Self-efficacy for smoking cessation vs. temporary abstinence: two aspects of a complex process
Received: 20-Mar-2020 / Accepted Date: 23-Jun-2020 / Published Date: 19-Jun-2020 DOI: 10.4172/2155-6105.1000394
Abstract
Introduction: Smokers receiving mental health care are particularly in need of tailored interventions. Objective: Study of patients enrolled in a specialized smoking cessation program based upon a 26-hour smoking abstinence period aimed better understanding of self-efficacy for smoking cessation and of the decision to quit.
Methods: A logistic regression predicting success/failure of abstinence included different variables. Self-efficacyfor temporary abstaining from smoking (TASE) and for permanent quitting (QSE) were distinguished.
Results: In 174 subjects enrolled at baseline, TASE was the only predictor of successful abstinence (OR=1.43; p=.001). Assessment of 138 subjects present 1 week after intervention showed increases in TASE and QSE (median
TASE from 8 to 10, p<.0001; median QSE from 8 to 9, p=.02). In subgroups of successful abstainers and of those engaging into smoking cessation, only TASE increased. Interestingly, for subjects who had planned a quit attempt
already before the intervention, 52% were still abstinent at 1 week vs. 87% of those who decided to quit during theintervention (p=.02).
Conclusion: A multicomponent program for all smokers can be a powerful method to increase self-efficacy, in particular for temporary smoking abstinence, and trigger unplanned quit attempts, shown here to be more successful than planned attempts.
Keywords: Addiction; Health psychology; Motivational; enhancement intervention; Psychiatry; Self-efficacy; Smoking; cessation; Temporary smoking abstinence
Keywords
Addiction; Health psychology; Motivational enhancement intervention; Psychiatry; Self-efficacy; Smoking cessation; Temporary smoking abstinence
Introduction
Smoking remains a major public health concern and its use has declined slowly in the general population but more slowly in vulnerable groups such as persons with mental illness [1-4]. A large body of knowledge is available concerning the addictive nature of smoking, such as mechanisms related to neural adaptation to nicotine [5], stimulation of the central reward pathway, environmental cues and situations stimulating reinforcement processes, as well as psychological aspects such as beliefs about smoking and stress reduction [6]. However, knowledge on the quitting process is still insufficient, as illustrated by the difficulty of increasing cessation rates in the general population [7] and relapse remains the more common outcome of cessation programs [8]. The process leading to stopping cigarette use always implies that the smoker makes the personal decision to quit. Pharmacological therapy or psychological support will not be effective without this individual step. Although it might not be sufficient, this motivation is a necessary condition to succeed [9,10]. Understanding how smokers decide to stop and put this decision into practice is thus relevant to improve smoking cessation.
Several elements influence smoking cessation attempts and success, such as severity of nicotine dependence, stage of change, previous attempts [11], intensity of craving, psychological distress [8], facilitating environments, etc. One of these elements is self-efficacy, a central concept in health behaviour theories, described as impacting behaviour changes such as stopping smoking [12]. However, despite numerous studies demonstrating links between self-efficacy and the adoption of new behaviours, the exact mechanism of this change remains unclear [13]. Self-efficacy may reflect for a large part actual smoking behaviour [14] and there is a debate whether an increase in self-efficacy is a cause or a consequence of ongoing behaviour change [15,16]. More research is needed to understand the essential relationship between behaviour and self-efficacy, the perception of one's capacity to control or modify habits. A behavioural experience, such as a quit attempt leading to success or failure, appears likely to influence a smoker's perception toward his ability to stop smoking. Many smokers say that they would like to stop but find it difficult, and often do not feel able to quit and become unmotivated. However, a successful stopping experience could increase confidence and self-efficacy. The extent of this effect would need to be explored both in smokers planning to quit as well as in smokers not thinking about stopping, before they undergo a positive abstinence experience.
This study took place in a mental health department providing an intensive 26-hour multicomponent intervention to promote smoking cessation among all smokers, independently of their intention to stop. This intervention is based on commitment to a 26-hour temporary smoking abstinence period to allow the experience of a "practice quit attempt", a technique also explored by others [17]. Such an approach lessens the stress of permanent cessation and repeated failures, while at the same time increasing the motivation to stop [18].
The aim of the study was to study predictors of a successful abstinence period and to examine associations with self-efficacy, quit attempts in the week after the intervention, and to deepen aspects concerning the fundamental issue of taking the decision to permanently stop smoking. We distinguished between self-efficacy to maintain TASE and self-efficacy to quit smoking permanently (QSE, quitting self-efficacy) as representing different aspects of the smoking cessation process.
Methods
Context
Subjects in this study were smokers receiving in- and outpatient mental health care in a public university hospital covering ages 18- 65 and who participated in a motivational intervention to promote smoking cessation. They came from a large range of facilities, including acute care or rehabilitation inpatient units or outpatient consultation or day care units, totalling 13 centres. Patients had a wide variety of diagnoses with a predominance of psychotic (41%) and mood (40%) disorders. Few patients with substance use disorders or mental disability as main diagnoses were included as they were followed by other specialised services.
Inclusion
All smokers who had smoked in the past week could participate regardless of their consumption level but they had to commit to try not to smoke during 26 hours. They also had to be in a stable mental condition allowing participation in the 26-hour program and its multiple activities. All participants were informed about the study and could decide to participate independently of enrolment. Consent forms were signed at the first evaluation. Exclusion criteria were insufficient understanding of questionnaires related to cognitive impairments or language barriers.
Subjects
During the 2010-2017 period, the intervention was proposed to 389 participants, of which 174 were included in the study (66.1% inpatients and 33.9% outpatients). 116 were not included because of refusal to participate in the study or cognitive or language impairment, 48 because they had already participated previously in the program, 43 because they did not show up after the first interview (worsening of psychiatric condition or improvement and discharge from hospital), 4 because they left the program in the first hours, and 4 were not smoking anymore at the time of the intervention. In the study sample of 174 subjects, 36 were absent at 1 week post-evaluation, leaving 138 subjects for pre-/post-intervention analyses.
Intervention
A motivational intervention encouraging smoking cessation, called "Day off", was proposed to all smokers from various psychiatric services, whatever their motivational level [19]. To induce positive experiences, this intervention offered the challenge of a 26-hour temporary smoking abstinence with attractive activities (thermal baths, restaurants) in a supportive context (support from peers and professionals) and optional nicotine replacement therapy (NRT), with doses adapted to usual smoking consumption and nicotine intake. The intervention also included an interactive session with smoking cessation specialists providing tobacco-related information and individual feedback on expired carbon monoxide (CO) levels as the intervention progressed.
Study design and measures
Evaluations were performed with the same instruments measuring psychological and tobacco-related variables at 3 time points: preintervention at about 1 week before the intervention, end-ofintervention after the 26-hour abstinence trial, and post-intervention 1 week later. Anxiety (Spielberger’s STAI-S [20]), depression (BDI-21 [21]) and well-being (WHO-5 index [22,23]) questionnaires were used for basic clinical assessment, relevant in the context of mental health. Tobacco dependence was evaluated with the Heaviness of Smoking Index (HSI) based on number of cigarettes per day and time to first cigarette [24,25] and motivation to quit with Biener’s contemplation ladder [26] and with interviews to determine the motivational stage according to the stage-of-change model. Expired CO levels were measured with a piCO+ Smokerlyzer (Bedfont UK). The first interview allowed to collect socio-demographic information and diagnoses were collected from medical charts. The decision to stop smoking was investigated by comparing subject's statements between pre- and postintervention, allowing to determine if they changed decision during the intervention about permanently quitting. Subjects were classified into 5 subgroups, ranging from "already firmly decided to stop smoking before intervention" to "has decided not to stop", and including “decided during intervention to quit”.
Self-efficacy for smoking cessation was measured by two 10-point scales anchored by wording, from “no, impossible” (1-2) to “yes, absolutely” (9-10). One scale assessed the perceived capacity to quit smoking in the long-term (QSE) (“I’m convinced that one day, whatever happens, I will stop smoking”) and another the perceived capacity to maintain temporary abstinence. Here participants had to rate the item "During the 26 hours of the “Day off”, I believe I will be able not to smoke" before the intervention and “If I would participate again in a “Day off”, I think I would be able not to smoke during the 26 hours” after the intervention. Analyses of TASE and QSE compared pre and post-intervention results and assessed associations with successful 26-hour abstinence, quit attempts in the week after the 26- hour intervention and time of decision to stop smoking. Results were further deepened for the subsample who had decided to stop smoking and differences were analysed according to whether this decision was made before or during the intervention.
Statistics
Predictors of success of temporary abstinence were analysed on the whole sample of 174 subjects. A multivariate logistic regression model assessed the combined influence of different factors on the success of the 26-hour abstinence. The adjusted odds ratio (OR) and 95% confidence intervals (95% CI) were reported for all independent variables entering the model simultaneously. Analyses for pre-/post comparisons of self-efficacy were performed on the 138 subjects present at both evaluations. Tests for change in ordinal scores were performed using the Wilcoxon signed-ranks test, the Mann-Whitney U-test was used for 2-group comparisons and the Kruskal Wallis test for 3-group comparisons. Relationships between the success of the quit attempt and the decision to stop taken during or before the intervention were examined on the 51 participants who made a quit attempt, using Fisher’s exact test. Statistical significance was set at 0.05 for all tests. Analyses were performed using SPSS version 22 (SPSS Inc, Chicago IL, USA).
Results
Baseline evaluation
The sample of 174 subjects had a median age of 35.5 (range: 17-64) and comprised 57.5% of males (n=100). Respectively 40.6%, 51.2% and 36.7% achieved compulsory, intermediate and higher education. The majority of participants (63.3%, n=107) received a disability pension or social aid. Half of the subjects (54.6%, n=95) were diagnosed with 2 or more psychiatric disorders. The main diagnoses were psychotic (41.4%, n=72) and mood disorders (40.2%, n=70). Median scores were 47 (20-77) for anxiety (STAI-S), 17 (0-52) for depression (BDI-21) and 12 (0-25) for well-being (WHO-5).
Tobacco consumption was high with a median of 20 (1-60) cigarettes per day, and presented a diversity of smoking profiles (33.6% >20 cigarettes per day and 32.4% ≤ 10 cigarettes per day). The Heaviness of Smoking Index (HSI) was 4 (1-7) and median expired carbon monoxide (CO) was 21 ppm (1-100). Level of motivation was fairly high as a quarter of participants (24.3%, n=41) made a quit attempt in the last 6 months, a half (51.7%, n=90) were in the pre-contemplation stage and the median Biener score was 7 (0-10).
Evaluation after 26-hour intervention
The majority of the 174 participants succeeded in smoking abstinence (biochemically verified via monoxide test with CO ≤ 6 ppm) for 26 hours (52.3%, n=91) while 37.4% (n=65) were abstinent during the first 9 hours (day program) and 8.8% (n=15) smoked one or more cigarettes during this time. The median CO measurement was 6 ppm (2-58) for the whole sample, 4 ppm (2-14) for abstinent subjects and 11 ppm (4-58) for those who smoked. Most participants (86.8%, n=151) used some form of NRT (fast- and/or slow-acting products: lozenges, gums, inhalers, patches).
Predictors of successful 26-hour smoking abstinence
Potentially predicting factors of successful 26-hour abstinence were entered into a logistic regression model: gender, age, diagnostic group, heaviness of smoking (HSI), motivation to quit (Biener’s contemplation ladder), anxiety (STAI-S), depression (BDI-21), wellbeing (WHO-5), TASE (self-efficacy for 26-hour smoking abstinence) and QSE (self-efficacy for quitting smoking). On the sample of 174 subjects, 30 had missing data (ie not answering one if the items on one of the questionnaires). Final analysis was performed on 144 subjects. Controlling for all other variables, self-efficacy and in particular TASE was the single most relevant predicting factor of successful smoking abstinence (OR=1.43; p=.001) (Table 1).
| Adjusted OR(1) | 95% CI | p-value | ||
|---|---|---|---|---|
| Gender | female (ref) | 1 | ||
| male | 1.24 | (0.57-2.70) | 0.59 | |
| Age | 1 | (0.97-1.03) | 0.83 | |
| Main diagnosis | other (ref) | 1 | ||
| substance use | 0.57 | (0.09-3.81) | 0.56 | |
| psychotic disorders | 0.48 | (0.15-1.58) | 0.23 | |
| mood disorders | 1.1 | (0.33-3.70) | 0.88 | |
| Heaviness of smoking index (HSI) | 0.89 | (0.89-0.71) | 0.32 | |
| Biener's scale | 1.06 | (0.91-1.24) | 0.46 | |
| Anxiety (STAI-S) | 1.04 | (0.99-1.09) | 0.11 | |
| Depression (BDI-21) | 0.99 | (0.94-1.05) | 0.73 | |
| Well-Being (WHO-5) | 1.01 | (0.93-1.10) | 0.77 | |
| Self-efficacy for temporary abstinence (TASE) | 1.43 | (1.15-1.77) | 0.001 | |
| Self-efficacy for permanent smoking cessation (QSE) | 1.06 | (0.86-1.32) | 0.59 |
* 30 had missing data in one of the items, logistic regression analysis performed on n=144
Table 1: Predictors of successful 26-hour smoking abstinence (n=174*).
Evaluation post intervention
At 1-week after intervention, 138 subjects were re-evaluated. Concerning smoking status, 2 subjects had missing data (for example inconsistent answers between self-report and CO measures). One third of the sample (37.5% n=51) had decided to make a quit attempt and two-thirds of them (66.7% n=34) or 19.5% of all participants, were not smoking at 1 week.
Concerning time of deciding to stop smoking, 134 subjects were evaluated (4 had missing data because of ambivalent answers and uncertainty, due to their psychiatric condition). 20.1% (n=27) of the subjects decided to stop smoking during the program, while 31 (23.1%) decided before the intervention to quit (10.4% (n=14) in continuation of the program and 12.7% (n=17) within 2 weeks after the intervention). A further 12.7% (n=17) were unsure about stopping and 44.0% (n=59) had decided not to stop.
Self-efficacy measures: TASE and QSE scores were not significantly different at pre-intervention (both medians 8 and 8, p=.70), but TASE was significantly higher than QSE (medians 10 and 9, p=.0004) after intervention. Self-efficacy increased between pre- and postintervention with a higher increase of TASE (median 8 to 10, p<.0001) than QSE (median 8 to 9, p=.02) (Table 2). TASE and QSE were highly correlated (TASEpre/QSEpre: Spearman’s rhô=.23, p=.002; TASEpost/ QSEpost: Spearman’s rhô=.38, p<.0001).
| pre-intervention | post-intervention | Test for change (Wilcoxon signed-ranks test); p-value | |
|---|---|---|---|
| Self-efficacy for temporary abstinence (TASE) | 8 (3-10) | 10 (3-10) | <.0001 |
| Self-efficacy for long-term smoking cessation (QSE) | 8 (1-10) | 9 (3-10) | 0.02 |
| Test for difference between TASE and QSE (Wilcoxon signed-ranks test); p-value | 0.7 | 0.0004 |
Table 2: Median values (observed range) of self-efficacy for temporary abstinence (TASE) and self-efficacy for long-term smoking cessation (QSE); n=138.
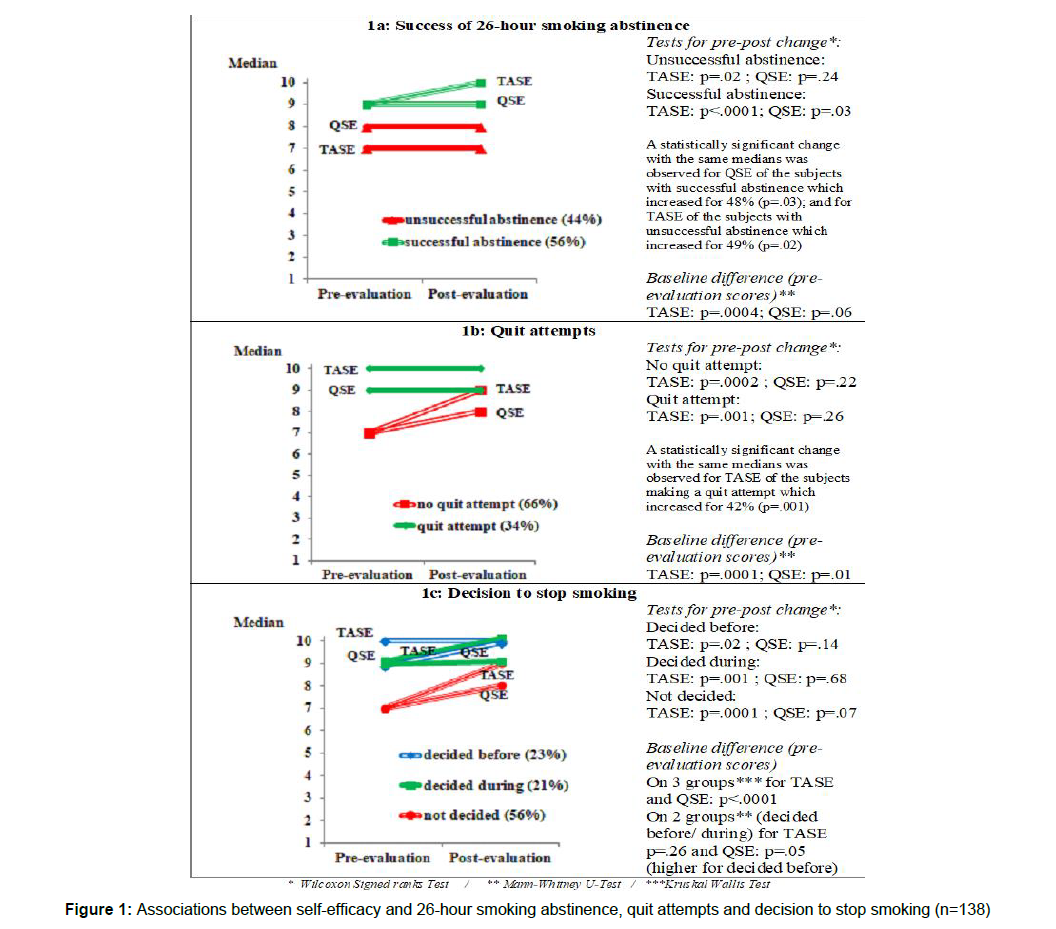
Self-efficacy at baseline and in particular TASE was higher for smokers who successfully abstained from cigarettes (Figure 1a). TASE significantly increased after the intervention for successful but also for unsuccessful abstainers. QSE increased only for successful abstainers. Figure 1 represents medians at pre and post intervention. In some cases a statistical significant change was observed despite identical medians, explainable by a lack of precision of this measure not reflecting the percentage of subjects increasing, or to ceiling effects.
All subjects deciding to stop smoking in continuation of the intervention were successful during the 26-hour abstinence trial. Baseline TASE and QSE self-efficacies were significantly higher for the subgroup deciding a quit attempt (Figure 1b). Results also showed that TASE increased for both subgroups, even for attempters who were already high at baseline. QSE did not significantly change in either subgroup.
Self-efficacy was compared between the 3 subgroups who decided to quit either before the intervention or during the intervention or remained undecided (Figure 1c). At baseline, TASE and QSE were significantly different between the 3 groups. In analyses comparing the 2 subgroups deciding to quit (before or during the intervention), baseline TASE were similar but QSE had higher levels for those who already decided to stop before (p=.05). Pre-/post- intervention comparisons showed that QSE did not change for any subgroup but that TASE changed for the 3 subgroups (before/during/not decided, respectively, p=.001, p=.02, p=.0001).
Quit attempts after 1 week and time of decision to stop smoking
Further analysis was conducted on the subgroup of participants who made a quit attempt following the program. Of the 51 subjects, 46 had complete data for both variables (Table 3). At 1 week, 87.0% (n=20) of smokers who decided to stop during the intervention were successful, whereas this proportion was only 52.2% (n=12) for those who had already decided to quit before the intervention (p=.02). This suggests that the chances of not smoking within 1 week were higher when the smoker made the decision to stop smoking during the program, deciding to extend his temporary smoking abstinence experience into firm smoking cessation.
| Successful quit attempt at 1 week | Unsuccessful quit attempt at 1 week | TOTAL | |
|---|---|---|---|
| Already decided before intervention to stop | |||
| N | 12 | 11 | 23 |
| % | 52.2 | 47.8 | 100 |
| Decided during intervention to stop | |||
| N | 20 | 3 | 23 |
| % | 87 | 13 | 100 |
| TOTAL | |||
| N | 32 | 14 | 46 |
| % | 69.6 | 30.4 | 100 |
Fisher’s Exact Test : p=.02
*4 had missing data on “time of decision to stop smoking”; analysis performed on n=46
Table 3: Associations between time of decision and successful abstinence (n=51*).
Discussion
The data confirmed the importance of self-efficacy: it was the strongest predictor of successful temporary smoking abstinence as compared to other variables such as those related to smoking, negative affects or psychiatric diagnosis. The study also showed that self-efficacy increased after this intervention.
Results confirmed that self-efficacy may be subdivided into different components, particularly temporary smoking abstinence and long-term smoking cessation (QSE). TASE increased significantly after intervention, regardless of success of 26-hour abstinence, making a quit attempt or time of decision about stopping, which was not the case for QSE. Even subjects who decided not to pursue smoking abstinence and stop after the intervention showed an important increase in TASE but not QSE.
At pre-intervention, participants who had decided before the intervention to stop smoking and those only deciding this during it had similar levels of TASE, but QSE was higher for those already planning to stop after the intervention. This suggests that QSE could be related, at pre-evaluation, to an already existing internal representation, plan or motivation to stop smoking in those prepared to stop. Literature suggests that quit attempts may be triggered by both planned (reflective) and spontaneous (impulsive) elements during the cessation process [27]. QSE might be more strongly related to planned or volitionnal aspects of the smoking cessation process.
The present program allowed a close observation of the smoking cessation decisional process and evolution during intervention. Indeed, for some participants, the decision to quit was made before starting the program, and they used the program as support at the very beginning of the quitting period. However, others only decided during the experience to make a quit attempt. They formed 20% of the sample, representing smokers with spontaneous or unplanned quitting, described as contrasted to planned quitting [28].
Our results showed that at 1 week, the success rate was higher for quit attempters with spontaneous than for those with planned quitting. Hypotheses to explain this include that those who had decided before had higher nicotine dependence, more difficulties in stopping and thus had planned since longer to quit. Another hypothesis could be that the moment the decision to stop is made corresponds to a moment of heightened self-efficacy induced by positive aspects such as success of the temporary abstinence experience. This is in line with studies showing that smoker self-concept and abstainer self-concept change over time and are related with success or failure to quit smoking [29].
According to the stage-of-change model, the subjects who had decided to quit before the intervention should be in an advanced motivational stage, with greater success rates of a quit attempt at 1 week post-evaluation than for those not having made this decision, but our observations did not support this assumption. The stage-ofchange model could give a preponderant weight to reflective aspects, as reflected in higher pre-QSE scores, without necessarily resulting in higher success of quit attempts than those engaging in an unplanned attempt. Our observations are compatible with a reciprocal effects model with effects of performance (for example successful abstinence) on self-efficacy and in a lesser extent effects of self-efficacy on performance [16].
Limitations of the study include high levels of smoking selfefficacy (both TASE and QSE) already before the intervention for those succeeding in the 26-hour abstinence period or making a quit attempt, suggesting a “chicken and egg” situation in which it is difficult to disentangle the effects of a prior decision to quit from the experience brought on by a successful temporary abstinence. Further limitations of the study include the very short follow-up period (1 week). This allowed to observe changes during intervention concerning decisions about smoking cessation and observe quit attempts at one week, but prohibited any inferences about success of long-term quitting. The absence of a control group and the multicomponent nature of the intervention did not allow to draw conclusions about factors leading to increase of self-efficacy, such as effects of group support, tobacco information provided or effects of NRT, used by a majority (87%) of participants. Inclusion of all smokers, representing a large range of smoking severity, complicates interpretation of results. Occasional or recent daily smokers who might not otherwise feel concerned by a more traditional smoking-cessation program were included, potentially leading to higher success rates of the 26-hour abstinence period and more quit attempts than on a highly dependent sample of help-searching smokers. Another limitation concerns the small number of subjects when the sample is subdivided, precluding the ability to test some hypotheses, for example comparison of smokers deciding during/ before to make a quit attempt according to nicotine dependence and other variables.
Notwithstanding the above, results were interesting in increasing smoking cessation self-efficacy and triggering a high number of unplanned quit attempts. The most important conclusions however may lie in highlighting some general characteristics of the intervention for future developments, in particular:
Inclusion of smokers in all stages of readiness to quit and not only those already motivated to quit, enabling to reach a larger group of smokers and to correct the “treatment default” which tends to offer smoking cessation services only to smokers motivated to stop [30].
Combination of a temporary abstinence period and adequate NRT, described as a powerful trigger for unplanned quit attempts [31], particularly relevant for psychiatric patients more frequently presenting with difficulties in planning quit attempts [28].
Integration of tobacco cessation and mental health care interventions, as tobacco use is a chronic disease like many psychiatric diseases. Long-term follow-ups allow the monitoring of both tobacco use and the psychiatric disorder on the same occasion, facilitating intervention before relapse [32]. On the other hand mental health treatment also increases the likelihood to quit smoking [1].
Conclusion
A multicomponent program for all smokers can be a groundbreaking strategy to build self-adequacy, specifically for brief smoking restraint, and trigger spontaneous quit endeavours, demonstrated here to be more effective than arranged endeavours.
Acknowledgements
We thank the following heads of services for their collaboration in the intervention: Dr Jean-Pierre Bachetta, Dr Javier Bartolomei, Dr Logos Curtis, Prof. Philippe Huguelet, Dr Othman Sentissi and the heads of the Department of Mental Health and Psychiatry, Prof. Panteleimon Giannacopoulos and Prof. Jean-Michel Aubry for support. We also thank the tobacco specialists Prof Dr JP Humair for revision of the manuscript and Ms Patricia Borrero (Directorate of Nursing Services, University Hospitals of Geneva) who provided interactive tobacco-information sessions for the inpatient groups.
References
- Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, et al. (2014) Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA, 311(2):172-182.
- Forman-Hoffman VL, Hedden SL, Miller GK, Brown K, Teich J, et al. (2017) Trends in cigarette use, by serious psychological distress status in the United States, 1998-2013. Addict Behav, 64:223-228.
- Steinberg ML, Williams JM, Li Y (2015) Poor Mental Health and Reduced Decline in Smoking Prevalence. Am J Prev Med, 49(3):362-369.
- Szatkowski L, McNeill A (2015) Diverging trends in smoking behaviors according to mental health status. Nicotine Tob Res, 17(3):356-360.
- Jensen KP, Sofuoglu M (2016) Stress response genes and the severity of nicotine withdrawal. Pharmacogenomics, 17(1):1-3.
- West R (2009) The multiple facets of cigarette addiction and what they mean for encouraging and helping smokers to stop. COPD, 6(4):277-283.
- Zhu SH, Lee M, Zhuang YL, Gamst A, Wolfson T (2012) Interventions to increase smoking cessation at the population level: how much progress has been made in the last two decades? Tob Control, 21(2):110-118.
- Piasecki TM (2006) Relapse to smoking. Clin Psychol Rev, 26(2):196-215.
- Borland R, Yong HH, Balmford J, Cooper J, Cummings KM, et al. (2010) Motivational factors predict quit attempts but not maintenance of smoking cessation: findings from the International Tobacco Control Four country project. Nicotine Tob Res, 12 Suppl, S4-11.
- Vangeli E, Stapleton J, Smit ES, Borland R, West R (2011) Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction, 106(12):2110-2121.
- Hyland A, Borland R, Li Q, Yong HH, McNeill A, Fong GT, et al. (2006) Individual-level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) Four Country Survey. Tob Control, 15 Suppl 3, iii83-94.
- Smit ES, Hoving C, Schelleman-Offermans K, West R, de Vries H (2014) Predictors of successful and unsuccessful quit attempts among smokers motivated to quit. Addict Behav, 39(9):1318-1324.
- Litt MD, Kadden RM (2015) Willpower versus "skillpower": Examining how self-efficacy works in treatment for marijuana dependence. Psychol Addict Behav, 29(3):532-540.
- Gwaltney CJ, Metrik J, Kahler CW, Shiffman S (2009) Self-efficacy and smoking cessation: a meta-analysis. Psychol Addict Behav, 23(1):56-66.
- Perkins KA, Parzynski C, Mercincavage M, Conklin CA, Fonte CA (2012) Is self-efficacy for smoking abstinence a cause of, or a reflection on, smoking behavior change? Exp Clin Psychopharmacol, 20(1):56-62.
- Talsma K (2018) I believe, therefore I achieve (and vice versa): A meta-analytic cross-lagged panel analysis of self-efficacy and academic performance. Learning and Individual Differences, 61:136-150.
- Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, et al, (2011) Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Arch Intern Med, 171(21):1901-1907.
- Burris JL, Heckman BW, Mathew AR, Carpenter MJ (2015) A mechanistic test of nicotine replacement therapy sampling for smoking cessation induction. Psychol Addict Behav, 29(2): 392-399.
- Keizer I, Gex-Fabry M, Croquette P, Khan A (2016) A short motivational program based on temporary smoking abstinence: Towards increased self-efficacy to quit in psychiatric inpatients. J Addict Res Ther 2016, 7(4):1-10.
- Spielberger CD (1984) State-trait Anxiety Inventory: A comprehensive Bibliography. Consulting Psychologists Press, Palo Alto, CA.
- Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol, 56(6):893-897
- Newnham EA, Hooke GR, Page AC (2010) Monitoring treatment response and outcomes using the World Health Organization's Wellbeing Index in psychiatric care. J Affect Disord, 122(1-2):133-138.
- Etter JF, Duc TV, Perneger TV (1999) Validity of the Fagerstrom test for nicotine dependence and of the Heaviness of Smoking Index among relatively light smokers. Addiction, 94(2): 269-281.
- Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T (1994) Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend, 34(3), 211-216.
- Biener L, Abrams DB (1991) The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol, 10(5):360-365.
- Smith AL, Carter SM, Dunlop SM, Freeman B, Chapman S (2017) Measured, opportunistic, unexpected and naive quitting: a qualitative grounded theory study of the process of quitting from the ex-smokers' perspective. BMC Public Health, 17(1):430.
- Moeller-Saxone K, Segan C (2016) The Role of Planning in Naturalistic Quitting Success Among People with Severe Mental Illness. Int J Ment Health Addiction, 14:526-538.
- Shadel WG, Mermelstein R, Borrelli B (1996) Self-concept changes over time in cognitive-behavioral treatment for smoking cessation. Addict Behav, 21(5), 659-663.
- Faseru B, Ellerbeck E F, Catley D, Gajewski B J, Scheuermann T S, et al. (2017) Changing the default for tobacco-cessation treatment in an inpatient setting: study protocol of a randomized controlled trial. Trials, 18(1):379.
- Jardin BF, Cropsey KL, Wahlquist AE, Gray KM, Silvestri GA, et al. (2014) Evaluating the effect of access to free medication to quit smoking: a clinical trial testing the role of motivation. Nicotine Tob Res, 16(7):992-999.
- Prochaska JJ, Hall SE, Delucchi K, Hall SM (2014) Efficacy of initiating tobacco dependence treatment in inpatient psychiatry: a randomized controlled trial. Am J Public Health, 104(8):1557-1565.
Citation: Keizer I, Wahl C, Croquette P, Gex-Fabry M, Khan AN (2020) Selfefficacy for smoking cessation vs. temporary abstinence: two aspects of a complex process. J Addict Res Ther 11:394. DOI: 10.4172/2155-6105.1000394
Copyright: © 2020 Keizer I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2508
- [From(publication date): 0-2020 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1712
- PDF downloads: 796