Screening, Optimization, and Antibacterial Activity of Cellulose: DegradingBacteria from Sewage Soil Waste
Received: 07-Jul-2021 / Accepted Date: 21-Jul-2021 / Published Date: 28-Jul-2021 DOI: 10.4172/2155-6199.1000002
Abstract
The present study was carried out to isolate, enhancement, identification, optimization of cellulose-degrading bacteria present in sewage soil waste, and screening for potential antibacterial activity. Three bacterial isolates were cultured at a large rate by Minimal Salt Medium (MSM) and showed the highest cellulase activity determined by congo red and iodine assay on Carboxy Methyl Cellulose (CMC) agar plate. Then various culture parameters such as pH, temperature, incubation period, substrate concentration, carbon, and nitrogen sources were optimized for the maximum cellulase secretion. Based on the morphological, cultural, and biochemical tests, the isolated strains were identified as Bacillus licheniformis, Bacillus sp. and Pseudomonas chlororaphis respectively. Bacillus licheniformis compared to other strains produced the highest cellulase enzyme (1 µ/ml) determined by the DNS method at neutral pH and 40ºC temperature on 24 hours incubation period. This bacterial strain was screened for the antibacterial activity determination against several bacterial pathogens and generated an inhibition zone ranging from 12-21 mm. The ethyl acetate extract of the selected strain showed the potential antibacterial activity against Escherichia coli and moderate activity against Shigella boydii and Bacillus cereus. The Minimum Inhibitory Concentration (MIC) of the tested extract was observed to be in the range of 44-62 µg/ml. Based on optimization, cellulose degradation, and antibacterial activity, Bacillus licheniformis was the potential species among the strains. The strain from sewage soil waste can be helpful for biodegradation and industrial application.
Keywords: Licheniformis; Cellulase; Polysaccharide; Pseudomonas; Shigella
Abbreviations
CMC: Carboxymethyl Cellulose; DMSO: Dimethyl Sulfoxide; LB: Luria-Bertani; DNS: 3,5-Dinitro Salicylic Acid
Introduction
Cellulose is a polysaccharide composed of a linear chain of several hundred to many thousands of β (1→4) linked D glucose units [1,2]. Glucose acts as the monomeric constituent of cellulose which is a product of photosynthesis [3]; thus, cellulose is also considered as a vast pool of chemically fixed carbon dioxide. It is the most abundant biological polymer present on Earth [4]. The use of cellulose for biofuel production involves the hydrolysis of cellulosic biomass, that is, saccharification, to form simple sugar monomers for the fermentation into bioethanol [5-7]. Cellulase is any of several important enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose, and some related polysaccharides. This enzyme breaks down the cellulose into simple sugars such as beta-glucose or shorter polysaccharides and oligosaccharides. The endoglucanase is an important enzyme that hydrolyzes β-1, 4 bonds in cellulose molecule; exoglucanase is another enzyme that cleaves the ends to release cellobiose and β-glucosidase convert it to glucose [8]. Cellulase enzymes have drowned much more attention because of their diverse type of application in several kinds of industres such as textile, food, feed, paper industries, etc [9-11]. It is also used in fermentation, fiber modification and pharmaceutical applications [12]. There have several cellulase producing fungi such as Rhizopus, Aspergillus, and Trichoderma species, and have some bacteria such as clostridium, bacillus, Cellulomonas, bacteroids, etc species that have been identified. However, due to the slow growth rate and a longer fermentation period of fungi compared to the bacteria [13], the cost for its production is yet to be high. Thus, to meet the global demand for cellulase, the isolation, screening, and characterization of cellulytic bacteria are still a highly active research area. The studies that have been done in past on isolation and characterization of cellulytic bacteria revealed that only a few numbers of bacteria are able to degrade cellulose into simple sugar in vitro [14,15]. Nature provides vast sources for cellulytic bacteria, among them, soil from the drain and sawmill area are cellulosic and the remaining bacteria are capable of degrading the cellulose molecule for their normal growth and development. This is why we focused our research work on screening, enhancement, isolation, and identification of cellulytic bacteria from different areas of sewage soil at Rajshahi City, Bangladesh. This microorganism produced some compound that has a strong antimicrobial activity.
Globalization interferes with infectious disease control at the national level while microbes move freely around the world, unhindered by borders, human responses to disease, and are a condition by jurisdictional boundaries [16]. According to WHO, significant progress has been made in controlling major infectious diseases. Around 43% of total death occurred in developing countries due to infectious disease in recent years [17]. Now days, a large variety of antibiotics are used to combat pathogenic microorganisms like viruses, bacteria, fungi, protozoan, and worms, which are responsible for numerous infectious diseases. This antibiotic is a chemical agent that is chiefly produced by microorganisms, and the antibacterial activity of these microorganisms can be determined by the discs diffusion method. The method was developed by Bondi and standardized by Bauer in 1966 for the susceptibility test. In this present study, the antimicrobial activity of the ethyl-acetate extract was studied by using the disc diffusion techniques to investigate the changes occurring in the bacteria, their possible relation to antibiotic susceptibility, resistance to antimicrobial agents and production of the bioactive compound by strain [18,19].
Materials and Methods
Isolation and screening of cellulytic bacteria
The soil wastes were collected from different sewage areas of Rajshahi city, Bangladesh. 1g collected sample was planned to serial dilution up to 10-6. The diluted sample was taken into Luria-Bertani (LB) medium. 0.2 ml of diluted each sample solution was transferred into carboxymethyl cellulose agar medium plate (composed of KH2PO4 0.5 g, MgSO4 0.25 g, cellulose 2.0 g, agar 15 g, gelatin 2 g, and distilled water l L, and at pH 6.8-7.2). The plates were then incubated at 37ºC for 24 hours and stored at 4ºC [20]. Then varieties of the bacterial colony were picked up from the plates. The cellulytic activity of the isolates was confirmed by several types of methods such as congo red, iodine solution, and filter paper degradation method [21,22]. The cellulytic bacteria showed a transparent clear zone into CMC agar plates inundated with congo red and iodine solution. The bacterial isolates were inoculated in a Minimal Salt Medium (MSM) for the enhancement of cellulytic bacteria.
Enhancement of cellulytic bacteria by Minimal Salt Medium (MSM)
100 ml of CMC (1.5 gm), 50 ml of trace element solution-6 (ZnSO4.7H2O 0.1 gm, MnCl2.4H2O 0.03 gm, CuCl2.2H2O 0.01 gm, NiCl2.6H2O 0.02gm, Na2MoO4.2H2O 0.03 gm and distilled water 1000 ml), 50 ml of trace element solution-4 (EDTA 0.5 gm, FeSO4.7H2O 0.2 gm, trace element solution-6 100 ml and distilled water 900 ml), 50 ml of minimal salt media (Na2HPO4.2H2O 3.5 gm, KH2PO4 1.0 gm, (NH4)2SO4 0.5 gm, MgCl2.6H2O 0.1 gm, Ca(NO3)2. 4H2O 0.05 gm, trace element solution-4 1 ml and distilled water 1000 ml) and 250 ml of distilled water were separately autoclaved and mixed by shaking vigorously to make a uniform suspension. Then the media (20 ml) was transferred into the different test tubes and inoculated with culture media (50 μl) and incubated at 37ºC for 3 days and optical density measured at 600 nm.
Identification of cellulytic bacteria: The isolated three cellulytic strains were characterized by morphological and biochemical tests.
Morphological characterization: Gram staining and microscopic observation were performed to characterize the isolated strains [23]. This technique was used to detect the gram-positive and gramnegative bacteria.
Biochemical characterization: The biochemical tests such as Fermentation test, Catalase test, Citrate utilization test, Methyl-red test, H2S production, and Voges-Proskauer test were carried out by following the standard method to characterize the bacterial isolates [24].
Optimization of the cultural parameters for maximum cellulase production
To optimize the cellulase production from the bacterial isolates, different cultural conditions such as pH scale (range 5.0-11.0), temperature (range 20ºC-45°C), incubation period (range 12-96 hours), and substrate concentration (range 0.5%-2.0%) were applied in the CMC broth media. Besides this, different carbon sources (wheat bran xylan, rice bran xylan, cellubiose, xylose, CMC, starch, chitin, and lactose) and nitrogen sources (yeast extract, peptone, tryptone, urea, ammonium phosphate, and sodium nitrite) were used and tested the highest cellulase activity after maximum enzyme production.
Enzyme activity test: The bacterial isolates which showed the maximum clear zone in the CMC agar plate was cultured in LB medium and incubated at 37ºC overnight. Then the culture was centrifuged at 4ºC and 12000 rpm for 20 minutes and the supernatant was used as a crude enzyme source for enzymatic assay.
DNS (3,5-dinitrosalicylic acid) test was used for enzyme activity test through the production of reducing sugars. CMC (1%) solution with 1N citrate buffer was used as a substrate. The mixture of the crude enzyme (150 μl) and citrate buffer (1 ml) into CMC substrate solution (1 ml) was incubated at 40ºC for 40 minutes. Then the DNS solution was added to end the reaction [25].The treated sample was boiled for 10 minutes, cooled in water for color stabilization and the optical density was measured at 540 nm. Finally, the standard calibration curve of glucose was plotted to determine the cellulase activity by releasing the amount of glucose from the degradation of carboxymethyl cellulose [12].
Determination of antibacterial activity of ethyl acetate extract
The strains (Bacillus licheniformis) showed the maximum enzyme activity after optimization of the cultural parameters that were tested for antibacterial activity. The selected pure strains were inoculated at CMC broth media at 40ºC and 4 mg of ethyl acetate extract of the bacterial isolates was dissolved in 2 ml ethyl acetate [26]. After this, three types of discs such as sample discs (5 mm in diameter), standard kanamycin disc (5 µg/disc) and solvent control disc (5 mm in diameter) were prepared for antibacterial screening. The sample solution of the desired concentration (100 µg/disc and 70 µg/disc) was applied on the sample disc in an aseptic condition. As an antibacterial activity, the inhibition zone diameter (mm) was measured against gram-positive bacteria (Staphylococcus aureus, Bacillus cereus) and gram-negative bacteria (Shigella dysenteriae, Escherichia coli, Shigella boydii) respectively..
Determination of Minimum Inhibitory Concentration (MIC)
The MIC values of the ethyl acetate extract of Bacillus licheniformis were determined against some known pathogenic bacteria (Staphylococcus aureus, Bacillus cereus, Shigella dysenteriae, Escherichia coli, Shigella boydii) by following the “Serial tube dilution technique”. The prepared inoculum during antibacterial activity was used to determine the MIC values. The stock solution was prepared by dissolving 1.024 mg of the compound in 2 ml DMSO solution. Thus the solution with a concentration of 512 µg/ml was obtained. Then the serial tube dilution method was performed step wisely to determine the minimum inhibitory concentration [27].
Results
Isolation and identification of cellulose producing bacteria
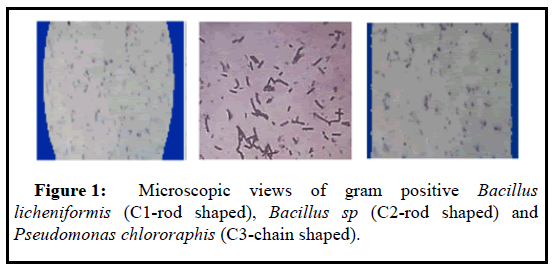
The pure bacterial strains were marked as C1, C2, C3 collected from sewage soil. In the microscopic observation, both C1 and C2 isolates were gram-positive and rod-shaped, but C3 strains were gram-positive and chain-shaped (Table 1 and Figure 1). The biochemical characterizations of the three isolates are represented respectively in Table 1.Based on the morphological and biochemical test, the isolated strains were identified as Bacillus licheniformis (C1), Bacillus sp (C2), and Pseudomonas chlororaphis (C3) respectively.
| Characteristics | C1 | C2 | C3 |
|---|---|---|---|
| Gram stain | + | + | + |
| Morphology | Rod shaped | Rod shaped | Chain shaped |
| Glucose fermentation | + | + | + |
| Galactose fermentation | + | + | + |
| Sucrose fermentation | + | + | + |
| Catalase test | + | + | + |
| Citrate utilization | - | + | - |
| Methyl-red test | - | - | - |
| H2S production test | - | - | - |
Voges-proskauer test |
- |
+ |
- |
Enhancement of cellulytic bacteria
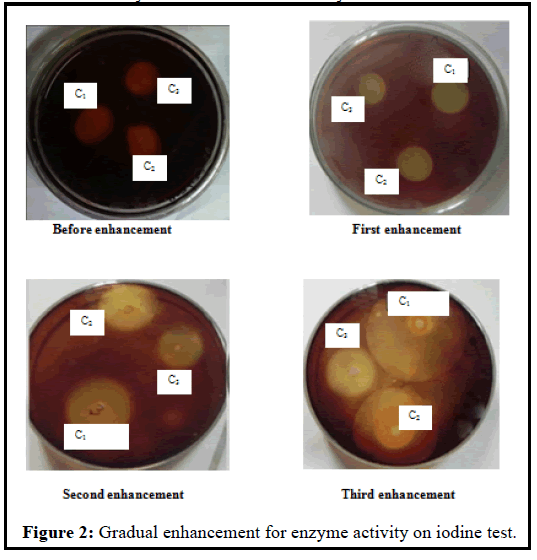
After the three times enhancement of isolated strains by MSM, the three isolates produced a larger zone of inhibition as compared to that produced before the enhancement of the isolates. The diameters of zone and cellulytic index produced by C1, C2, and C3 at the different steps of the enhancement are shown in Table 2. Iodine test showed that Bacillus licheniformis (C1) isolate formed the largest cellulytic index (8.00) at third enhancement and lowest cellulytic index (2.67) before enhancement. Again, the C2 isolate had the largest cellulytic index (5.00) at second enhancement and lowest cellulytic index (1.57) before enhancement and on contrast, the C3 isolate made the largest cellulytic index (3.66) at second and third enhancement and lowest cellulytic index (1.8) before enhancement. It was observed that the cellulytic activity of the three strains gradually increased in the enhancement process (Figure 2).
| Isolated bacteria | Diameter of colony (mm) | Diameter of zone (mm) | Cellulytic index | |
|---|---|---|---|---|
| Before enhancement | C1 | 3 | 11 | 2.67 |
| C2 | 3.5 | 9 | 1.57 | |
| C3 | 2.5 | 7 | 1.8 | |
| First enhancement | C1 | 2.75 | 13 | 3.72 |
| C2 | 2 | 12 | 5 | |
| C3 | 2 | 9 | 3.5 | |
| Second enhancement | C1 | 3 | 18 | 5 |
| C2 | 3 | 16 | 4.3 | |
| C3 | 3 | 14 | 3.66 | |
| Third enhancement | C1 | 4 | 36 | 8 |
| C2 | 7 | 28 | 3 | |
| C3 | 3 | 14 | 3.66 |
Table 2: Cellulytic index of isolated cellulytic bacte.
Optimization of culture conditions and enzyme activity test
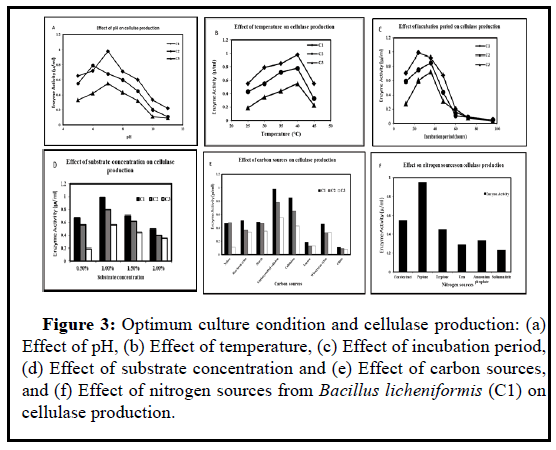
The optimum cultural condition and highest enzyme activity for the bacterial isolates are showed in Figures 3a-3f. It was observed that the C1 strain (Bacillus licheniformis) produced the highest cellulase (1 µmoleml-1min-1) than C2 (Bacillus sp) and C3 (Pseudomonas chlororaphis) within 24 hours incubation period at temperature 40ºC and pH 7.0. The bacterial strains C2 and C3 yielded 0.8 µmoleml-1min-1 and 0.6 µmoleml-1 min-1 enzymes respectively in pH 6.0 and pH 7.0 on 36 hours incubation period at 40ºC temperature. These bacterial strains showed high enzyme activity at 1% carboxymethyl cellulose (Carbon source) and peptone (Nitrogen source).
Figure 3: : Optimum culture condition and cellulase production: (a) Effect of pH, (b) Effect of temperature, (c) Effect of incubation period, (d) Effect of substrate concentration and (e) Effect of carbon sources, and (f) Effect of nitrogen sources from Bacillus licheniformis (C1) on cellulase production.
Determination of antibacterial activity of ethyl acetate extract
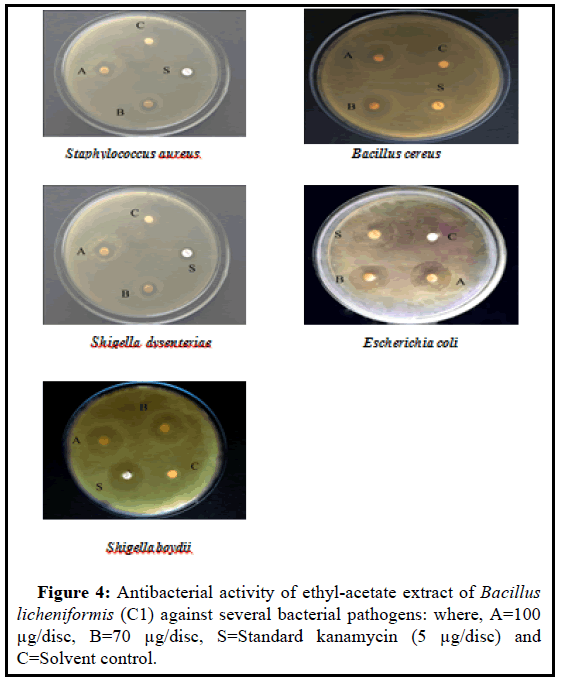
Details of the antibacterial activity of ethyl acetate extract (Bacillus licheniformis) are presented in Table 3 and Figure 4. The inhibition zone diameter were 12, 18, 13, 21, 20 mm (100 µg/discs), and 10, 15, 12, 19, 19 mm (70 µg/discs) against gram-positive and gram-negative bacteria, respectively. The maximum activities were observed against Escherichia coli for the tested extract.
| Test bacteria | Diameter of zone of inhibition (mm) of the ethyl-acetate extract | Standard kanamycin discs (5 µg/disc) | |
|---|---|---|---|
| 100 µg/disc | 70 µg/disc | ||
| Staphylococcus aureus | 12 | 10 | 10 |
| Bacillus cereus | 18 | 15 | 10 |
| Shigella dysenteriae | 13 | 12 | 9 |
| Escherichia coli | 21 | 19 | 22 |
| Shigella boydii | 20 | 19 | 20 |
Table 3: Results of antibacterial activity of ethyl-acetate extract of Bacillus licheniformis (C1).
Determination of minimum inhibitory concentration
The results of MIC in details are presented in Table 4. The MIC values of ethyl acetate extract of Bacillus licheniformis (C1) strain were varied between 44-62 µg/ml. The lowest MIC (44 µg/ml) was observed against Escherichia coli, and Shigella boydii, whereas MIC value for Staphylococcus aureus, Bacillus cereus and Shigella dysenteriae were 62, 55, and 62 µg/ml, respectively.
| Bacteria | Ethyl-acetate extract (ug/ml) |
|---|---|
| Staphylococcus aureus | 62 |
| Bacillus cereus | 55 |
| Shigella dysenteriae | 62 |
| Escherichia coli | 44 |
| Shigella boydii | 44 |
Table 4: MIC values of ethyl acetate extract against the tested pathogenic bacteria.
Discussion
In the present research, the bacterial isolates were characterized by morphological and biochemical assays and were optimized in different cultural conditions, and then the antibacterial activity of the highest enzyme secreting strain (Bacillus licheniformis) was performed. The bacterial strains were isolated from sewage waste and were screened for cellulase activity by congo red, iodine solution, and filter paper degradation method [21,22]. The bacterial isolates were identified as Bacillus licheniformis (C1), Bacillus sp(C2), and Pseudomonas chlororaphis (C3) respectively based on the morphological, cultural and biochemical assays [23-28]. It was observed that Bacillus licheniformis (C1) formed the highest cellulytic index (8.00) at third enhancement and lowest cellulytic index (2.67) before enhancement (Table 2 and Figure 1).
For the maximum enzyme production, the bacterial strains were cultured in different conditions such as pH, temperature, incubation period, substrate concentration, different carbon, and nitrogen sources. Bacillus licheniformis was optimized at pH 7.0 and 40ºC temperature on 24 hours incubation period, and then produced 1 µ/ml enzyme at the optimized condition (Figure 3). After the optimization, the enzyme activity was determined by performing the DNS test. CMC (Carbon source), and peptone (Nitrogen source) were considered as the best media source for the optimized cellulase secretion. In the previous study, it was observed that the maximum production of endoglucanase from the microorganisms such as Cellulomonas, Bacillus and Micrococcus spp. isolated from coir retting effluents of the estuarine environment was obtained at 40ºC temperature and neutral pH [28]. In the recent study, Bacillus sp produced the highest enzyme (1.65 µ/ml) at pH 3.5 and 35ºC on 24 hours incubation period [29].
Considering the highest enzyme activity, Bacillus licheniformiswas selected for antibacterial activity. The ethyl acetate extract of the selected strain was tested against five pathogenic bacteria Staphylococcus aureus, Bacillus cereus, Shigella dysenteriae, Escherichia coli, and Shigella boydii, respectively.The maximum activities were observed against Escherichia coli for the tested extract (Table 3 and Figure 4). It was observed that the MIC value range of ethyl acetate extract of Bacillus licheniformis strain was from 44-62 (µg/ml) are presented in Table 4 and Figure 4 that is more significant than the range reported by the previous study [30]. These indicate that the ethyl acetate extract of Bacillus licheniformis strain has the antibacterial activity.
Conclusion
The present study reveals that the isolated bacterial strains from sewage waste (soil) are optimized at different cultural parameters. The isolated strain Bacillus licheniformis showed stronger antibacterial activity against several pathogenic bacteria for maximum enzyme production. This study suggests that Bacillus licheniformis can be useful in the bioremediation of solid organic waste and the pharmaceutical industry.
Limitation
There is one limitation of the present study. The molecular characterization of the cellulose degrading bacteria was not performed due to the limitation of lab facilities.
Declarations
Authors’ contributions
MM performed the experiments, analyzed, and interpreted the data. FI, NR performed conceptualization, data analysis, methodology, manuscript writing, and critical revision of the manuscript. JM analyzed and interpreted the data. All authors read the manuscript and approved the final version.
Funding
This study did not receive any external funding.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44(22): 3358-3393.
- Romeo T. (2008) Bacterial biofilms. Springer Berlin Heidelberg, Berlin, Heidelberg.
- Â Nevins DJ. (1995) Sugars: their origin in photosynthesis and subsequent biological interconversions. Am J Clin Nutr 61(4):915S-921S.
- Â Pauly M, Keegstra K. (2018) Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J 54(4):559-568.
- Pauly M, Keegstra K. (2018) Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J 54(4):559-568.
- Whitaker JR. (1990) New and future uses of enzymes in food processing. Food Biotechnol 4(2): 669-697.
- Lynd LR, Wyman CE, Gerngross TU (1999) Biocommodity engineering. Biotechnol Prog 15(5): 777-793.
- Lai D, Deng L, Li J, Liao B, Guo Q, Fu Y (2011) Hydrolysis of cellulose into glucose by magnetic solid acid. Chem Sus Chem 4: 55-58.
- Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011)Biomass pretreatment: Fundamentals toward application. Biotechnol Adv 29(6): 675-685.
- Cavaco-Paulo A (1998) Mechanism of cellulase action in textile processes. Carbohydr Polym 37(3): 273-277.
- Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18(5): 355-383.
- Gyalai-Korpos M, Nagy G, Mareczky Z, Schuster A, Réczey K, et al. (2010) Relevance of the light signaling machinery for cellulase expression in Trichoderma reesei (hypocrea jecorina). BMC Res Notes 3: 330.
- Shanmugapriya K, Saravana PS, Krishnapriya, Manoharan M, Mythili A, et al. (2012) Isolation, screening and partial purification of cellulase from cellulase producing bacteria. Int J Adv Biotechnol Res 3(1): 509-514
- Nakamura K, Kirin B.C.L K, Kitamura (1982) Isolation and identification of crystalline cellulose hydrolyzing bacterium and its enzymatic properties. J Ferment Technol J 60(4): 343-348.
- Saha S, Roy RN, Sen SK, Ray AK (2006) Characterization of cellulase-producing bacteria from the digestive tract of tilapia,Oreochromis mossambica (Peters) and grass carp, Ctenopharyngodon idella (Valenciennes). Aquac Res 37(4): 380-388.
- Doi RH (2008) Cellulases of mesophilic microorganisms: cellulosome and noncellulosome producers. Ann N Y Acad Sci 1125: 267-279.
- Stepanović S, Antić N, Dakić I, Švabić-Vlahović M (2003) In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol Res 158(4): 353-357.
- Bondi A, Spaulding EH (1947) A routine method for the rapid determination of susceptibility to penicillin and other antibiotics. Am J Med Sci 213(2): 221-225.
- Carballo JL,Hernández-Inda ZL, Pérez P, GarcÃa-Grávalos MD (2002) A comparison between two brine shrimp assays to detect in vitrocytotoxicity in marine natural products. BMC Biotechnol 17.
- Bauer AW, Kirby WMM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45(4): 493-496.
- Yin L, Huang P, HLin H (2010) Isolation of cellulase-producing bacteria and characterization of the cellulase from the isolated Bacterium cellulomonas sp. YJ5. J Agric Food Chem 58(17): 9833-9837.
- Andro T, Chambost JP, Kotoujansky A, Cattaneo J, Bertheau Y, et al. (1984) Mutants of erwinia chrysanthemi defective in secretion of pectinase and cellulase. J. Bacteriol 160(3): 1199-1203.
- Kim J, Hur S, Hong J (2005) Purification and characterization of an alkaline cellulase from a newly isolated alkalophilic Bacillus sp. HSH-810. Biotechnol Lett 27: 313-316.
- Apun K, Jong BC, Salleh MA (2000) Screening and isolation of a cellulolytic and amylolytic Bacillus from sago pith waste. J Gen Appl Microbiol 46(5): 263-267.
- Levine ND (1975) Bergey’s manual of determinative bacteriology. J Protozool 22(1): 7-7.
- Miller GL (1959) Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem 31(3): 426-428.
- Kumar SP, Al-Dhabi NA, Duraipandiyan V, Balachandran C, Praveen Kumar P, et al. (2014) In vitro antimicrobial, antioxidant and cytotoxic properties of Streptomyces lavendulae strain SCA5. BMC Microbiol 14: 291.
- RodrÃguez-Tudela JL, Barchiesi F, Bille J, Chryssanthou E, Cuenca-Estrella M, et al. (2003) Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin Microbiol Infect 9(8): i-viii.
- Immanuel G, Dhanusha R, Prema P, Palavesam A (2006) Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. Int J Environ Sci Technol 3: 25-34.
- SCD Sharma, Shovon MS, Jahan MGS, Asaduzzaman AKM, Khatun B,et al. (2012) Antibiotic sensitivity and antibacterial activity of Micrococcus sp SCS1 7.
Citation: Islam F, Miah M, Mahato J, Roy N (2021) Screening, Optimization, and Antibacterial Activity of Cellulose: Degrading Bacteria from Sewage Soil Waste. JBRBD 12:S5:002. DOI: 10.4172/2155-6199.1000002
Copyright: © 2021 Islam F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2326
- [From(publication date): 0-2021 - Apr 17, 2025]
- Breakdown by view type
- HTML page views: 1620
- PDF downloads: 706