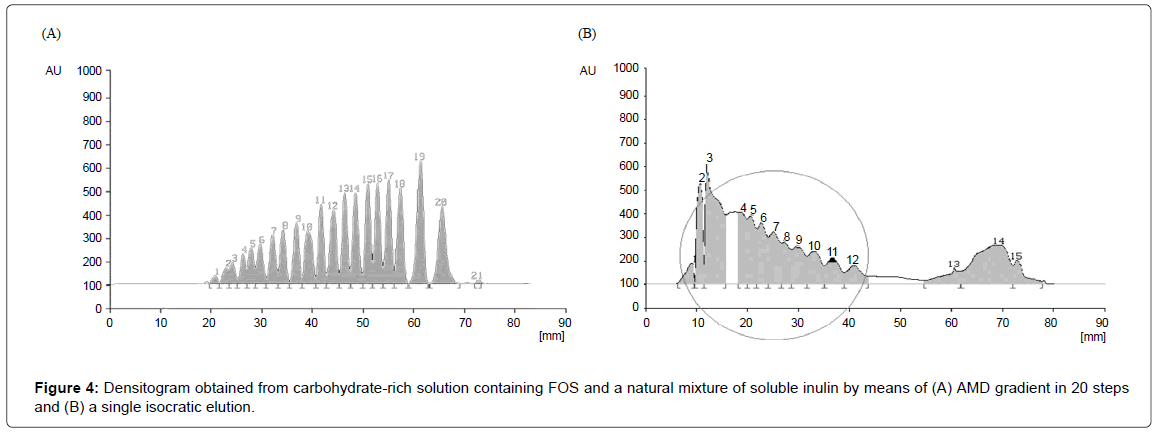
Using the same procedure, AMD gradient has been set up and optimized also for carbohydrates mixture composed by glucose, fructose, sucrose, FOS and inulin. Because the soluble inulin fractions were expected to have higher DP, the polarity of the solvent mixture has to be adjusted as to be able to separate all the components in the same development space. Because of the lack of pure standards of oligosaccharides and inulin higher than DP5 (fructosyl-nystose), a plot similar to the one shown in Figure 1 could not be built: the correct polarity interval of eluent was achieved by trial and error experiments. The 20 steps optimized separation with amounts of water ranging from 25 to 15% water and correspondingly from 75 to 85% of acetone:acetonitrile 1:1 is depicted in Figure 4A. Quality of AMD separation for 21 components is shown in comparison with the results of a simple isocratic elution with acetone:acetonitrile (1:1)-water (86:14) of the same mixtures as a control (Figure 4B).
Method development for xylose and XOS
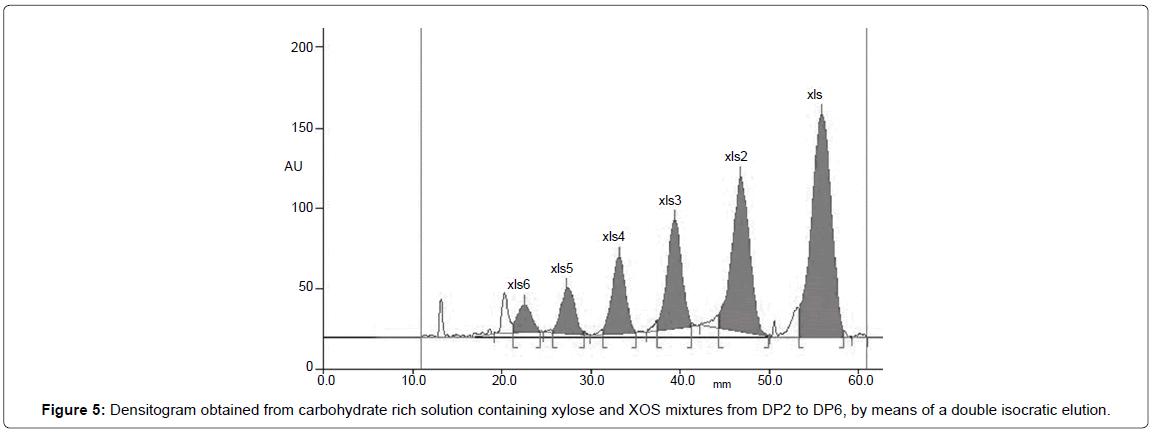
In the case of XOS mixture and their corresponding principal monomer, xylose, method set up and optimization were based on previous results obtained by Reiffova and Nemcova [30] for fructooligosaccharides separation. Two subsequent isocratic elution with butanol:ethanol:water (5:3:2) as mobile phase, have permitted to obtain satisfactorily results on XOS mixture containing oligomers up to DP6 (Figure 5).
Selection of the optimum mobile phases for separation on thin layer was based on the furthest migration distance for xylose, because this is the basic units of XOS with the lowest molecular masses. The first position on densitogram (the utmost spots from start) in line belongs to monosaccharide with the lowest molecular mass, then gradually other components of XOS chain with increasing molecular masses.
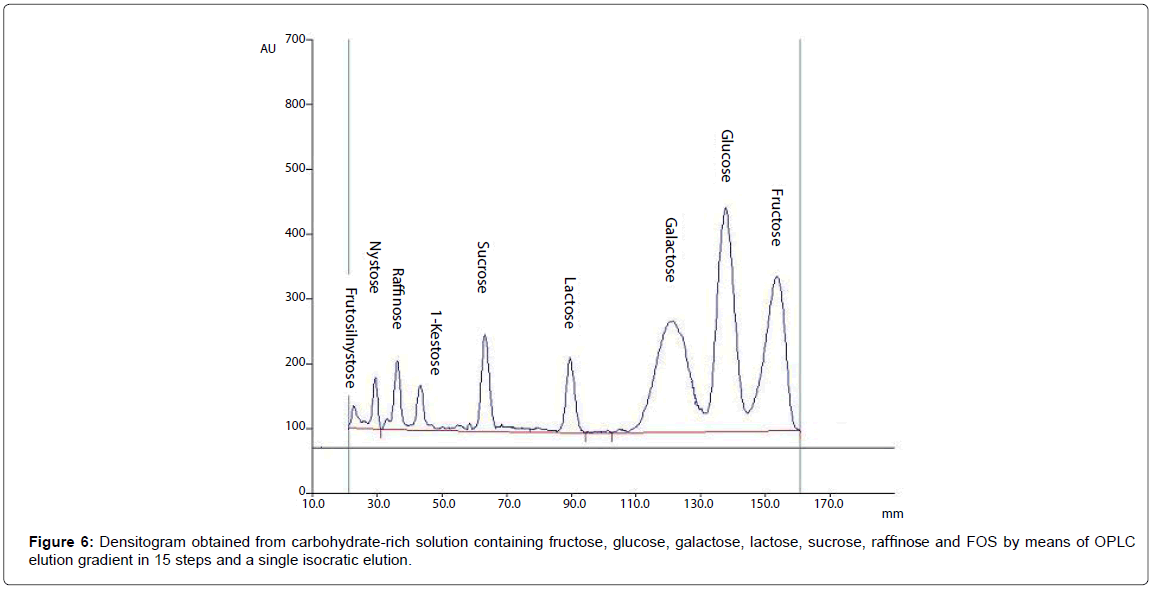
OPLC method development for glucose, fructose, lactose, raffinose and FOS
As a result of digestion and absorption in the upper gastrointestinal tract, human colon where Bifidobacteria usually growth, is an environment rich of carbohydrates at different grade of hydrolysis, depending on the overall metabolic activities of the other microbial intestinal community [31]. Therefore, B. adolescentis MB239 is able to ferment different carbohydrates, from poly- and oligo-saccharides to di- and mono-saccharides, and present at different concentrations.
In this latter case examined, the growth solution for fermentation contained only a mixture of low molecular weight carbohydrates, as glucose, fructose, sucrose, lactose, raffinose, 1-kestose, nystose and fructosyl-nystose. Because of acetonitrile-water has been reported to enable selective separation of carbohydrate on silica plates, overpressured development of our mixture with acetonitrile:water 85:15 was tried on OPLC plates [32]. A series of trial and error experiments indicated that to improve peak resolution, over-running development with a mobile phase volume up to 10000 μL was necessary. A densitogram obtained from a fermentation medium is shown in Figure 6, from which it is apparent that all the sugars are perfectly separated under these conditions.
Retention-structure relationships
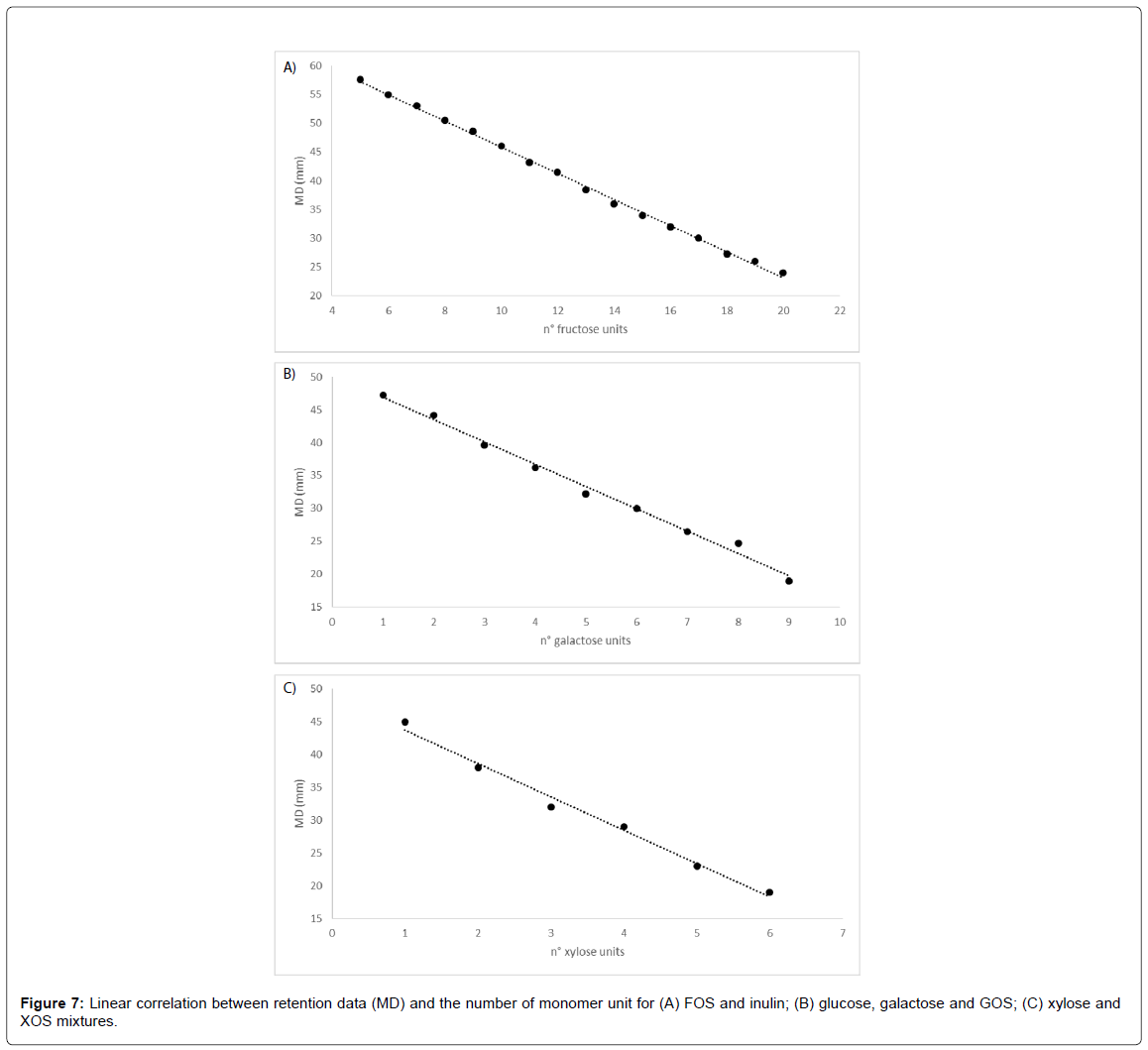
Carbohydrates separation and quantification is of particular importance when they are used as carbon sources in fermentation processes, because only from quantification it is possible to obtain data related to process yield and productivity. On the other hand, especially when carbohydrates of natural origin are used, even if purchased as commercial mixtures, their composition are often reported as approximate percentage and present a high level of heterogeneity from a sample to another. For this reason, precise identification of mixture components could be of great importance, taking into account that microorganisms has different specific growth rate depending on chemical characteristics of the carbon sources, as molecular weight or chemical complexity.
Even when separation has been optimized, identifying the peaks or defining as well as possible the correspondence between the peaks and the chemical structure of components has to be considered. The lack of pure standard substances of GOS, XOS, FOS higher than DP5, and inulin emphasized the need for different approaches to method development. Several studies on retention behavior of molecules have been carried out in the past [33,34] and have revealed linear relationship between chemical structure and retention (Martin’s correlation) [35].
Chemical structure of any given fractions of GOS, XOS and FOS/ inulin can be regarded as a simple chain of the corresponding monomer (galactose, xylose and fructose) attached to a glucose head unit (except of XOS, where the head is xylose as well) and correlated to migration distance (MD) measured in millimeters. MD represents the distance between the final and starting (deposition) point of band migration on the plate.
In all cases examined, retention was found to be linearly dependent on the number of monomer units, with correlation coefficient R2 of 0.9982, 0.9923 and 0.8830 for FOS (Figure 7A), GOS (Figure 7B) and XOS (Figure 7C).
Quantitative assessment of the methods
For quantitative assessment of the method developed and proposed to characterize carbon sources in fermentation media, calibration models have been constructed for each analyte, using the standard solutions deposited on the plates. For each method, in accordance with Ferenczi-Fodor et al. [36] statistical parameters were calculated on the basis of calibration plots. For the sake of brevity, as an example, correlation coefficients of calibrations (R2), the upper limits of linearity, the estimated limits of detection (LOD) and the limit of quantification (LOQ) are reported in Table 2 for xylose and XOS mixture. It is worthwhile noting that the satisfactory statistical performance of the method could make HPTLC an important alternative method to HPLC or other techniques applied to fermentation monitoring.
| Sugar | R2 | Upper limit sugar of linearity [ppm] | LOD [ppm] | LOQ [ppm] |
|---|---|---|---|---|
| Xylose | 0.9857 | 80 | 9.75 | 32.49 |
| XOS-DP2 | 0.9979 | 80 | 5.36 | 17.88 |
| XOS-DP3 | 0.9925 | 80 | 5.30 | 17.65 |
| XOS-DP4 | 0.9979 | 80 | 6.94 | 23.13 |
| XOS-DP5 | 0.9955 | 80 | 9.16 | 30.53 |
| XOS-DP6 | 0.9939 | 110 | 27.33 | 91.10 |
Table 2: Statistical data from the calibration plots for xylose and XOS mixture.
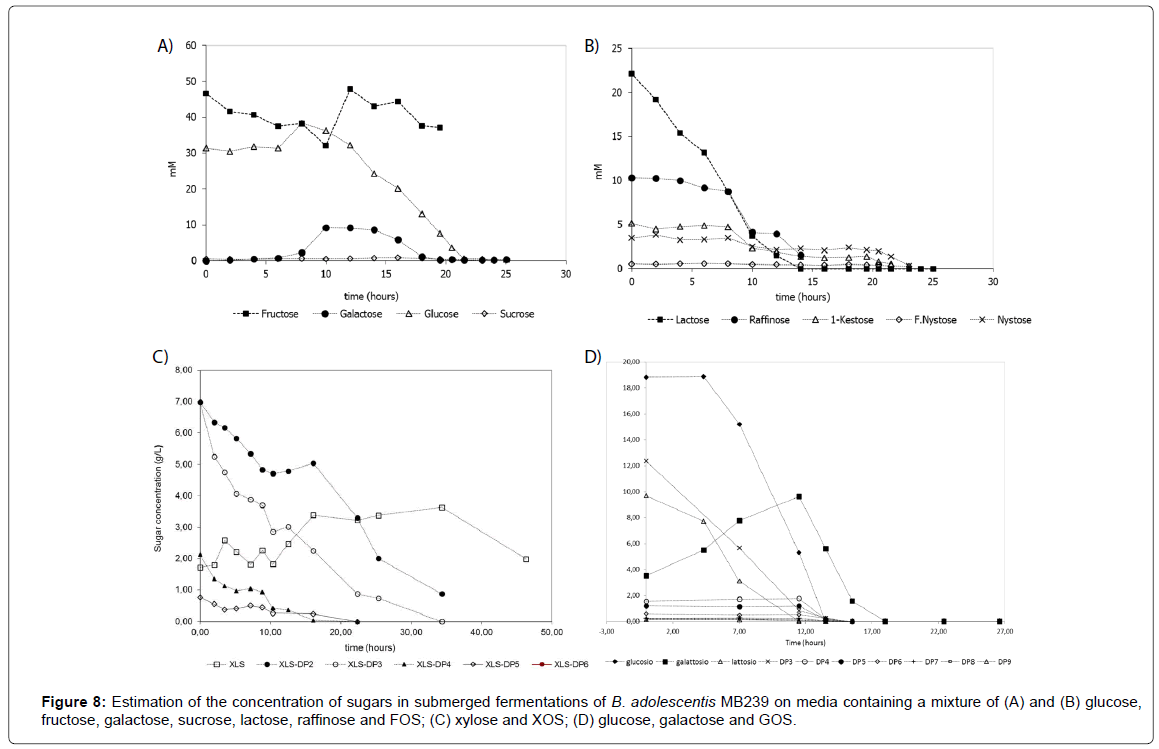
Applying quantitative determination to real fermentation processes of B. adolescentis MB239 in the presence of carbohydrates mixtures analyzed, a complete monitoring of sugars depletion trends was carried out. Figure 8 shows some examples of such different trends over time. In particular, Figures 8A and 8B, represent the same fermentation on nine carbohydrates mixture, but divided in two figures, as to avoid visual overlapping. It is clearly shown that while FOS have been consuming (Figure 8B), fructose concentration has been increasing (Figure 8A), because fructose units has progressively formed at a rate higher than rate of consumption, resulting in a net accumulation over time. In Figure 8C, fermentation of XOS (DP2-DP6) and xylose has been reported. As in the previous case, it is worthwhile noting an accumulation of monomer (xylose) during the process, up to 35 hours, when it remained the unique carbon source available. Finally, in Figure 8D trends of depletion of glucose, galactose, lactose and GOS mixtures have been reported.
Conclusion
HPTLC alone, and in combination with AMD and OPLC instrumental techniques, seems to lead to new possibilities in analysis of carbohydrates in fermentations chemistry. The possibility of performing a direct and sensitive method and the ability to simultaneously detect many different analytes on a single plate, without time-consuming pretreatment, is the major advantage of this technique compared to other methods of analysis commonly used in fermentation monitoring. Culture solutions simply have to be centrifuged or filtered to remove particulate matter, properly diluted and applied to the plate. The analytical interest of this application is principally connected to the possibility to obtain very detailed data about the relative consumption of carbohydrates over time in complex mixtures, and could represents an effective tool for obtaining more information about physiological status or metabolic behavior of cells.
References
- Erickson B, Nelson, Winters P (2012) Perspective on opportunities in industrial biotechnology in renewable chemicals. Biotechnol J 7: 176-185.
- Parekh S, Vinci VA, Strobel RJ (2000) Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol 54: 287-301.
- Peters D (2006) Carbohydrates for fermentation. Biotechnol J 1: 806-814.
- Saxena RC, Adhikari DK, Goyal HB (2009) Biomass-based energy fuel through biochemical routes: a review. Ren Sust Energy Rev 13: 167-178.
- Godge RK, Siddheshwar SS, Somawanshi SB, Dolas RT, Pattan SR (2013) Overview on Fermentation technique and its Application towards industry. IJRPLS 1: 92-101.
- Woodley JM, Breuer M, Mink D (2013) A future perspective on the role of industrial biotechnology for chemicals production. Chem Eng Res Des 91: 2029-2036.
- Watson D, Motherway MOC, Schoterman MH, Neerven RJ, Nauta A, et al. (2013) Selective carbohydrate utilization by lactobacilli and bifidobacteria. J Appl Microbiol 114: 1132-1146.
- Horwitz W (1980) Official methods of analysis. Association of Official Agricultural Chemists. Washington, DC 534.
- Hayashi T, Sakurada I, Honda K, Motohashi S, Uchikura K (2012) Electrochemical detection of sugar-related compounds using boron-doped diamond electrodes. Anal Sci 28: 127-133.
- Blunden CA, Wilson MF (1985) A specific method for the determination of soluble sugars in plant extracts using enzymatic analysis and its application to the sugar content of developing pear fruit buds. Anal Biochem 151: 403-408.
- Koubaa M, Mghaieth S, Thomasset B, Roscher A (2012) Gas chromatography-mass spectrometry analysis of 13C labeling in sugars for metabolic flux analysis. Anal Biochem 425: 183-188.
- Di Stefano V, Avellone G, Bongiorno D, Cunsolo V, Muccilli V, et al. (2012) Applications of liquid chromatography-mass spectrometry for food analysis. J Chromatogr A 1259: 74-85.
- Arfelli G, Sartini E (2014) Characterisation of brewpub beer carbohydrates using high performance anion exchange chromatography coupled with pulsed amperometric detection. Food Chem 142: 152-158.
- Ghebregzabher M, Rufini S, Monaldi B, Lato M (1976) Thin-layer chromatography of carbohydrates. J Chromatogr 127: 133-162.
- Poole FC, Poole SK (1994) Instrumental thin-layer chromatography. Anal Chem 66: 27A-37A.
- Fuchs B, Süss R, Teuber K, Eibisch M, Schiller J (2011) Lipid analysis by thin-layer chromatography--a review of the current state. J Chromatogr A 1218: 2754-2774.
- Srivastava MM (2011) An Overview of HPTLC: A Modern Analytical Technique with Excellent Potential for Automation, Optimization, Hyphenation, and Multidimensional Applications. High-Performance Thin-Layer Chromatography. Springer, Berlin, Germany. Pp. 3-24.
- Günther M, Schmidt PC (2005) Comparison between HPLC and HPTLC-densitometry for the determination of harpagoside from Harpagophytum procumbens CO(2)-extracts. J Pharm Biomed Anal 37: 817-821.
- Zlatkis A, Kaiser RE (2011) HPTLC-high performance thin-layer chromatography. Pp. 9.
- Montero CM, Dodero MCR, Sanchez GAG, Barroso CG (2004) Analysis of Low Molecular Weight Carbohydrates in Food and Beverages: A Review. Chromatographia 59: 15-30.
- Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, et al. (2005) Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl Environ Microbiol 71: 6150-6158.
- Amaretti A, Bernardi T, Tamburini E, Zanoni S, Lomma M, et al. (2007) Kinetics and metabolism of Bifidobacterium adolescentis MB 239 growing on glucose, galactose, lactose, and galactooligosaccharides. Appl Environ Microbiol 73: 3637-3644.
- Poole C (2014) Instrumental Thin-Layer Chromatography. Elsevier
- Tyihak E, Mincsovics E, Kalasz H (1979) New planar liquid chromatographic technique: overpressured thin-layer chromatography. J Chromatogr 174: 75-81.
- Ferenczi-Fodor K, Végh Z, Renger B (2006) Thin-layer chromatography in testing the purity of pharmaceuticals. TrAC 25: 778-789.
- Bernardi T, Tamburini E, Vaccari G (2005) Separation of Complex Fructo-oligosaccharides (FOS) and inulin mixtures by HPTLC-AMD. J Planar Chromatogr 18: 23-27.
- Kiridena W, Poole CF (1998) Structure-driven retention model for solvent selection and optimization in reversed-phase thin-layer chromatography. J Chromatogr A 802: 335-347.
- Abbott SR (1980) Practical aspects of normal-phase chromatography. J Chromatogr Sci 18: 540-550.
- Spangenberg B, Poole CF, Weins C (2011) Quantitative thin-layer chromatography: a practical survey. Springer Science.
- Reiffová K, Nemcová R (2006) Thin-layer chromatography analysis of fructooligosaccharides in biological samples. J Chromatogr A 1110: 214-221.
- Rinne MM, Gueimonde M, Kalliomäki M, Hoppu U, Salminen SJ, et al. (2005) Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Immunol Med Microbiol 43: 59-65.
- Tamburini E, Bernardi T, Bianchini E, Pedrini P (2009) Fermentation Monitoring Based on HPTLC-OPLC: The Effect of a Complex Biological Matrix on Quantitative Performance. J Planar Chromatogr 22: 1-6.
- Kaliszan R (1987) Quantitative structure-chromatographic retention relationships. John Wiley and Sons, New York.
- Héberger K (2007) Quantitative structure-(chromatographic) retention relationships. J Chromatogr A 1158: 273-305.
- Martin AJP (1950) Some theoretical aspects of partition chromatography. Biochem Soc Symp 3: 4-25.
- Ferenczi-Fodor K, Végh Z, Nagy-Turák A, Renger B, Zeller M (2001) Validation and quality assurance of planar chromatographic procedures in pharmaceutical analysis. J AOAC Int 84: 1265-1276.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 22997
- [From(publication date):
August-2015 - Nov 24, 2024] - Breakdown by view type
- HTML page views : 17916
- PDF downloads : 5081