Screening of Toxoplasma gondii Antibodies in Pregnant and Aborted Women Attending Wad Medani Maternity Teaching Hospital and Um Algura Hospital Using Toxo-Latex Agglutination and Electro-Chemiluminescence Immunoassay (ECLIA)
Received: 05-May-2017 / Accepted Date: 01-Jun-2017 / Published Date: 05-Jun-2017
Abstract
Background: Toxoplasmosis is an infection caused by an obligate intracellular parasite Toxoplasma gondii in final and intermediate host. Toxoplasma gondii is commonly associated with congenital infections that are not clinically apparent. The infection in the first trimester pregnancy may cause severe congenital anomalies or even foetal loss. In congenitally infected children can cause devastating effects including eye blindness, neurological impairment and mental retardation. The parasite distributed world-wide, in Sudan the prevalence was reported to be 34.1% and in Gezira state it was 41.7%.
Objectives: This study aimed to diagnose T. gondii infection inpregnant and aborted women by Toxo-Latex agglutination test and Electro-chemiluminescence immunoassay (ECLIA) for IgG and IgM antibodies.
Materials and Methods: Total 100 samples of venous blood collected from pregnant and aborted women, 37 and 63 samples were from participants in Wad Medani and Um Algura, respectively. These samples were diagnosed using Toxo-Latex agglutination test for IgG antibodies, Electro-chemiluminescence immunoassay for IgG antibodies and Electro-chemiluminescence immunoassay (ECLIA) for IgM antibodies.
Results: The results showed that a seropositivity of Toxoplasma IgG antibodies by Toxo-Latex agglutination test was 69% and 52.6% by Electro-chemiluminescence immunoassay.The Toxoplasma IgM seropositivity was (5.1%). There was significant difference between two methods (P<0.0001). The sensitivity and specificity of Toxo-Latex agglutination test was (94%) and (57.8%) respectively with positive predictive value (71.2%) and negative predictive value (89.6%). The seroprevalence of Toxoplasma IgG antibodies showed (67.6%) in Wad Medan and (69.8%) in Um Algura by Toxo-Latex agglutination test while by Electro-chemiluminescence immunoassay was 43.2% and 54% in Wad Medani and Um Algura respectively. The seroprevalence of Toxoplasma IgM antibodies was (7.9%) and this present in Um Algura. The high prevalence noted among age group 26-35. There is relation between the positive results with clinical symptoms; strong correlation with risk factors specially eating undercooked meat (71%), consumption of raw meat (68.1%), and contact with cats (52.1%). There was negative correlation between the seropositivity and parity number.
Conclusion: The Electro-chemiluminescence immunoassay remain high specific and sensitive method for diagnosis of Toxoplasmosis, but a high cost of both apparatus and test reagents may prevent applicability in rural area, so the Toxo-Latex agglutination test may be useful in screening for disease due to simple applicability. The study recommended that the screening of Toxoplasma gondii should be done to all pregnant women to prevent disease progression, control cats, avoiding eating raw and undercooked meat and drinking filtrated water.
Keywords: Toxoplasmosis; Sudan; Pregnant women; Toxoplasma gondii; ECLIA
66388Introduction
Toxoplasma gondii is an animal coccidian parasite that causes toxoplasmosis, with congenital toxoplasmosis being the most serious form of infection in human [1-4]. Toxoplasmosis is considered one of the Neglected Parasitic Infections, a group of five parasitic diseases that have been targeted by Centre for Disease Control (CDC) for public health action [5].The parasite is a heteroxenous in which the definitive hosts (cat and lynx) produce oocysts, whereas, human and other mammalian like rodents, chickens, lambs act as intermediate hosts and other developmental stages of Toxoplasma gondii are found [6].
The immune response to T. gondii involves both innate and adaptive immune responses, by coordinated series of cellular interactions between the parasite, enterocytes, monocytes, Dendritic Cells (DCs), macrophages, Natural Killer cells and neutrophils. DCs play a central role in immune response by stimulating of both innate and adaptive immune response. IFN-γ dependent cell immunity plays a major role in resistance against both acute and chronic T. gondii infection, with IFN-γ activated effector cells in both hemopoietic and nonhemopoietic cell compartments controlling T. gondii via several antimicrobial mechanisms. CD8+ T cells are crucial for protection against T. gondii [7-9].
Infection by T. gondii is usually asymptomatic in immunocompetent humans, but serious or lethal complications may occur when the parasite is transmitted to the fetus via transplacental route by its asymptomatic infected mother, resulting in congenital toxoplasmosis [1,10-12]. Severe toxoplasmosis occurs in immunocompromised adults that develop either acute infection or reactivation from quiescent tissue cysts [13]. Congenital transmission may occur when an uninfected mother acquires primary infection during pregnancy. In pregnant women, the disease is often asymptomatic or have only mild symptoms, infection may cause spontaneous abortion, still birth, or serious foetal damage. The gestational age at which the infection is contracted is a key variable affecting the clinical foetal outcome [14]. Psychiatric disorders, poor impulse control, personality aberrations or neurocognitive impairment attempt were marginally more frequent among individuals with T. gondii seropositivity [15].
The seroprevalence of T. gondii in human population varies greatly among different countries, geographical areas within the same country, and among the ethnic groups living in the same area [16]. T. gondii has a cosmopolitan distribution due to presence of many animals that can accommodate the parasite and follow its dissemination [17,18]. Several studies showed that different socio-demographic determinants, geographical locations and ethnicity have significant effects on Toxoplasma seropositivity [19-22].
The seroprevalence of T. gondii was reported to be 85.4% for anti- Toxoplasma gondii antibody in Ethiopia [23]; in Southeastern Turkey anti-Toxoplasma IgG and IgM antibody was found to be 53.3% and 1% respectively [24]; in the Malaysia was 19.9% [25], followed by the Indian 22.40% [26] and 15.2% in pregnant women 17.3% in control subjects for anti-T. gondii IgG antibodies, while in case of IgM 2.9% in pregnant women and 3.8 in controls in Eastern China [27]. In Sudan, toxoplasmosis was reported to occur long time ago [28]. Moreover, toxoplasma infection was also reported at different infection rates [29,30].
Association between toxoplasma infection and immunocompromised patient was also documented [19]. Serological testing for anti-Toxoplasma antibodies is the mainstay for the diagnosis of toxoplasmosis. Diagnosis of acute maternal infection is mainly based on detection of sero-conversion or four fold rise in IgM antibodies levels which appear sooner after infectionthan IgG antibodies and disappear faster than IgG after recovery [2,11]. Sabin and Feldman did the first serological test for determination of prevalence of disease using extracellular dye into live trophozoite by complement-fixing antibodies to T. gondii that led to the development of the Sabin-Feldman dye-test for serodiagnosis [31]. Today there are effective tests for the diagnosis of toxoplasmosis that are based on different methods such as: enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence test Diagnosis of toxoplasmosis has been improved by the emergence of molecular technologies to amplify parasite nucleic acids. Among these, polymerase chain reaction (PCR)-based molecular techniques have been useful for the genetic characterization of T. gondii and the diagnosis of congenital toxoplasmosis and disseminated toxoplasmosis and it was preferred due to its excellent sensitivity and specificity to determine the presence of parasites in clinical sample in much reduced time [32]. In addition Toxo-Latex agglutination test usually give a high positive rate comparing to other techniques [33].
Patients and Methods
Patients
A hospital based cross-sectional study conducted in 100 pregnant and abortive women were selected randomly during the period November 2014 to January 2015. 37 blood samples were taken from women in Wad Medani Maternity Teaching hospital while the remaining samples about 63 samples were taken from Um Algura hospital.
Methods
Blood samples (3 ml) were aseptically withdrawn from each of the study participants into EDTA container. The plasma was separated from the whole blood by centrifugation at 3000 rpm for 5 minutes and the plasma stored at -20ºC until used.
For the screening of T. gondii antibody plasma samples were analysed by Toxo-Latex agglutination test for the qualitative and semiquantitative detection of anti-toxoplasma antibodies which used Latex particles coated with inactivated T. gondii soluble antigen (Spinreact) and Electro-chemiluminescence immunoassay (ECLIA) by Cobas modular platform (Cobas e 411 analyzer) an immunoassay for the in vitro quantitative determination of IgG and IgM antibodies to Toxoplasma gondii in human serum or plasma. The samples were analysed according to manufacturer's instructions. The evaluation of anti-toxoplasma IgG and IgM antibodies is part of TORCH screening. TORCH is a group pathogens known to cause hazardous congenital infections and include; Toxoplasmosis, syphilis, hepatitis B, hepatitis E, Coxsackie virus, Epstein–Barr virus (EBV), human parvovirus, varicella zoster, Rubella, Cytomegalovirus (CMV) and Herpes simplex virus (HSV).
Toxo-latex agglutination test: The reagents and samples were allowed to reach room temperature because the sensitivity of the test may be reduced at low temperatures. About 50 μL of the sample and one drop of each Positive and Negative controls was added into separate circles on the slide test Toxo-Latex reagents were mixed vigorously or on a vortex mixer before add 25 μL of this reagent next to the samples to be tested. Drops were mixed using a stirrer for each sample. The slides then mixed via rotating for 4 minutes. Then the slide was examined macroscopically for the presence or absence of visible agglutination. The presence of agglutination indicates an antibody concentration equal or greater than 4 IU/mL (Latex agglutination test kit - SPINREACT, TOXO_LATEX Slide Agglutination, Code 1201002).
Electro-chemiluminescence immunoassay (ECLIA): Preparation and loading of samples were done according to Cobas Modulator Plateform. The total duration of assay is 18 minutes, as mention below:
First incubation: 10 μL of sample were automatically pre diluted 1:20 with Elecsys Diluent Universal. T. gondii -specific recombinant antigen labelled with a ruthenium complex was added. Anti-Toxo IgG and IgM antibodies present in the sample react with the ruthenium-labelled T. gondii -specific recombinant antigen.
Second incubation: Biotinylated monoclonal h-IgM-specific antibodies and streptavidin-coated micro particles were added. The complex became bound to the solid phase via interaction of biotin and streptavidin.
The reaction mixture was aspirated into the measuring cell where the microparticles were magnetically captured onto the surface of the electrode. Unbound substances were then removed with ProCell. Application of a voltage to the electrode then induced chemiluminescent emission which is measured by a photomultiplier.
The cut off values for positive and negative results were determined according to manufacture procedure.
Toxoplasma gondii IgG: >1 (non reactive), >_ 1.0 - > (Intermediate), >_ 30 (Reactive).
Toxoplasma gondii IgM: >0.8(non reactive), >_0.8 - >1.0 (Intermediate), >_1 (Reactive).
Ethical considerations
The Ethical approval and permission obtained from Gezira State Ministry of Health Authorities and informed consent obtained from the participants.
Result analysis
The results were analysed by Statistical Package for the Social Sciences (SPSS) program version 16, a crosstab and correlation was done. Also, Medical Calculator (MedCalc) was used to calculate the specificity, sensitivity and predictive value by a Receiver Operating Characteristic curve (ROC).
Results were determined automatically by the Elecsys software by comparing the electrochemiluminescence signal obtained from the reaction product of the sample with the signal of the cutoff value previously obtained by Toxo IgG and IgM calibration (Cobas411, serial No. 0868-16 manufactured by Hitachi high technologies corporation, Tokyo- Japan).
Results
In present study, hundred women were enrolled; 37 women (37%) were examined at Wad Medani and 63 women (63%) at Um Algura. The pregnant participant were (74%) while the aborted once were (26%) of the examined population. The participants were categorized into three age groups; 15-25 (59%), 26-35 (36%) and 36-45 (5%) and the age rang was 15-38 years with mean of 24 ± 5.6.
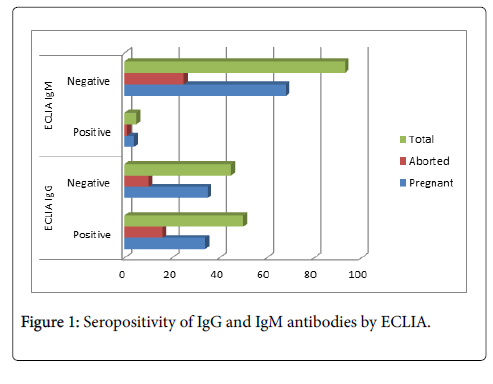
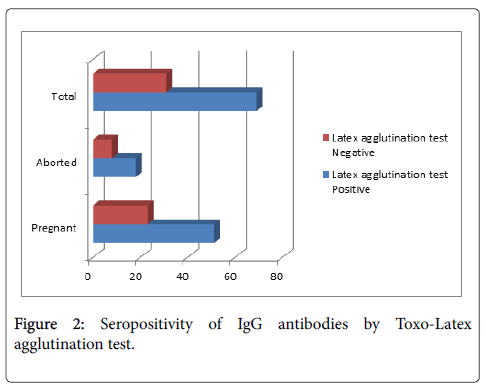
The seropositivity of anti-Toxoplasma gondii IgG antibodies among study population was 52.6% by Electro-chemiluminescence immunoassay (ECLIA) and 69% by Toxo-Latex agglutination test which include 34 cases (68%), 51 cases (69%) in pregnant and 16 cases (61.5%), 18 cases (69.2%) in aborted women and (Figures 1 and 2). While the seropositivity of anti-Toxoplasma gondii IgM antibodies among study population was 5.1% in which pregnant women were 4 cases (5.6%) and aborted women were 1 case (3.9%) by Electro-chemiluminescence immunoassay (ECLIA) (Figure 1). The seropositivity of IgG in Wad Medani was 67.6% and 50% while in Um Algura was 69.8% and 54% by Toxo-Latex agglutination test and ECLIA respectively (Table 1). In contrast, the seropositivity of IgM was 0% in Wad Medani and 8% in Um Algura by ECLIA (Table 2).
| Study area | Toxo-Latex agglutination test | P-value | ECLIA | P-value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | 0.492 | Positive | Negative | 0.44 | |
| Wad Medani | 25 (67.6%) | 12 (32.4%) | 16 (50%) | 16 (50%) | ||
| Um Algura | 44 (69.8%) | 19 (30.2) | 34 (54%) | 29 (46%) | ||
| Total | 69 | 31 | 50 | 45 | ||
Table 1: Seropositivity of IgG antibodies among study area by both technique.
| Maternal status | Toxo-Latex agglutination test | P-value | |
|---|---|---|---|
| Positive | Negative | 0.104 | |
| Wad Medani | 0 (0%) | 35 (100%) | |
| Um Algura | 5 (8%) | 58 (92%) | |
| Total | 5 | 93 (94.9) | |
Table 2: Seropositivity of IgM antibodies among study area by ECLIA.
The seropositivity of IgG among age group showed 39 cases (66.1%) and 24 cases (43%) in age group 15-25; 28 cases (77.8%) and 23 cases (67.6%) in age group 26-35 and 2 cases (40%) and 3 cases (60%) in age group 36-45 by by Toxo-Latex agglutination test and ECLIA respectively (Table 3).
| Age group | ECLIA IgG | Toxo-Latex agglutination test | ||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| 15-25 years | 24 (43%) | 32 (57%) | 39 (66.1%) | 20 (33.9%) |
| 26-35 years | 23 (67.6%) | 11 (32.4%) | 28 (77.8%) | 8 (22.2%) |
| 36-45 years | 3 (60%) | 2 (40%) | 2 (40%) | 3 (60%) |
| Total | 50 | 45 | 69 (69%) | 31 (31%) |
| P-value | 0.045 | 0.925 | ||
Table 3: Seropositivity of IgG antibodies among age group by both technique.
The seropositivity of IgG antibodies correlated to contact with animal and their products showed that 36 cases (73.5%) and 27 cases (68.7%) in women whom contact with cats; 47 cases (72.3%) and 34 cases (54%) in women whom consumed raw meats and 49 cases (66.2%) and 35 cases (50%) in women whom eat undercooked meat (Table 4) by Toxo-Latex agglutination test and ECLIA respectively. The Seropositivity of IgG in women whom had history of miscarriage were 20 cases (71.4%) and 16 cases (61.5%) while in women drink infiltrated water were 26 cases (66.7%) and 21 cases (53.8%) by Toxo-Latex agglutination test and ECLIA respectively (Table 5).
| Risk factor | ECLIA IgG | P-value | Toxo-Latex agglutination test | P-value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| Contact with cats | 27 (58.7%) | 19 (41.3%) | 0.173 | 36 (73.5%) | 13 (26.5%) | 0.233 |
| Raw meat | 34 (54%) | 29 (46%) | 0.44 | 47 (72.3%) | 18 (27.7%) | 0.226 |
| Undercooked meat | 35 (50%) | 35 (50%) | 0.266 | 49 (66.2%) | 25 (33.8%) | 0.223 |
Table 4: Seropositivity of IgG antibodies correlated animal risk factors.
| Risk factor | ECLIA IgG | P-value | Toxo-Latex agglutination test | P-value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| History of miscarriage | 16(61.5%) | 10 (38.5%) | 0.202 | 20 (71.4%) | 8(28.6%) | 0.471 |
| Infiltrated water | 21 (53.8%) | 18 (46.2%) | 0.505 | 26 (66.7%) | 13 (33.3%) | 0.426 |
Table 5: Seropositivity of IgG antibodies correlated to other risk factors.
There was negative correlation between the seropositivity of Toxoplasma IgG antibodies with parity number i.e. infection rate was decreased with increasing in number of parity (Tables 7 and 8). The seropositivity of IgG antibodies correlated to sign and symptoms showed that 40 and 28 cases of fever (74% and 52%), 9 and 7 cases of rash (69.2% and 53.8%), 3 and 1 cases of inflammation of eyes (60% and 20%), 11 and 9 cases of pneumonia (73.3% and 60%), 2 and 2 cases of history of still birth (66.7% and 66.7%) and one case history of child blindness (100%) by Toxo-Latex agglutination test and ECLIA respectively (Table 6).
| Symptoms | ECLIA IgG | P-value | Toxo-Latex agglutination test | P-value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| Fever | 28 (52.8%) | 25 (47.2%) | 0.166 | 40 (74%) | 14 (26%) | 0.565 |
| Rash | 7 (53.8%) | 6 (46.2%) | 0.629 | 9 (69.2%) | 4 (30.8%) | 0.582 |
| Eye inflammation | 1 (20%) | 4 (80%) | 0.495 | 3 (60%) | 2 (40%) | 0.15 |
| Pneumonia | 9 (60%) | 6 (40%) | 0.475 | 11 (73.3%) | 4 (26.7%) | 0.368 |
| Still birth | 2 (66.7%) | 1 (33.3%) | 0.676 | 2 (66.7%) | 1 (33.3%) | 0.54 |
| Child blindness | 1 (100%) | 0 (0%) | 0.69 | 1 (100%) | 0 (0%) | 0.526 |
Table 6: Seropositivity of IgG antibodies correlated to sign and symptoms.
| Parity Number | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | 37 | 12 | 6 | 6 | 2 | 2 | 3 | 0 | 1 | 69 |
| Negative | 16 | 7 | 3 | 0 | 1 | 1 | 2 | 1 | 0 | 31 |
| Total | 53 | 19 | 9 | 6 | 3 | 3 | 5 | 1 | 1 | 100 |
Table 7: Seropositivity of Toxoplasma gondii IgG antibodies correlated to parity number by Toxo-Latex agglutination test.
| Parity Number | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | 26 | 7 | 5 | 3 | 2 | 1 | 4 | 1 | 1 | 50 |
| Negative | 24 | 11 | 4 | 2 | 1 | 2 | 1 | 0 | 0 | 45 |
| Total | 50 | 18 | 9 | 5 | 3 | 3 | 5 | 1 | 1 | 95 |
Table 8: Seropositivity of Toxoplasma gondii IgG antibodies correlated to parity number by ECLIA.
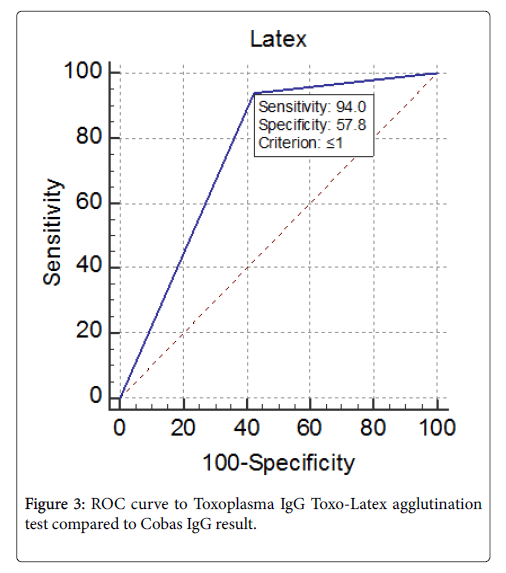
Specificity and sensitivity of Toxo-Latex agglutination test compared to Electro-chemiluminescence immunoassay was done. In this study, we considered that Electro-chemiluminescence immunoassay is a gold standard method for anti-Toxoplasma gondii IgG antibodies and we used it to detect sensitivity and specificity of Toxo-Latex agglutination test. The curve show the specificity of Toxo-Latex agglutination test was 57.8% while the sensitivity was 94% (Figure 3).
Discussion
Toxoplasma gondii is an intracellular protozoan which distributed worldwide. The hypothesis of this study is to compare the Toxo-Latex agglutination test with Electro-chemiluminescence immunoassay (ECLIA) for detection of toxoplasmosis to detect sensitivity and specificity with the predictive values to evaluation the reliability of this test as its common diagnostic tool for the disease. The current study aimed to screen of toxoplasmosis in one of the most affected immunocompetent population; pregnant women.
Seropositivity of Toxoplasma IgG antibodies by Toxo-Latex agglutination test was 69% of 100 of population which while seropositivity of IgG by Electro-chemiluminescence immunoassay was 52.6% of 95 of population; which constitute 69% and 49.3% among pregnant women while 69.2% and 61.5% among aborted women by Toxo-Latex agglutination test and ECLIA respectively. Our results indicate high prevalence rate of toxoplasma IgG among population and may be due to the past infection since the IgG antibody usually persists for the life of the host [34], and in accordance with that reported in some geographical area such as Sudan [29,30,35,36], Baghdad city in Iraqi [14] and in Ahmedabad in Pakistan [37]. In these studies the seroprevalence of IgG antibodies is similar to our results. While our data are disagree with that published from Ghana [38].
The seropositivity of IgM was evaluated by Electro-chemiluminescence immunoassay (ECLIA), 5 cases out of 98 were positive and considered as 5.1%. Of these positive samples, 4 samples with mixed with IgG antibodies and this may be either due to persisting IgM or unspecific stimulation immune system or the infection is most likely established for more than one year since specific IgM may be detected for 18 months or more [34]. In accordance with documented data from Iraqi, Sudan, Palestine and Saudi Arabia [14,36,39,40] respectively, and disagree with that reported from Sudan, Pakistan, Ghana and Sri Lanka [35,37,38,41] respectively whom considered high and absence of Toxoplasma IgM prevalence 41.3%, 88.7 and 0% with respectively. The only one sample which IgM positive and IgG negative by Electro-chemiluminescence immunoassay (ECLIA) indicate a recent infection classified in age group 15-25, locality in rural area, no correlation with sign and symptoms and correlate to risk factor eating raw meat and contact with cat.
The seroprevalence of Toxoplasma IgG antibodies by Toxo-Latex and Electro-chemiluminescence immunoassay (ECLIA) tests were 67.6% and 43.2% in Wad Medani while in Um Algura were 69.8% and 54%. Also, the seroprevalence of IgM correlated to location is 7.9% in Um Algura and 0% in Wad Medani. This showed a slightly high seroprevalence of IgG in rural area than urban area and the seroprevalence of IgM present in rural area and absence in urban area since the risk factors in rural area is more than urban area such as contact with cat, eating raw meat, eating undercooked meat, drinking infiltrated water and personal hygiene decreased in rural area. In accordance with [33] which indicate high prevalence of IgM anti- Toxoplasma in rural area of Kurdistan.
The high IgG seropositivity reported in age group 26-35 (63%), this due to women in this age are high risky and the most fertile period of childbearing age. In accordance with literature data indicating that seroprevalence increased by age [29,30,35,42].
There were a correlation of Toxoplasma IgG seroprevalence to a sign and symptoms by Toxo-Latex and Cobas tests and Toxoplasma IgM antibodies, this sign mainly fever may be due to disease or other cases such as malaria since the collection was done in high transmitted season of malaria. There is a strong correlation between abortion and seropositivity of both IgG and IgM results (25%) reflect the most serious outcome among those populations at risk, this agreed with that reported by Ebadi P [43] whom found that the prevalence of IgG seroprevalence among women with history of abortion was 17.5%.
There were a correlation of Toxoplasma IgG antibodies to risk , this strong correlation indicate that all risk factors and possible rout of transmission involved in infection with history of miscarriage been a factor of incomplete treatment and this due to personal hygiene and environmental condition. This agreed with [29] in Sudan whom found that a contact with cats, eating raw meat, and eating soil [36] and in Sudan whom found correlation with consumption of raw meat. And disagreed with [35] in Khartoum, Sudan whom found that there are no correlation between the disease and risk factors.
There was strong negative correlation between seropositivity of Toxoplasma IgG and IgM antibodies; in women with no pregnancy have a high seroprevalence of IgG and IgM and this agreed with that published from Nigeria [16].
The high prevalence of IgG and present of IgM antibodies indicate the present of infection in risk group and the remaining women may be in risk of acquiring infection with the presence of all possible rout of transmission.
In the evaluation of Toxo-Latex agglutination test, the ROC curve involved assessing the specificity, sensitivity, negative predictive value, positive predictive value and calculation of the area under the curve. There are a significant difference between the two methods (Significance level P=<0.0001). Toxo-Latex agglutination test showed 94% sensitivity and 57.8% specificity with positive predictive values of 71.2% where a negative predictive value was 89.6%. This may be due to cross reactivity such as hepatocellular disease; manufacturing and transporting of the reagents. To the best of our knowledge there was no available study in assessing Latex agglutination test compared to Cobas e411 test, but this agreed with that reported by Narmin [33] which compared the Toxo-Latex with ELISA test for IgG and their results showed a high prevalence of IgG by Toxo-Latex than ELISA.
Conclusion
The study concluded that the seropositivity of Toxoplasma IgG antibodies by latex agglutination test was 69% and 52.6% by Electro-chemiluminescence immunoassay which slightly increased in rural area, and the Toxoplasma IgM seropositivity was (5.1%) and present in rural area. There was a significant difference between the two methods (P<0.0001). The sensitivity and specificity of Toxo-Latex agglutination test was (94%) and (57.8%) respectively with positive predictive value (71.2%) and negative predictive value (89.6%). The high prevalence noted among age group of 26-35 years. There was correlation with risk factors specially eating undercooked meat (71%), consumption of raw meat (68.1%), and contact with cats (52.1%). There was negative correlation between the seropositivity and parity number.
This study is recommended to screen Toxoplasma gondii during pregnancy which could have an impact to prevent progression of disease if present and subsequently prevent complication on pregnant woman and her fetus.
Acknowledgement
The authors are indebted to the staff of Um-Elgura Hospital and Wad Medani Obstetrics and Gynaecology Teaching Hospital for their cooperation. Also we acknowledged the technical assistance provided by the staff of the laboratories at National Cancer Institute and the Faculty of Medical Laboratory Sciences in University of Gezira.
Competing Interests
The authors declare that they have no competing interests.
References
- Liesenfeld O, Montoya JG, Tatahineni NJ, Davis M, Brown BW, et al. (2001) Confirmatory serologic testing for acute toxoplasmosis and rate of induced abortions among women reported to have positive Toxoplasma immunoglobulin M antibordy titers. Am J ObsteGynecol 184: 140-145.
- Montoya JG, Rosso F (2005) Diagnosis and management of toxoplasmosis. Clinics in Perinatology 32: 705-726.
- Remington JS, McLeod R, Thulliez P, DEsmonts G (2005) Toxoplasmosis. In: Remington JS, Klein J, eds. Infect Dis Fetus and Newborn Infant, 6th Edn., Philadelphia, WB Saunders.
- Remington JS, Thulliez P, Montoya JG (2004) Recent developments fordiagnosis of toxoplasmosis. Minireview. J Clin Microbiol 42: 941-945.
- Center for Disease Control and Prevention (2017) Parasites-Toxoplasmosis (Toxoplasma Infection).
- Frenkel JK, Dubey JP, Miller NL (1970). Toxoplasma gondii in cats: fecal stages identified as coccidian oocysts. Science 167: 893-896.
- Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC (2005) Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect Immun 73: 695-702.
- John B, Harris TH, Tait ED, Wilson EH, Gregg B, et al. (2009) Dynamic Imaging of CD8 (+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog 5: e1000505.
- John B, Weninger W, Hunter CA (2010). Advances in imaging the innate and adaptive immune response to Toxoplasma gondii. Future Microbiol 5: 1321-1328.
- Pinon JM, Dumon H, Chemla C, Franck J, Petersen E, et al. (2001) Strategy for diagnosis of congenital toxoplasmosis: evaluation of methods comparing mothers and newborns and standard methods for postnatal detection of immunoglobulin G, M, and A antibodies. J Clin Microbiol 39: 2267-2271.
- Rorman E, Zamir CS, Rilkis I, Ben-David H (2006). Congenital toxoplasmosis—prenatal aspects of Toxoplasma gondii infection. Reprod Toxicol 21: 458-472.
- Ajzenberg D, Yera H, Marty P, Paris L, Dalle F, et al. (2015) Genotype of 88 Toxoplasma gondii Isolates Associated with Toxoplasmosis in Immunocompromised Patients and Correlation with Clinical Findings. J Infect Dis 199: 1155-1167.
- Mohammed TK (2011) Seroprevalence of Toxoplasma gondii among pregnant women in Baghdad City. Inst Med Technol.
- Sugden K, Moffitt TE, Pinto L, Poulton R, Williams BS, et al. (2016). Is Toxoplasma Gondii Infection Related to Brain and Behavior Impairments in Humans? Evidence from a Population-Representative Birth Cohort. PLoS ONE 11: e0148435.
- Deji-Agboola AM, Busari OS, Osinupebi OA, Amoo AOJ (2011) Seroprevalence of Toxoplasma gondii Antibodies among Pregnant Women Attending Antenatal Clinic of Federal Medical Center, Lagos, Nigeria. Int J Biol Med Res 2: 1135-1139.
- Tlamcani1 Z, Lemkhenete Z, Lmimoun BE (2013) Toxoplasmosis: The value of molecular methods in diagnosis compared to conventional methods. J Microbiol Infect Dis 3: 93-99.
- Meerburg BG, Kijlstra A (2009) Changing climate changing pathogens: Toxoplasma gondii in North-Western Europe. Parasitol Res 105: 17-24.
- Ibrahim AM, Bushara SH, Rakib NK, Zoalnorain SH, Adam DawoudAbakar, et al. (2015) Seroprevalence and Analysis of Some Risk Factors Associated with Human Toxoplasmosis among HIV Patients Attending Bashyer University Teaching Hospital, Sudan. European Acad Res 3: 6198- 6215.
- Hill D, Dubey JP (2002) Toxoplasma gondii: transmission, diagnosis andprevention. Clin Microbiol Infect 8: 634-640.
- Gilbert RE, Tookey PA, Cubitt WD, Ades AE, Masters J, et al. (1993) Prevalence of toxoplasma IgG among pregnant women in West Londonaccording to country of birth and ethnic group. BMJ 306: 185.
- Falusi O, French AL, Seaberg EC, Tien PC, Watts DH, et al. (2002) Prevalence and Predictors ofToxoplasma Seropositivity in Women with and at Risk for Human Immunodeficiency Virus Infection. Clin Infect Dis 35: 1414 –1417.
- Gelaye W, Kebede T, Hailu A (2015) High prevalence of anti-toxoplasma antibodies and absence of Toxoplasma gondii infection risk factors among pregnant women attending routine antenatal care in two Hospitals of Addis Ababa, Ethiopia. Int J Infect Dis 34: 41–45.
- Yentur Doni N, Simsek Z, Gurses G, Yildiz Zeyrek F, Demir C (2015) Prevalence and associated risk factors of Toxoplasma gondii in female farmworkers of southeastern Turkey. J Infect Dev Ctries 9: 87-93.
- Brandon-Mong GJ, Mat Seri NA, Sharma RS, Andiappan H, Tan TC, et al. (2015) Seroepidemiology of Toxoplasmosis among People Having Close Contact with Animals. Front Immunol 6: 143.
- Singh S, Munawwar A, Rao S, Mehta S, Hazarika NK (2014) Serologic Prevalence of Toxoplasma gondii in Indian Women of Child Bearing Age and Effects of Social and Environmental Factors. PLoS Negl Trop Dis 8: e2737.
- Cong W, Dong XY, Meng QF, Zhou N, Wang XY, et al. (2015) Toxoplasma gondii Infection in Pregnant Women: A Seroprevalence and Case-Control Study in Eastern China. Biomed Res Int. 2015: 170278.
- Carter F, Fleck D (1966) The incidence of Toxoplasmaantibodies in the Sudanese. Trans R Soc Trop Med Hyg 60: 539-543.
- Mohamed K, Ahmed AA, Elrayah IE (2013) Prevalence and risk factors for Toxoplasma gondii infection in humans from Khartoum State, Sudan. J Public Health Epidemiol 2: 60-66.
- Hameed A (1991) Sero-epidemiology of toxoplasmosis in Gezira, Sudan. J Trop Med Hyg 94: 329-332.
- Sabin AB, Feldman HA (1948) Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoan parasite (Toxoplasma). Science 108: 660-663.
- Tlamcani Z, Lemkhenete Z, Lmimoun BE (2013) Toxoplasmosis: The value of molecular methods in diagnosis compared to conventional methods. J Microbiol Infect Dis 3: 93-99.
- Hamad NR, Kadir MA (2013) Prevalence and comparison between the efficacy of different techniques for diagnosis of Toxoplasma gondii among women in erbil province-Iraqi Kurdistan. Ann Intl Interdis Conf: 24-26.
- Gillespie S, Pearson RD (2001) Principle and Practice of Clinical Parasitology. John Wiley and Sons Ltd, England: 369-398.
- Abdel-Raouff M, Elbasheir MM (2014) Sero-prevalence of Toxoplasma gondii infection among pregnant women attending antenatal clinics in Khartoum and Omdurman MaternityHospitals, Sudan. J Coastal Life Med: 496-499.
- Elnahas A, Gerais AS, Elbashir MI, Eldien ES, Adam I (2003) Toxoplasmosis in pregnant Sudanese women. Saudi Med J 24: 868-870.
- Sood N, Soni S, Egad MV, Gupta P (2004) Serprevalence of Toxoplasma gondii in Women with Bad Obstetric History in Ahmedabad. Gujarat Medical J 64: 35-37.
- Ayii I, Edu SAA, Apea-Kubi KA, Boamah D, Bosompemi KM (2009) Sero-epidemiology of toxoplasmosis amongst pregnant women in the greater Accra region of Ghana, Ghana Medical J 43: 107-114.
- Al-Hindi AI, Lubbad AMH (2009) Seroprevalence of toxoplasmosis among Palestinian aborted women in Gaza. Annals of Alquds medicine 5: 39-47.
- Al-Harthi SA, Jamjoom MB, Ghazi OH (2006) Seroprevalence of Toxoplasma gondii Among Pregnant Women in Makkah, Saudi Arabia. Umm Al-Qura Uni J Sci Med Eng 18: 217-227.
- Subasinghe SDLP, Karunaweera ND, Kaluarachchi A, Abayaweera CA, Gunatilake MH, et al. (2010) Toxoplasma gondii seroprevalence among two selected groups of pregnant women. Sri Lankan J Inf Dis 1: 9-17.
- Dubey JP, Beattie CP (1988) Toxoplasmosis of Animals and Man. Boca Raton, FL: CRC Press 100: 500-501.
- Ebadi P, Solhjoo K, Bagheri K, Eftekhar F (2011) Seroprevalence of toxoplasmosis among the women with recurrent spontaneous abortion in comparison with the women with uncomplicated delivery. J Jahrom Uni of Med Scis 9: 32-36.
Citation: Mohamed MY, Abakar AD, Talha BA, Nour BYM (2017) Screening of Toxoplasma gondii Antibodies in Pregnant and Aborted Women Attending Wad Medani Maternity Teaching Hospital and Um Algura Hospital Using Toxo-Latex Agglutination and Electro-Chemiluminescence Immunoassay (ECLIA). Arch Parasitol 1: 105.
Copyright: © 2017 Mohamed MY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 5220
- [From(publication date): 0-2017 - Jul 11, 2025]
- Breakdown by view type
- HTML page views: 4218
- PDF downloads: 1002