Editorial Open Access
Secondary Metabolites (Pterosin F and B) From Pteridium aquilinum
Monica Butnariu*
Banat’s University of Agricultural Sciences and Veterinary Medicine “Regele Mihai I al Romaniei” from Timisoara, 300645, Calea Aradului, Romania
- *Corresponding Author:
- Monica Butnariu
Banat’s University of Agricultural Sciences and Veterinary
Medicine “Regele Mihai I al Romaniei” from Timisoara, 300645
Calea Aradului, Romania
Tel: +400-256-277464
E-mail: monicabutnariu@yahoo.com
Received date: August 31, 2015 Accepted date: September 02, 2015 Published date: September 05, 2015
Citation: Butnariu M (2015) Secondary Metabolites (Pterosin F and B) From Pteridium aquilinum. J Ecosys Ecograph 5:e123. doi:10.4172/2157-7625.1000e123
Copyright: © 2015 Butnariu M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
Secondary metabolites with active potential of fern are phenolic compounds, ptaquiloside (C20H30O8), pterosins (group of sesquiterpenes and norsesquiterpenes), hydrogen cyanide, and also produces carcinogenic compounds, etc. contains flavonoids, which have antibiotic properties, compounds that may have potential against fungal agents, pests for organic production.
Keywords
Secondary metabolites; Fern; Phenolic compounds; Antibiotic; Organic production
Introduction
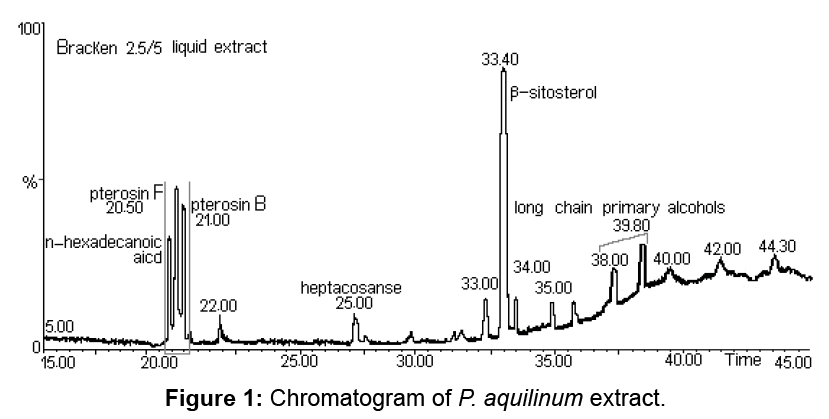
The name of western bracken fern is Pteridium aquilinum (L.) Kuhn. Bracken fern is considered a single, worldwide species, some authors disagree. There are two recognized subspecies: aquilinum (typicum) in the Northern Hemisphere and caudatum in the Southern Hemisphere. The chromatogram of P. aquilinum extract emphasizing the chemical composition is shown in Figure 1.
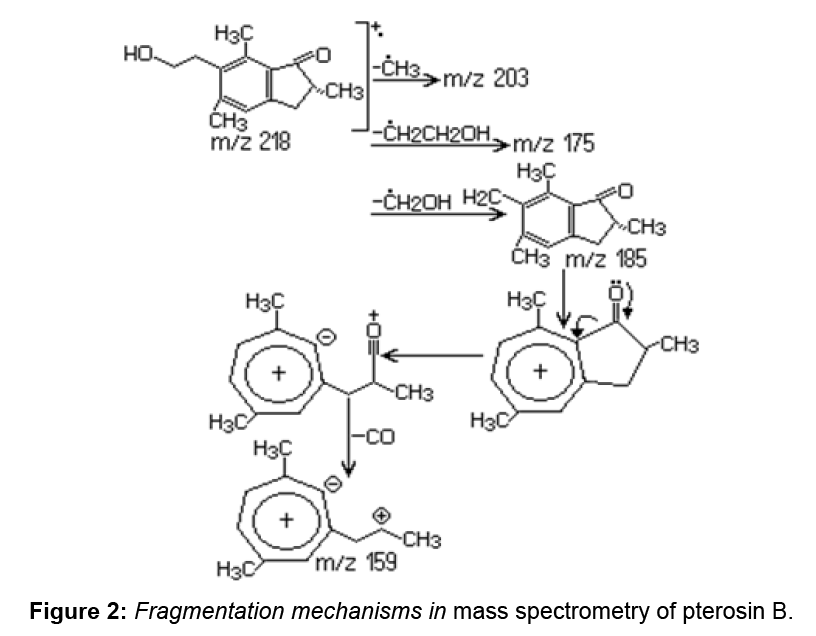
Although Ptaquiloside derived from fern are found in large quantities, this compound is difficult to quantify due to its instability. Pterosin B, also known as a final product of the ptaquiloside decomposition, is used to quantify the ptaquiloside. Pterosin B (C14H18O2) has the basic structure of indanone and may be generated by decomposition of ptaquiloside. In P. aquilinum extract pterosin B was identified by the GC–MS analysis at 21.02 min. For pterosin B, the major ions were observed at 187, 203 and 218 m/z rates.
Decomposition of benzyl side chain is a process in which the m/z 187, m/z 203 and m/z 218 ions are formed due to the loss of methyl and ethyl groups. The results are confirmed by the information obtained by other authors through GC–MS analysis for pterosin B [1]. Along with pterosin B, pterosin F (C14H17OCl) is also detected in P. aquilinum extract.
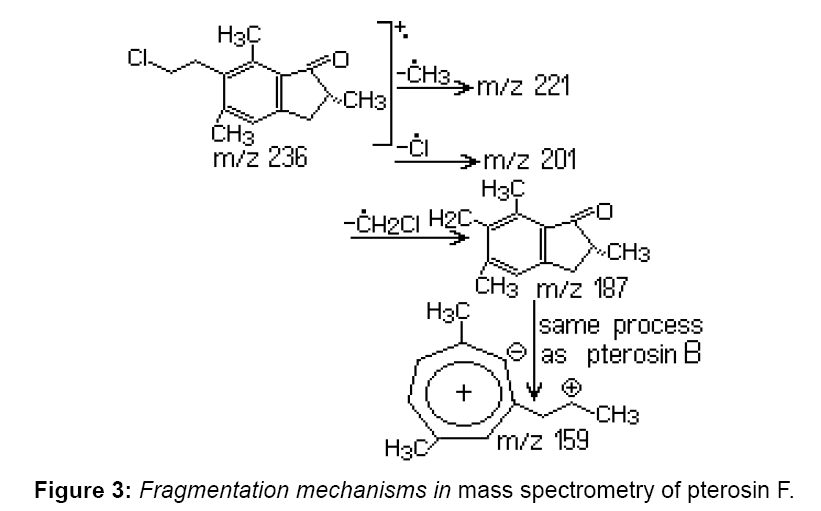
Pterosin F has a structure similar to pterosin B (Figure 2), with the exception that the hydroxyl group at the side chain is replaced by chlorine. Pterosin F appears in the chromatogram at 20.57 min, identification was achieved by GC–MS. Characteristic ions were identified at m/z 187, 201 and 236. Provided the decomposition of the benzyl side chain, for pterosin B the m/z 187 ion is formed. The loss of chlorine from the side chain causes the formation of an m/z 201 ion. The m/z 236 molecular ion, characteristic to m/z 236 and 238 rate reflects the relative abundance of chlorine isotopes (35Cl: 37Cl=3:01). The presence of pterosin F extract indicates that the discussed substance exists in the leaves of P. aquilinum before extraction (Figure 3).
In previous studies, pterosin F was found in methanol extract of young P. aquilinum leaves [2]. It has not been earlier established whether pterosin F is synthesized or it is a product of a metabolic precursor activated in leaves of P. aquilinum. Several studies suggest that pterosin B is formed from ptaquiloside by decomposition in presence of light [1]. It is possible that ptaquiloside is a source for pterosin B in leaves. Another compound indanone-type of compounds, pterosin A, was isolated from fern and we took into consideration that its precursor is also ptaquiloside, named dennstoside [1].
For pterosin F, precursor and the biosynthetic mechanism are unknown. These compounds are stored in the root cells for subsequent release. P. aquilinum root exudates released in the soil, could be the reason for the inhibitory effect on the development of other species.
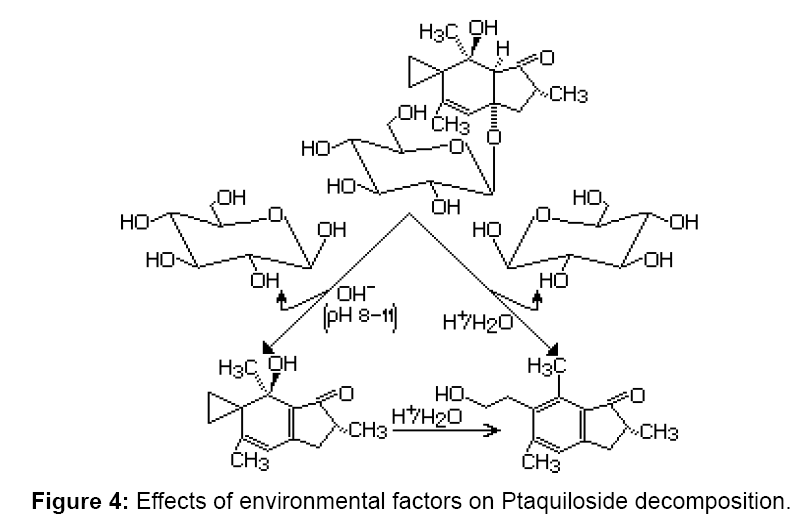
This (root exudates) is the more probable means by which fern allelochemicals are released into the soil, which explains the higher inhibitory activity (both on germination and growth) of extracts, which also inhibited the root growth of P. pratensis. This probably indicates interferences between P. aquilinum allelochemicals and growth phytohormones [3]. For example, depending on the pH of the soil, ptaquiloside may decompose according to the following reaction scheme (Figure 4). Ptaquiloside in alkaline pH between 8 and 11 loses one molecule of D–glucose, and in aqueous solutions at acidic pH loses D–glucose, converting into pterosin B.
Conclusions
It is possible that these effects are related to the metabolism, concentration and/or sensitivity of various plant hormones, but it also encompasses the environmental conditions.
References
- Attya M, Nardi M, Tagarelli A, Sindona G (2012) A new facile synthesis of D4–pterosin B and D4–bromopterosin, deuterated analogues of ptaquiloside. Molecules 17:5795-5802.
- Kaur H, Kaur R, Kaur S, Baldwin I, Inderjit T (2009) Taking ecological function seriously: Soil Microbial Communities Can Obviate Allelopathic Effects of Released Metabolites. PLoS One 4:e4700.
- Lee YP, Hsu FL, Kang JJ, Chen CK, Lee SS (2012) Metabolism of (2S)–pterosin A: Identification of the phase I and phase II metabolites in rat urine. Drug Metabolism and Disposition 40: 1566-1574.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 14784
- [From(publication date):
September-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10187
- PDF downloads : 4597