Second Case Report of Myxofibrosarcoma of Larynx
Received: 10-Jan-2015 / Accepted Date: 27-Jan-2015 / Published Date: 05-Feb-2015 DOI: 10.4172/2161-119X.1000186
Abstract
A 47 year-old woman admitted to the hospital with progressive hoarseness. On the physical examination of larynx, a 2.5 cm mass was detected on the left arytenoid area. The tumor was totally excised and histopathologic diagnosis was consisted with myxofibrosarcoma of intermediate grade. Myxofibrosarcoma is common soft tissue sarcoma mainly occur in the extremities. Head and neck region is seldom becoming a host for soft tissue sarcomas including myxofibrosarcoma. Here in, we present the second case of laryngeal MFS with its clinicopathological features, differential diagnosis, treatment modalities and prognostic factors with the review of the literature.
Introduction
Head and neck (HN) sarcomas constitute about 5-10% of all sarcomas and only 1% of all HN tumor types. According to the limited work published to date about HN sarcomas, histological subtype is a significant prognostic factor in addition to tumor size, Histological Grade (HG), depth of invasion, HN location and surgical resection margin status [1]. Fibrosarcoma, undifferentiated pleomorphic sarcoma, liposarcoma, leiomyosarcoma, rhabdomyosarcoma, Kaposi sarcoma, malignant peripheral nerve sheath tumor and synovial sarcoma are most commonly seen histological subtypes of sarcomas in this region [2].
Myxofibrosarcoma (MFS), which is the most common sarcoma in elderly patients predominantly located in extremities, is seen rarely in HN region. Here in, we reported the second case of laryngeal myxofibrosarcoma with review of the literature.
Clinical Summary
A 47 year-old woman admitted to the hospital with progressive hoarseness despite medical therapy. On the physical examination of HN, a 2.5 cm well circumscribed mass was detected on the left arytenoid area. The tumor was totally excised. The specimen was fixed with 10% formaldehyde and embedded in paraffin. Five mm cut sections prepared and stained with Hematoxylin and Eosin.
Pathological Findings
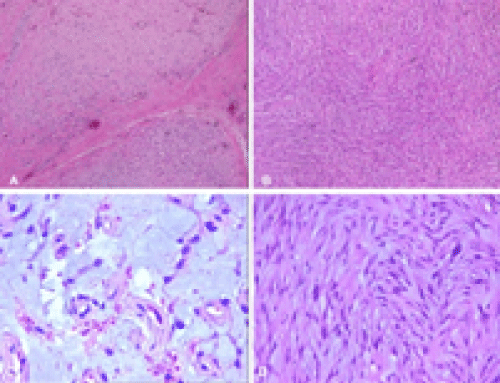
On histopathological examination a multinodular tumor was detected under laryngeal epithelium (Figure 1A). It was consisted of mildly to moderately cellular myxoid and fibromyxoid areas. There is a fascicular pattern particularly in cellular areas (Figure 1B and C). The tumor cells have spindle somehow round nuclei with occasional inclusions and multinuclear tumor cells were also seen. Nuclear atypia varied mild to moderate (Figure 1D). The cytoplasm of the cells was scant and some of them were multivacuolated. There was no necrosis and mitotic figures were infrequent.
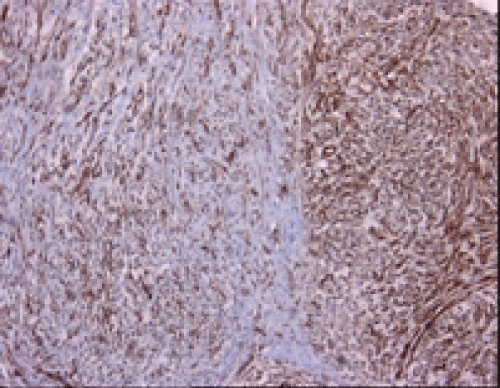
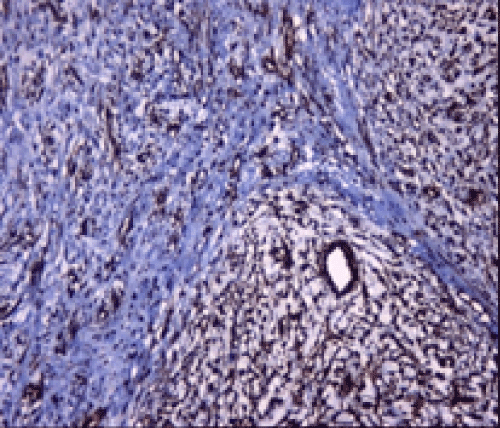
Immunohistochemical studies showed that the tumor cells were immunoreactive with Vimentin (Thermo Scientific, V9, 1:600) and Smooth Muscle Actin (SMA) (Thermo Scientific, 1A4, 1:3500) (Figures 2 and 3), whereas negative with CD34 (Thermo Scientific, QBEND/10, 1:100), S-100 (Thermo Scientific, 4C4.9, 1:600), Neurofilament (NF) (Thermo Scientific, 2F11, 1:100), Desmin (Thermo Scientific, D33, 1:50) and Caldesmon (Thermo Scientific, h-CALD,1:400). Final histopathological diagnosis was MFS of intermediate HG and surgical margins were tumor positive. The patient refused further therapy and is not under follow-up now.
Discussion
MFS is one of the most common sarcoma in the extremities of elderly patients. It is most likely also that the myxoid variant of malignant fibrous histiocytoma introduced by Weiss et al. [3]. In this report they showed that it was appropriate to separate myxoid form of MFH from the usual non-myxoid form because of the more favorable prognosis of myxoid variant. Later in 1977, Angervall et al., instead use the term MFS, which underlined the myxoid matrix and implies fibroblastic origin [4]. Currently, the World Health Organization recognizes MFS as a distinct entity with distinctive clinicopathological features [2].
They arise frequently in dermal and subcutaneous tissues [5]. They are seen only rarely on the trunk, in the HN area, on the hands and feet. Abdominal and retroperitoneal location is extremely rare [2]. In HN area orbit, maxillary sinus, thyroid, hypopharynx, nasopharynx, external auditory canal involvement have been published previously [6-11]. To date, there is only one report of a case of MFS arising from larynx in English literature [12]. In this report, the patient was 79-year-old man whose tumor was located in the right vocal fold. Histopathological examination of incisional biopsy showed MFS of low grade and the tumor was resected totally with negative surgical margins. No further therapy was applied. The patient was placed under intimate follow-up. Grossly, superficially located neoplasm consists of multiple variably gelatinous/firm nodules whereas deeply located ones tend to form a single mass. Tumor necrosis is often found in high grade tumors.
Although histopathological appearance of MFS can vary according to grade of tumor, all cases share distinct morphologic features such as multinodular growth pattern and a myxoid stroma composed of hyaluranic acid. Low grade tumors are predominantly hypocellular and composed of only a few tumor cells with atypical, enlarged, hyperchromatic nuclei. Mitotic figures are infrequently found. Presence of prominent, curvilinear, elongated vessels with accumulation of tumor cells and/or inflammatory cells around them is a characteristic finding. So called pseudolipoblasts which implies vacuolated neoplastic fibroblastic cells are frequently present.
High grade lesions composed of solid areas with fascicular growth pattern. Bizarre, multinucleated giant cells and pleomorphism in tumor cells, numerous mitosis, areas of hemorrhage and necrosis are common findings. Morphologic features of MFS of intermediate HG range between low and high grade tumors. In order to the present tumor has both solid and myxoid areas, the degree of nuclear atypia was mild to moderate and there were no necrosis, we concluded it was of intermediate HG. Immunohistochemically, the tumor cells stain diffusely for vimentin and focal immunoreaction with muscle specific actin and/or alpha-SMA may be seen. Differential diagnosis of low and intermediate grade MFS should be made from both benign and malignant myxoid soft tissue lesions such as nodular fasciitis, myxoma, myxoid neurofibroma, myxoid neurothekeoma, angiomyxoma and myxoid liposarcoma.
The most difficult distinction is from low-grade fibromyxoid sarcoma. Clinically, this tumor occurs in young patients, originated from deep locations, has a tendency for multiple recurrences and distant metastasis in the case of the tumor is incompletely excised. It is commonly originated from lower extremities however rare HN involvement has been described [13,14]. Morphologically, it is composed of cytologically bland spindle cells arranged in a whorled pattern and set in an alternatively myxoid and a more prominent collagenous stroma. In contrast, MFS is always predominantly myxoid and the tumor cells show more cytological atypia than those of lowgrade fibromyxoid sarcoma.
According to the limited published data about prognostic factors in MFS, status of surgical margins as well as size and HG of tumor are significant predictors of survival. The incidence of local recurrence (LR) is definitely high in the case of the tumor excision with positive surgical margins. Furthermore in a study of Haglund et al., patients developed LR whom had close (<1 cm) as well as positive surgical margins [15].
It is obvious that HG is a prognostic factor but the way of its effect is controversial. Sanfilippo et al. showed [16]. HG correlated with metastases but not with LR whereas intermediate and high grade MFS was found to develop LR in another study [15]. Additionally, patients whose tumor size of greater than 5cm have unfavorable prognosis [16].
Like their soft tissue counterparts the primary goal of treatment of HN sarcomas including MFS, should be en-bloc resection with the margin of normal tissue [17]. The highly infiltrative nature of sarcomas makes difficulties in their complete resection. Sufficient excisional treatment of HN tumors is further limited by significant anatomic constraints and results high rate of margin positivity. Although it is not established that there is a survival benefit of adjuvant radiotherapy, some authors believed that adjunctive radiotherapy should be applied after resection of especially intermediate and high grade tumors even if they had negative surgical margins [15]. The role of chemotherapy is still undefined in such cases.
The majority of malignant tumors of larynx are of epithelial origin. Sarcomas of the larynx are relatively rare tumors represent a heterogeneous group of tumors with distinct prognostic implications. Although there are only one report of MFS of larynx and limited number of papers of other HN regions in English literature, MFS should be keep in mind when a pathologist face a myxoid mesenchymal lesion in larynx. Due to it has a more favorable prognosis as compared to other sarcomas, adequate excision of the tumor could be the sufficient treatment of choice for most of this type of sarcomas.
References
- Smith VA, Overton LJ, Lentsch EJ (2012) Head and neck soft tissue sarcomas: unique lack of significance of synchronous node metastases. J SurgOncol 106: 837-843.
- World Health Organization. Hypopharynx, larynx and trachea. In: Barnes L, Eveson JW, Reichart P, Sidransky D,editors. Pathology&Genetics of Head and Neck tumors. Lyon: IARCPress; 2005.p.147-49.
- Weiss SW, Enzinger FM (1977) Myxoid variant of malignant fibrous histiocytoma. Cancer 39: 1672-1685.
- Angervall L, Kindblom LG, Merck C (1977) Myxofibrosarcoma. A study of 30 cases. ActaPatholMicrobiolScandA 85A: 127-140.
- Mentzel T, Calonje E, Wadden C, Camplejohn RS, Beham A, et al. (1996) Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J SurgPathol 20: 391-405.
- Zhang Q, Wojno TH, Yaffe BM, Grossniklaus HE (2010) Myxofibrosarcoma of the orbit: a clinicopathologic case report. OphthalPlastReconstrSurg 26: 129-131.
- Ito H (1965) [2 cases of myxofibrosarcoma originating from the maxillary sinus]. Jibiinkoka 37: 549-553.
- Kouassi YM, Tanon-Anoh MJ, Doukouré B, Assouan C, Buraïma F, et al. (2010) [Thyroid localization of myxofibrosarcoma: first case in Africa]. Med Trop (Mars) 70: 70-72.
- Nishimura G, Sano D, Hanashi M, Yamanaka S, Tanigaki Y, et al. (2006) Myxofibrosarcoma of the hypopharynx. AurisNasus Larynx 33: 93-96.
- Jérôme-Marson V, Uro-Coste E, Lacoste-Collin L, Gomez-Brouchet A, Serrano E, et al. (2003) [Extraskeletalmyxoidchondrosarcoma of the nasopharynx]. Ann Pathol 23: 253-257.
- VON LEDEN H (1951) Myxofibrosarcoma of the external auditory canal. Ann OtolRhinolLaryngol 60: 258-259.
- Gugatschka M, Beham A, Stammberger H, Schmid C, Friedrich G (2010) First case of a myxofibrosarcoma of the vocal folds: case report and review of the literature. J Voice 24: 374-376.
- Papadimitriou JC, Ord RA, Drachenberg CB (1997) Head and neck fibromyxoid sarcoma: clinicopathological correlation with emphasis on peculiar ultrastructural features related to collagen processing. UltrastructPathol 21: 81-87.
- GarcÃÂa JJ, Folpe AL (2010) The impact of advances in molecular genetic pathology on the classification, diagnosis and treatment of selected soft tissue tumors of the head and neck. Head Neck Pathol 4: 70-76.
- Haglund KE, Raut CP, Nascimento AF, Wang Q, George S, et al. (2012) Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int J RadiatOncolBiolPhys 82: 361-367.
- Sanfilippo R, Miceli R, Grosso F, Fiore M, Puma E, et al. (2011) Myxofibrosarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann SurgOncol 18: 720-725.
- Bentz BG, Singh B, Woodruff J, Brennan M, Shah JP, et al. (2004) Head and neck soft tissue sarcomas: a multivariate analysis of outcomes. Ann SurgOncol 11: 619-628.
Citation: Ocak GA, Ozbudak IH, Toru HS, Derin AT (2015) Second Case Report of Myxofibrosarcoma of Larynx. Otolaryngology 5:186. DOI: 10.4172/2161-119X.1000186
Copyright: © 2015 Ocak GA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author andsource are credited
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14643
- [From(publication date): 3-2015 - Apr 06, 2025]
- Breakdown by view type
- HTML page views: 10099
- PDF downloads: 4544