Research Article Open Access
Sensitive Spectrophotometric and Conductometric Methods for the Determination of Candesartan Using Acidic Dyes
| Alaa S Amin1*, Hanna M Salah2, Gamal H Ragab2 and Inass S Kamel2 | |
| 1Chemistry Department, Faculty of Science, Benha University, Benha, Egypt | |
| 2Analytical Chemistry Department, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt | |
| *Corresponding Author : | Alaa S. Amin Chemistry Department, Faculty of Science Benha University, Benha, 2345, Egypt Tel: 0020105090599 Fax: 0020132222578 E-mail: asamin2005@hotmail.com |
| Received February 25, 2015; Accepted March 06, 2015; Published March 13, 2015 | |
| Citation: Amin AS, Salah HM, Ragab GH, Kamel IS (2015) Sensitive Spectrophotometric and Conductometric Methods for the Determination of Candesartan Using Acidic Dyes. Biochem Physiol 4:153. doi:10.4172/2168-9652.1000153 | |
| Copyright: © 2015 Amin AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Two simple, sensitive, accurate, rapid spectrophotometric and conductometric methods were developed for the determination of candesartan (CAND) in raw material and in its pharmaceutical preparation. The proposed methods depend upon the reaction of bromocresol green (BCG) or bromocresol purple (BCP) with candesartan in phosphate buffered solution to form stable colored ion-pair complex, which was extracted in chloroform. The yellow colored complexes were determined at λmax 415, 405 nm with BCG, BCP, respectively. Using conductometric titration, candesartan could be evaluated in acetone. The optimizations of various experimental conditions were described. The results obtained showed good recover of 100.14 (n=6) with relative standard deviation of 0.62 (n=6). Applications of the proposed methods to representative pharmaceutical formulations are successfully presented compared with official methods.
| Keywords |
| Candesartan determination; Spectrophotometry; Conductometry; Ion-pair complexation; Dosage forms analysis |
| Introduction |
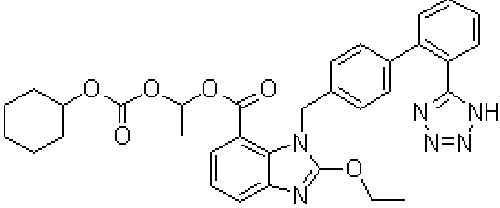
| Candesartan cilexetil, 2-Ethoxy-3-[21-(1H-tetrazol-5-yl) biphenyl-4-yl]-3H-benzoimidazole-4-carboxylic acid 1-cyclohexyloxy carbonyloxy ethyl ester, is prodrug that is hydrolyzed during absorption from the gastrointestinal tract to the active form candesartan (Figure 1). The absolute bioavailability for candesartan is about 40% when candesartan cilexetil is given as a solution and about 14% when given as tablets [1]. Peak plasma concentrations of candesartan occur about 3 to 4 hours after oral doses as tablets. Candesartan is more than 99% bound to plasma proteins. It is excreted in urine and bile mainly as unchanged drug and a small amount of inactive metabolites [1]. The terminal elimination half-life is about 9 hours [1]. Candesartan is not removed by hemodialysis [1]. Angiotensin II receptor antagonists (angiotensin II receptor blockers) are used in the management of hypertension; they may have a particular role in patients who develop cough with ACE inhibitors. Some are also used in diabetic nephropathy and in the management of heart failure [2,3]. Their mode of action is by selective blockade of angiotensin I receptors thus reducing the pressor effects of angiotensin II [4-8]. |
| A number of methods for the determination of candesartan were reported, stripping voltammetric [9-11] and high performance liquid chromatography [12-20], capillary electrophoresis [21], NMR [22] high performance thin layer chromatography [23], densitometry [24] spectrophotometry [14,25], derivative spectrophotometry [26], spectrofluorimetry [27,28]. The development of conductometric methods for candesartan is worthwhile. |
| In this work, two different techniques for the simple and accurate determination of the drug were investigated. bromocresol green, bromocresol purple are used to form ion-pair complexes with candesartan with good chromophore. The purpose of the present investigation is to develop sensitive, simple, accurate, lower detection and quantification limits and precise spectrophotometric and conductometric methods for the determination of candesartan and to apply the procedures to its dosage form. |
| Materials and Methods |
| Apparatus |
| The pH values of the buffer solutions were measured using an HANNA pH-meter. The absorption spectra for all measurements were carried out using Shimadzu 1601 PC double beam UV-VIS spectrophotometer, equipped with 1cm quartz cells, a fixed slit width (2.0 nm) was connected to an IBM-PC computer, loaded with Shimadzu UVPC software which was equipped with HP desk jet printer. Conductometer model 470 JENWAY was used for all the conductometric measurements. |
| Reagents |
| Analytical and medical use grade reagents and deionized water was used to prepare all solution. Candesartan was obtained from (Global Napi Pharmaceutical, 6th of October, Giza, Egypt) with 99.8% purity. |
| A stock standard candesartan of 100 μg/ml was prepared by dissolving an exact weight (0.01 g) of the pure drug in least amount of acetone then complete the volume to 100 ml with water in a 100 ml calibrated flask. Stock solution of bromocresol green or bromocresol purple (Aldrich product) 5x 10-3 M solution was also prepared. BCG prepared by dissolving appropriate weight in 100 ml acetone, while BCP prepared by dissolving in 4.0 drops of 1.0 M NaOH then complete to the mark with deionized water |
| Pharmaceutical preparation solutions |
| An accurately 100 mg from a composite of the mixed contents of 10 tablets was weight, and then the procedure as for stock standard solution of the studied drug powder was followed. |
| General Procedures |
| Spectrophotometric procedure: An aliquot containing (2.0-10), and (6.0-20) μg/ml of the investigated drug in case of BCG, BCP, respectively, was transferred into a 10 ml calibrated flask, 1.2 ml of BCG (flask 1) or 0.5 ml of BCP (flask 2) were added then 2.5 ml phosphate buffer (pH= 4.5) was added to (flask 1) or 0.5 ml of phosphate buffer (pH= 3.0) was added to (flask 2). The mixture was shacked for 5.0 min at room temperature; then, the complex formed was extracted with 5.0 ml chloroform. The organic layer filtered over anhydrous sodium sulphate into 10 ml volumetric flasks, and then the volumes were completed to 10 ml with chloroform. Each sample was repeated for six times to studied the reproducibility. The absorbance of the colored solutions were measured at 415, 405, against blank similarly prepared. Under experimental conditions above described, standard calibration graphs for candesartan was constructed by plotting the absorbance versus concentration and the regression equations were computed and recorded in Table 1. |
| Conductometric procedure: A volume containing 0.1-1.5 mg of drug was transferred to a 50 ml calibrated flask and made up to the mark with acetone. The contents of the calibrated flask were transferred to a beaker and the conductivity cell was immersed. 5 × 10-3 M bromocresol green solutions were then added from a microburette and the conductance was measured subsequent to each addition of reagent solution and after thorough stirring. The conductance reading taken 2.0 min after each addition which was corrected for dilution [29] by means of the following equation, assuming that conductivity is a linear function of dilution. |
| Amount of the drug (mg) = VMR / N |
| Where V = Volume (ml) of the titrant consumed in the titration, M = Relative molecular mass of the analyte, R = Molarity of the titrant, and N = Number of moles of the titrant consumed per one mole of the analyte. |
| Ω−1 (Correct) = Ω−1 (obs) [v1 + v2/ v1] [29] |
| Where Ω−1 (obs) is the observed electrolytic conductivity, v1 the initial volume ad v2 the volume of reagent added. A graph of corrected conductivity versus the volume of added titrant was constructed and the end-point determined. |
| Stoichiometric Relationship |
| Job’s method of continuous variation was employed; a 5 × 10−3 M standard solution of CAND and reagent solution were used. A series of solution were prepared in which the total volume of drug and reagent was kept at 1.0 ml. The reagents were mixed in various proportions and diluted to volume in a 10 ml calibrated flask with the appropriate solvent following the above mentioned procedures. |
| Assay procedure for Candesartan Formulations |
| Fourteen tablets of each Candesar 8 tablets and Atacand 16 tablets were powdered and mixed well. An accurately weighed amount of the powder equivalent to 50 mg of candesartan cilexetil of each was transferred into two separate 100 ml volumetric flasks. 75 ml of methanol were added, sonicated for 0.5 h, completed to volume with methanol, to obtain 0.5 mg/ml stock solution, and filtered. The solution was diluted to the same concentrations of the appropriate working solutions and proceeded according to the procedure mentioned above. The nominal content of candesartan cilexetil in each tablet was calculated from the calibration graph prepared above as described in the general procedure. |
| Procedure for spiked urine |
| A 5.0 ml of urine was transferred into a 100 ml separating funnel. Spike with increasing quantities of CAND to give 1.0 mg/100ml. A 1.0 ml of HClO4 was added and shacked well, then extracted with 5.0 ml of chloroform for three times, and then passed the chloroform layer over anhydrous sodium sulfate. The extract under reduced pressure was evaporated till dryness. The residue in 5.0 ml of (1:4) (dimethylformamide (DMF): methanol) was dissolved and then proceeded as described under general procedure. The nominal content of the drug was determined from the corresponding regression equation. |
| Procedure for spiked plasma |
| A 1.0 ml of equine plasma was transferred into a 100 ml separating funnel and spiked with increasing quantities of CAND to give 1.0 mg/100 ml. A 1.0 ml of HClO4 was added and shacked well. Then, extracted with 5.0 ml of chloroform for three times, and passed the chloroform layer over anhydrous sodium sulfate. The extract under reduced pressure was evaporated till dryness. The residue was dissolved in 5.0 ml of (1:4) (dimethylformamide (DMF): methanol) and then proceeded as described under general procedure. Determine the nominal content of the drug from the corresponding regression equation. |
| Results and Discussion |
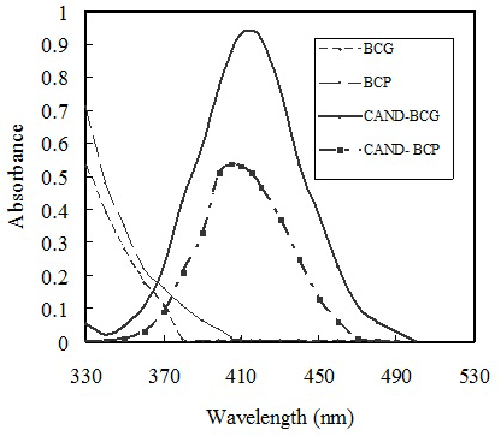
| The absorption spectrum of extracted solution containing CAND with the reagent (BCG or BCP) exhibits new absorption band at longer wavelength than that drug and dye alone (Figure 2). The new broad absorption band in the visible region (yellow color) after addition of drug to a fixed concentration of dye indicates the formation of ionpair complex. Investigations were carried out to establish the most favorable conditions for the ion pair complex formation of candesartan with bromocresol green or bromocresol purple to achieve maximum color development and/or sharp end point, to determine CAND. The influence of some variables on the reaction has been tested as follow: |
| Optimum conditions for Spectrophotometric Method |
| Effect of pH: In order to establish the optimum pH value for each ion –pair formed CAND was allowed to react with BCG or BCP in aqueous phosphate buffered solution on of pH’s range (2.0 -12.0). The absorbance intensity was measured at its λmax. The highest absorbance value at pH was 4.5 using BCG and 3.0 using BCP. Furthermore, the amount of phosphate buffer solution added was examined and found to be 2.5 ml in case of BCG and 0.5 ml in case of BCP. |
| Effect of reagent volume: When various volumes of 5 × 10-3 M BCG or BCP were added to a fixed concentrations of CAND, 1.2 and 0.5 ml of BCG, and BCP, respectively, were found to be sufficient for the production of maximum and reproducible color intensity. Higher concentration of reagent decreased the absorbance and color intensity of the formed ion-pair. |
| Effect of shaking time: The time required for complete color development of ion-pair formed between CAND and (BCG or BCP) was investigated. Allowing the reactants to be shacked for different time intervals, it was observed that time needed for maximum color intensity is 5.0 min in case of BCG, BCP. |
| Effect of temperature: The optimum reaction temperature was determined by complete color development was attained and permitting quantitative analysis to be carried out with good reproducibility. The best temperature for BCG, and BCP was found at room temperature (25 ± 2°C). |
| Effect of extracting solvents: The polarity of the solvent affects both extraction efficiency and absorbance intensity. The results using different solvents (benzene, chloroform, carbon tetrachloride, 1,2-dichloromethane) showed that chloroform is the best solvent for extraction of the ion-pair complex formed in case of BCG or BCP. Chloroform was selected due to its higher sensitivity and the considerably lower extraction of the reagent itself. |
| Effect of sequence of addition: The most suitable sequence was drug, reagent (BCG or BCP), then the buffer media (phosphate) for the production of the highest color intensity, while the other sequences produced lower absorbance values. |
| The stoichiometry of the complex: The stoichiometry of the complex formed between BCG and BCP with CAND was investigated at the optimum experimental conditions, by the continuous variation method. The result indicates the existence of 1:1 charge transfer complex at a definite λmax recorded in Table 1. The conditional stability constants (log K), calculated with Harvey and Manning equation applying the data obtained from the continuous variation method (Table 1). |
| Conditions for conductometric titrations |
| Investigations were carried out to establish the most suitable conditions for the ion associates formation of candesartan with BCG to attain sharp endpoint. The optimum conditions for performing the titration in a quantitative manner were elucidated. |
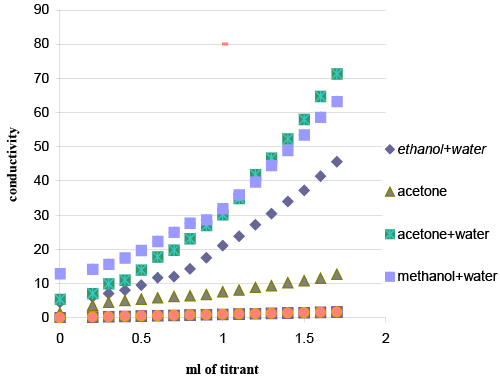
| Reaction medium: Titrations in different media were attempted (Figure 3) to obtain the best results. Preliminary experiments were occurred in acetone drug solution (prepared by dissolving 100 μg drug in 1.0 ml acetone), with acetone reagent solution, acetone/ water (50%) drug solution with acetone/water (50%) reagent solution, methanol/ water (50%) drug solution with methanol/water (50%) reagent solution, Preliminary experiments showed that procedure in acetone was the most suitable for successful results. |
| Reagent concentration: Different concentrations of BCG solutions were tried ranging from 2 × 10−2 to 5 × 10−4 M. The optimum concentration of the reagent was 5 × 10−3 M in titration of the studied drug to achieve a constant and highly stable conductance reading within 2.0 min of mixing. Concentrations less than these limits led to more time was needed to obtain constant conductance values and unstable readings. |
| Representative titration curve is shown two straight lines are obtained, intersecting at the endpoint; the first branch ascending, and the second has conductance values that would slightly increase after the equivalence point. The increase of conductance may be attributed to the formation of ion pair in solution as a result of the complexation reaction. |
| The conductometric titrations of different volumes of 5 × 10−3 M BCG solution were performed. The results show maximum in the conductance curve at drug-reagent molar ratio of (1:1). |
| The conductance measured before the addition of the titrant (volume of BCG equals zero) is mainly due to the hydrogen ions mobility. The data show that accurate results were obtained with good recoveries and low standard deviation values. The optimum concentration ranges for determination of the cited drug were in the range of 0.1–1.5 mg/ml. At such ranges, sharp inflections and stable conductance reading were obtained. |
| Method Validation |
| Using spectrophotometric method: At the optimum conditions of pH, reagent volume, shaking time, temperature, extracting solvent, and sequence of addition; CAND react with anionic dyes to form ionpair complexes, which are often colored and subsequently measured colorimetry. These characters are applied for the determination of CAND, through measuring the absorbance of the formed colored ion-pair at corresponding optimum wavelength using BCG or BCP. Various parameters affecting the reaction development were studied. A calibration graph was constructed using standard solution of CAND. Under the optimum conditions, a linear relationship existed between the absorbance and concentration of the drug in the concentration range (2-12) for BCG and (6-20) for BCP. The correlation coefficient slopes and intercepts, standard deviation, relative standard deviation and relative error of the calibration data for CAND are calculated. The reproducibility of the method was determined by running six replicate samples, each containing 10 μg/ml of CAND. At this concentration, the correlation coefficient was found to be <0.9998 % as recorded in (Table 1). The mean molar absorptivity, Sandell sensitivity, detection and quantification limits are calculated from the standard deviation of the absorbance measurements obtain from Beer’s law and recorded in Table 1. |
| The detection limit (LOD) for the proposed method was calculated using the following equation according to the ICH |
| LOD = 3 σ / S |
| Where σ = the standard deviation of replicate blank responses (under the same conditions as for sample analysis), and S = the slope of the calibration curve. The limit of quantification (LOQ) is defined as according to the previous equations, the LODs and LOQs were calculated as in Table 1. Their values confirm the sensitivity of the proposed method. |
| LOQ = 10 σ / S |
| In order to determine the accuracy and precision of the proposed method, solutions containing one concentration of candesartan were prepared and analyzed in six replicates. The relative standard deviation (RSD %) as precision and percentage relative error (Er %) and accuracy of the suggested method was calculated at 95% confidence levels and can be considered satisfactory. |
| The interday and intraday precisions and accuracy results are shown in (Table 2). The analytical results for accuracy and precision show that the proposed method has good repeatability and reproducibility. |
| Using conductometric method: In order to address the validity of the proposed method, statistical analysis of the data obtained from its application on candesartan in the pure form and in formulation was performed. The results revealed in tables showed that the proposed method is satisfactorily accurate, precise, and reproducible over a concentration range of 0.1–1.5 mg. Results for the determination of the studied drug using the previously mentioned method were compared with results from reported one. |
| Analytical Applications |
| The suggested methods were applied successfully to the determination of CAND in commercial tablet. Six replicate determinations were made. Table 3 shows that satisfactory recovery data were obtained and the assay results were in good agreement with the label claims. Moreover, to check the validity of the proposed methods, dosage forms were tested for possible interference with standard addition method. There was no significant difference between slopes of calibration curves and standard addition methods at two methods. Therefore, it is concluded that the excipients in dosage forms of CAND such as starch, lactose, talc, stearic acid, titan dioxide, yellow iron oxide were not found to have any interference in the analysis of CAND, indicating high selectivity of the proposed methods. At 95% confidence level the calculated t- and F-values did not exceed the theoretical values indicating no significant difference between the two proposed methods and the reported method using the first-derivative spectrophotometric method (D1-method) [228-246 nm] for CAND (Table 3). |
| Analysis of biological fluids |
| The high sensitivity of the proposed method allowed the determination of CAND in biological fluids. The proposed method was further applied to the in-vitro determination of CAND in spiked human urine and equine plasma. CAND is readily absorbed from the gastro-intestinal tract. The half-life time is about 10–12 h. CAND is extensively metabolized without significant hypoglycemic activity. The usual initial dose is 40–80 mg daily, gradually increased if necessary, up to 320 mg daily. Doses of more than 160 mg daily are given in two divided doses. Following oral ingestion of a single 80 mg CAND dose gives a final plasma concentration of about 1.6 μg/ml. This value lies within the working concentration range of the proposed methods, thus it could be successfully applied to the determination of CAND in spiked human urine and plasma over the specific concentration range. The results are abridged in Table 3. The mean percentage recoveries for CAND in spiked urine and plasma are 99.33 to 100.54 ± 0.43 to 0.95 (n = 6). The method involved extraction of the drug using chloroform. |
| Conclusion |
| Spectrophotometric and conductometric analysis are of major interest in analytical pharmacy since it offers distinct possibility in the assay of a particular component in dosage formulations. In the present study, the maximum color development of CAND with BCP or BCG ion-pair complex was instantaneous. No heating or standing was needed. These methods do not involve procedural steps and do not take more operator time and expertise like HPLC and other methods. In terms of simplicity, rapidity, sensitivity, expense and free from interference by common additives and excipients, the methods could be considered superior in comparison with the previously reported methods, especially with those based on chromatography, or other reported spectrophotometric methods. The reagents utilized in the proposed methods are cheaper, readily available and the procedures do not involve any critical reaction conditions or tedious sample preparation. |
| The method is unaffected by slight variations in experimental conditions such as time, reagent concentration or temperature. The proposed methods gave results with good accuracy to permit determination of low concentrations. The wide applicability of the described procedure for routine quality control is well established by the assay of CAND in pure form, as well as in tablets dosage forms. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14004
- [From(publication date):
June-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9561
- PDF downloads : 4443
