Screening for Resistance Sources in Local and Exotic Hot Pepper Genotypes to Fusarium Wilt (Fusarium oxysporium) and Associated Quality Traits in Ethiopia
Received: 05-May-2018 / Accepted Date: 12-May-2018 / Published Date: 19-May-2018 DOI: 10.4172/2329-8863.1000367
Abstract
Fifty-four hot pepper germplasm (49 local accessions and 5 AVRDC genotypes) were evaluated for resistance to fusarium wilt in a greenhouse at Melkassa Agricultural Research Centre in Ethiopia. A completely randomized design with three replications was used. Each local accession was also analyzed for associated quality traits of capsaicin and oleoresin content. The overall results indicated that disease incidence ranged from 8 (PBC-731) to 100% for Acc-15. Most local collections exhibited higher disease incidences percent than AVRDC genotypes with an overall mean DI <25% of Fusarium wilt. Among the 49 local accessions, however, only two accessions were found resistant (1-10%) while three were moderately resistant (11-20%). Assessment of the same accessions for resistance to the disease using severity index (SI) showed that 11 accessions and genotypes were found resistant (<10% DSI) whereas nine genotypes moderately resistant (10-20% SI), 10 genotypes were moderately susceptible (21-40% SI), four accessions were found susceptible (41-60%) and the rest 20 accessions were highly susceptible >60%. Based on severity rating (1-5 scale), two accessions (Acc-39 and PBC-731) were highly resistant to wilt with severity ratings of 1 and 14 accessions were found resistant with severity rating scale of 2 whereas 38 accessions were susceptible with severity scale of >3. Capsaicin content percentage ranged from 0.16-0.55%, and heat unit ranged 26372 to 88775 SHU, for Acc-32 and Acc-24, respectively. Oleoresin content in international color unit and ASTA value ranged from 32,800 to 118,840 cu and 82-296 ASTA with an overall mean of 69,704 ICU and 172 ASTA value. The highest color quality colors of greater than 250 ASTA was exhibited by 10 accessions (Acc-4, Acc-5, Acc-6, Acc-7, Acc-24, Acc-27, Acc-33, Acc-34, Acc-39 and Acc-31). This study identified resistance accessions with desirable qualities among the 49 local accessions. These materials also had good wilt resistance potential and could be used as source parents in the future hybridization and for simultaneous selection for Fusarium wilt resistance and high processing quality traits in hot pepper improvement program.
Keywords: Hot pepper; Fusarium wilt; Oleoresin; Capsaicin
Introduction
Hot pepper (Capsicum annum L. ) is one of the most economically important vegetable crops in Ethiopia. Cultivars with elongated-fruit with mildly to highly pungent types are the most preferred. The crop is widely grown in an altitude ranging from 1400-1900 meters above sea level under rain fed and irrigated conditions. Its use is primarily as the main component of daily diet of majority of Ethiopians and also considered as a high value commodity, which has huge potential for improving the income and hence the livelihoods of thousands of small holder farmers [1]. Ethiopia is one of a few African countries that produce capsaicin and oleoresin for the export market from locally selected materials that contributed substantially to the national economy. In spite of tremendous potential use, good scope for processing, available export market as a spice powder and oleoresin and a wide range of available genetic resource, little effort has been made to improve quality and productivity of pepper, in the country [2]. Among biotic factors that affect pepper production in Ethiopia, Fusarium wilt that is caused by F. oxisporium is one of the most economically important diseases, and it accounts for yield losses of up to 80% [3]. In recent years, the importance of the disease has been increasing and is given considerable attention by hot pepper producers and other stakeholders.).
Unfortunately, commercially acceptable varieties in Ethiopia are very susceptible to this disease. It can, however, be hypothesized that resistant hot pepper germplasm against Fusarium wilt could exist either in the land races that have been cultivated by small scale farmers for many or exotic accessions imported by different research organizations from different countries. In the locally cultivated land races or in varieties released by local research organizations, gene flow is expected to be high due to uncontrolled pollination of the crop and its high rate out-crossing.
Therefore, it is imperative to collect, characterize, evaluate and identify Fusarium wilt resistant materials that could be used as sources of resistance for introgressing resistant traits into commercially acceptable hot pepper cultivars. On the other hand, most resistant cultivars lack most important quality attributes of horticultural characteristics necessary to compete with widely acceptable susceptible cultivars [4]. Therefore, development of new resistant hot pepper cultivars could also have additional attributes for improving quality of hot pepper. So far, no systematic research has been conducted on hot pepper accessions available in Ethiopia and exotic germplasm introduced from different sources. Thus, the objective of this study was, to screen exotic and local genotypes for resistance to Fusarium wilt and quality traits (capsaicin and oleoresin contents) and come up with potential parents that could be used in the future improvement program.
Materials and Methods
Collection of Fusarium disease samples
Field survey was carried out during the 2015 cropping season in the major pepper growing areas in the central rift valley of Ethiopia. The major hot-pepper producing areas such as Hawasa, Butajira, Koka, Ziway, Bishoftu and Wonji were purposely selected. Farm fields at each selected area were systematically sampled to collect disease specimens. Following all accessible roads, every hot pepper farm was selected and assessed. Highly infected pepper plants showing typical wilting symptoms (leaf yellowing and dropping and/or partial or complete plant wilting) were collected for isolation and identification of F. oxysporium . The specimens were up rooted and kept separately in a polyethylene bag and placed in an ice box with a temperature of about 4°C and brought to Melkassa Agricultural Research Centre (MARC), Plant Pathology Laboratory.
Isolation and identification
Potato Dextrose Agar (PDA) was prepared by diluting 39 g of PDA in 1000 ml of distilled water. This was autoclaved for 20 minutes at 120 lb pressure. To restrict bacterial growth, PDA was amended with 100 mg/l streptomycin and then poured into Petri plates. The infected roots and stems were washed, sterilized, and then placed in the PDA medium. After five days of incubation at 25 ± 2°C, colonies of fungus were transferred to fresh PDA media. Sub culturing was done till distinct/pure culture was obtained. Identification of fungi was done based on the cultural characteristics and microscopic examination using the Standard manuals [5].
Acquisition of genotypes and screening for resistance
Fifty-four hot pepper genotypes of 49 locally collected accessions from different agro-ecological zones in Ethiopia described, in Table 1 and 5 AVRDC genotypes (ICPN-916, Melka Shote, Melka Awaze, Melka Zala and PBC-731 were evaluated for their reaction to Fusarium wilt in the green house.
| S No | Acc. | Disease Incidence | R | Disease Severity | R | S No | Acc. | Disease Incidence | R | Disease Severity |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Acc-1 | 0.82(0.96) | HS | 0.66(0.72) | HS | 28 | Acc-28 | 0.81(0.94) | HS | 0.65(0.70) |
| 2 | Acc-2 | 0.88(1.1) | HS | 0.70(0.78) | HS | 29 | Acc-29 | 0.84(0.99) | HS | 0.84(1.10) |
| 3 | Acc-3 | 0.74(0.83) | HS | 0.59(0.63) | S | 30 | Acc-30 | 0.76(0.86) | HS | 0.60(0.65) |
| 4 | Acc-4 | 0.23(0.23) | MS | 0.09(0.09) | R | 31 | Acc-31 | 0.41(0.41) | S | 0.24(0.24) |
| 5 | Acc-5 | 0.24(0.24) | MS | 0.09(0.10) | R | 32 | Acc-32 | 0.23(0.23) | MS | 0.09(0.10) |
| 6 | Acc-6 | 0.27(0.27) | MS | 0.11(0.11) | MR | 33 | Acc-33 | 0.23(0.23) | MS | 0.09(0.10) |
| 7 | Acc-7 | 0.94(1.22) | HS | 0.94(1.22) | HS | 34 | Acc-34 | 0.41(0.43) | S | 0.25(0.25) |
| 8 | Acc-8 | 0.91(1.15) | HS | 0.91(1.14) | HS | 35 | Acc-35 | 0.41(0.42) | S | 0.25(0.25) |
| 9 | Acc-9 | 0.92(1.16) | HS | 0.92(1.17) | HS | 36 | Acc-36 | 0.28(0.28) | MS | 0.11(0.11) |
| 10 | Acc-10 | 0.81(0.95) | HS | 0.81(0.94) | HS | 37 | Acc-37 | 0.33(0.34) | S | 0.20(0.20) |
| 11 | Acc-11 | 0.76(0.86) | HS | 0.61(0.65) | HS | 38 | Acc-38 | 0.34(0.34) | S | 0.20(0.21) |
| 12 | Acc-12 | 0.26(0.26) | MS | 0.09(0.10) | R | 39 | Acc-39 | 0.09(0.18) | R | 0.02(0.02) |
| 13 | Acc-13 | 0.90(1.12) | HS | 0.90(1.12) | HS | 40 | Acc-40 | 0.25(0.26) | MS | 0.10(0.08) |
| 14 | Acc-14 | 0.96(1.31) | HS | 0.96(1.30) | HS | 41 | Acc-41 | 0.46(0.48) | S | 0.28(0.31) |
| 15 | Acc-15 | 1.00(1.61) | HS | 1.10(1.60) | HS | 42 | Acc-42 | 0.41(0.41) | S | 0.24(0.25) |
| 16 | Acc-16 | 0.85(1.02) | HS | 0.85(1.02) | HS | 43 | Acc-43 | 0.35(0.36) | S | 0.21(0.22) |
| 17 | Acc-17 | 0.84(1.01) | HS | 0.84(1.10) | HS | 44 | Acc-44 | 0.41(0.41) | S | 0.24(0.24) |
| 18 | Acc-18 | 0.93(1.21) | HS | 0.93(1.20) | HS | 45 | Acc-45 | 0.38(0.39) | S | 0.23(0.23) |
| 19 | Acc-19 | 0.35(0.36) | S | 0.20(0.21) | MR | 46 | Acc-46 | 0.20(0.21) | MR | 0.08(0.09) |
| 20 | Acc-20 | 0.76(0.87) | HS | 0.60(0.65) | HS | 47 | Acc-47 | 0.37(0.38) | S | 0.22(0.22) |
| 21 | Acc-21 | 0.84(1.02) | HS | 0.84(1.10) | HS | 48 | Acc-48 | 0.81(0.95) | HS | 0.81(0.95) |
| 22 | Acc-22 | 0.86(1.04) | HS | 0.86(1.04) | HS | 49 | Acc-49 | 0.25(0.25) | MS | 0.10(0.11) |
| 23 | Acc-23 | 0.94(1.22) | HS | 0.94(1.22) | HS | 50 | ICPN-916 | 0.26(0.27) | MS | 0.10(0.11) |
| 24 | Acc-24 | 0.95(1.25) | HS | 0.95(1.25) | HS | 51 | Melka Shote | 0.26(0.27) | MS | 0.10(0.11) |
| 25 | Acc-25 | 0.46(0.48) | S | 0.28(0.28) | MS | 52 | Melka awaz | 0.20(0.20) | MR | 0.08(0.09) |
| 26 | Acc-26 | 0.83(0.99) | HS | 0.66(0.73) | HS | 53 | Melka zala | 0.14(0.14) | MR | 0.06(0.05) |
| 27 | Acc-27 | 0.78(0.89) | HS | 0.61(0.67) | HS | 54 | PBC-731 | 0.08(0.08) | R | 0.02(0.02) |
| Mean | 0.56 | 0.45 | 0.56 | 0.45 | ||||||
| CV (%) | 33 | 35 | 33 | 35 | ||||||
| LSD (0.05) | 0.6 | 0.7 | 0.6 | 0.7 |
Table 1: Mean diseases incidence and severity index along with resistance levels of 54 hot pepper genotypes to Fusarium wilt evaluated at MARC in, 2015/16. Numbers in parenthesis are transformed values. R=resistance level, HR=highly resistant, R=resistant, MR=moderately resistant, MS=moderately susceptible, S=susceptible, HS=highly susceptible, CV (%)=coefficient of variation in percent and LSD (0.05)=least significant difference at P<0.05.
Seedlings of all accessions were raised in the green house in a 52.3 cm × 25.9 cm × 6.1 cm plastic trays, having 12 individual cells with water draining holes below. A growing media that comprised soil, compost and sand at a ratio of 2:1:1, respectively. Each media component was properly sieved in a 0.5 mm sieve size, mixed manually and autoclaved at 120°C for 90 min and spread on a clean concrete floor at room temperature. The pepper seeds were carefully drilled in each cell (2 seeds per cell) and each tray was covered with a thin layer of dry grass. The trays were watered twice a day using a watering can with relatively fine nozzles. The experiment was laid out in a Complete Randomized Design (CRD) with three replications using eight seedlings per plot. The roots of 45 days old pepper seedlings were pruned gently 2 hrs prior to inoculation, and trays that contained these seedlings were placed in water-filled plastic boxes to saturate the roots. The fungus inoculums were prepared by considering 500 conidia per ml resulting in a final concentration of 10,000 conidia per tray. Root inoculation was performed following procedures described by Black et al. [6]. Two hundred ml of spore suspension was prepared in which twenty-ml of spore suspension was poured into each box containing seedling tray. Then immediately placed in a box and incubated for 12 hrs in the green house. Then the seedlings were taken from the box and kept in the green house to evaluate the reaction of accessions with the control seedlings treated only with distilled water.
Determination of capsaicin and oleoresin content
Mature dry fruits were harvested and dried at an ambient condition for 3-5 days under shade. Then ground to a fine powder with mortar and pestle. Ten and four gram of pepper powder for capsaicin and oleoresin respectively was extracted in 100 ml of 96% methanol, in 250 ml Erlenmeyer flask covered with black markings tape and put a funnel into the neck of the flask. The extraction was performed in 5 hours on temperature of 78°C. Then filtered before use and kept in brown glass bottle. Then 2 or 3 ml of filtrate were transferred into 100 ml volumetric flask and the flask was immediately filled up to volume with methanol. Then absorption at the maximum 460 nm was read against pure solvent in a Spectrophotometer. The apparatus capsaicin content in Scoville heat unit (SHU) was calculated in (mg/g) of powder) based the following formula: SHU=Absorbance × IF × 1.61 × 107
For determination of oleoresins content, the absorption of samples was read at the maximum 460 nm against pure solvent in a Spectrophotometer. The oleoresins content in (mg/g of powder) calculated based the following formula:
ICU=Absorbance × IF × 1.64/weight of sample
ASTA=ICU/weight of sample
Where, IF is the deviation factor of the spectrophotometer (0.60).
Data collection procedures and analysis
The plants were monitored every two weeks for disease symptoms of (leaf yellowing and chlorosis, shedding from the base, stunting and total plants wilt and death). Then the diseases developments were evaluated based on different criteria.
Disease severity rating scale was rated based on a 5-point scale as per AVRDC pepper disease compendium [6]. Such as 0=no visible infection, vigorous, healthy=R; 1=slight leaf yellowing=MR; 2=old lower leaf yellowing and plant wilting=MS; 3=lower leaves shading and stunted plants=S; 4=all the leaves shedding and the stem collapsed and few plants death and HS; 5=Total plant death
Disease incidence (DI) percentage, was calculated as the proportion of infected plants per plot and expressed as a percentage [7].
Disease Incidence (%)=(Number of infected plants per row/ Total number of plants per variety) × 100
The incidence was calculated and grading was done on the basis of wilt incidence (%) as:
• Highly resistant (HR): 0% wilting.
• Resistant (R): 1-10% wilting.
• Moderately Resistant (MR): 11-20% wilting.
• Moderately Susceptible (MS): 21-30% wilting.
• Susceptible (S): 31-50% wilting.
• Highly susceptible: >50% wilting.
The disease severity index (DSI) was calculated according to the formula given by Galanihe et al. [8].
DSI (%) = Σ{(P × Q)}/(M × N)] × 100
Where P=severity score, Q=number of infected plants having the same score; M=Total number of plants observed, N=Maximum rating scale number. The computed Diseases Severity Index (DSI %) of each material were categorized as: Highly resistant (HR)=0%; Resistant (R)=DSI% <10%; Moderately resistant (MR)=10-20%; MS=moderately susceptible, 21-40%; Susceptible (S)=41-60%; highly susceptible (HS) >60%. Data on wilted plants were recorded every two weeks throughout the duration of the experiment for 30 days. Disease incidence and severity (DSI) were transformed using the ARCSIN function so as to normalize variability. The transformed data were subjected to analysis of variance using all analysis of variance for quantitative characters using (SAS 9.2 software package, 2004). A probability value of LSD <0.05 was used as a bench mark to examine variations among the accessions.
Results
Isolation and identification of Fusarium
Typical characteristics of F. oxsporium isolates were recovered from almost all plants that showed typical symptoms of Fusarium wilt. The isolates were characterized based on morphological characteristics ranging from cottony white to pink pigmentation on PDA. The majority of isolates were characterized as white color mycelium and few isolates showed other pigmentations such as violet, yellow, grey and color intensity that varies from time to time depending upon the period of incubation.
Reaction of pepper genotypes to Fusarium wilt diseases
Wilt incidence percent: The analysis of variance for Fusarium wilt incidence, severity index and scale showed significant (P<0.05) differences among the genotypes considered in the study. The overall disease incidence ranged from 8 to 100%, with the lowest values recorded for pepper genotypes Acc-39 and AVRDC genotypes, PBC-731, and the highest value registered for Acc-15.
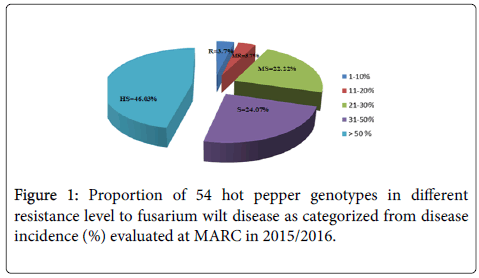
Higher disease incidence was recorded from the locally collected genotypes than exotic ones. Accordingly, 48% of the local collections showed greater than 70% DI out of which, nine (18%) genotypes (Acc-7, Acc-8, Acc-9, Acc-13, Acc-14, Acc-15, Acc-18, Acc-23 and Acc-24) exhibited high wilt incidence (>90%) level. From the AVRDC genotypes, PBC-731 was the least infected with Fusarium wilt as the disease incidence level was only 8%, followed by genotypes Melka Zala and Melka Awaze with 14 and 20% incidences, respectively. However, from the local genotypes, Acc-39 was not severely infected in the green house and showed almost equal levels of response to Fusarium as that of the AVRDC genotype PBC-731, which showed <10% incidence level than all the other local and exotic genotypes. This was followed by eight local genotypes (Acc-4, Acc-5, Acc-12, Acc-32, Acc-33, Acc-40, Acc-46 and Acc-49) with incidence levels of <25%, as compared to all the others that gave incidence levels of >30%. Based on the DI percentage, only PBC-731 and Acc-39 showed <10% DI and could be considered to be resistant to Fusarium wilt (Table 1).
One local accession, Acc-46 and two AVRDC genotypes Melka Awaze and Melka Zala which showed 14 to 20% DI could be considered as moderately resistant. Eleven genotypes (Acc-4, Acc-5, Acc-6, Acc-12, Acc-32, Acc-33, Acc-36, Acc-40, Acc-49, Melaka Awaze, Melka Zala with DI of 21- 30% considered as moderately susceptible. Meanwhile, 13 genotypes (Acc-19, Acc-25, Acc-31, Acc-34, Acc-35, Acc-37, Acc-38, Acc-41, Acc-42, Acc-43, Acc-44, Acc-45 and Acc-47) showed DI percent from 31-50% considered as susceptible and the remaining 25 local genotypes with DI >50% were found to be highly susceptible (Table 1).
Considering the two extremes the lowest incidence among local accession was recorded on Acc-39 (9%), followed by Acc-46 (20%), while the highest was recorded on genotypes Acc-15 and Acc-14 with high incidences level of 100% and 96%, respectively. Therefore, from the local genotypes Acc-4, Acc-5, Acc-12, Acc-32, Acc-33, Acc-40, Acc-46 and Acc-49 and introduced genotypes Melka Awaza, Melka Zala and PBC-731 had the least wilt incidences in that order. The overall mean percent DI of (56%) was recorded from local materials compared to the introduced genotypes (19%). There was no accession and genotype without diseases symptom, but high variability existed within and between genotypes (Figure 1).
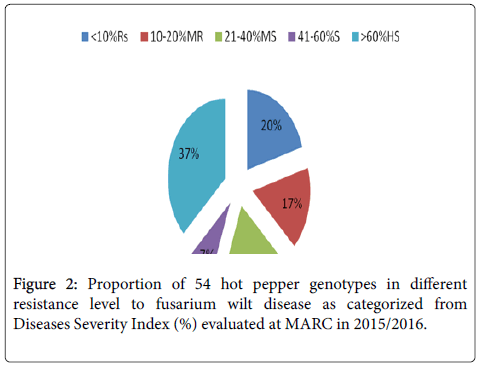
Disease severity index: Disease severity indices varied significantly (P<0.05) among genotype and local genotypes, based on severity index rating genotypes were categorized into 5; where, 11 genotypes (Acc-4, Acc-5, Acc-12, Acc-32, Acc-33, Acc-39, Acc-46, Acc-49, Melaka Awaze, Melka Zala and PBC-731) were found resistant (<10% DSI) with the AVRDC lines PBC-731 and local accession, Acc-39, being the highest resistant with severity index of 2%, whereas nine genotypes (Acc-6, Acc-19, Acc-36, Acc-37, Acc-38, Acc-40, Acc-49, Melaka Awaze and Melka Zala) moderately resistant (10-20% SI) and 10 genotypes (Acc-25, Acc-31, Acc-34, Acc-35, Acc-41, Acc-42, Acc-43, Acc-44, Acc-45, and Acc-47) moderately susceptible (21-40% SI), four genotypes (Acc-3, Acc-20, Acc-27, and Acc-30) were found susceptible (41-60%) and the rest 20 genotypes were highly susceptible >60% (Acc-1, Acc-2, Acc-7, Acc-8, Acc-9, Acc-10, Acc-13, Acc-14, Acc-15, Acc-16, Acc-17, Acc-18, Acc-21, Acc-22, Acc-23, Acc-24, Acc-26, Acc-28, Acc-29, and Acc-48) (Figure 2). However, 25 genotypes had severity index scores above the overall mean of (45%) with Acc-15 (100%) and Acc-14 (96%) found to be severely infected genotypes followed by genotypes Acc-7, Acc-8, Acc-9, Acc-13, Acc-14, Acc-15, Acc-18, Acc-23, Acc-24. In general, 24 genotypes and five exotic genotypes had severity indices less than the overall mean of 45%, with four genotypes, PBC-731 and Acc-39, Melka Zala and Acc-46, showing the least severity index. Nine local genotypes (Acc-39, Acc-46, Acc-32, Acc-33, Acc-12, Acc-4, Acc-5, Acc-46, Acc-49) and all AVRDC genotypes registered the lowest severity indices of <10%.
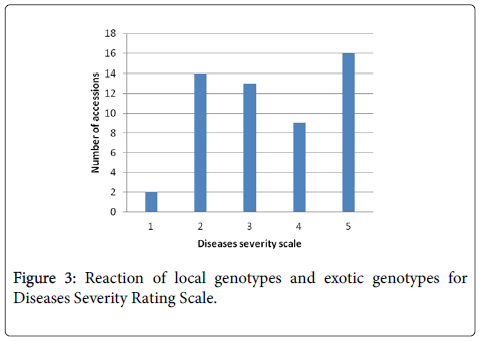
Diseases severity scale: Based on disease severity scale (1-5) 54 genotypes categorized in to resistant (1-2), moderate (3) and susceptible (4-5). Thus, Acc-39 and AVRDC line PBC-731 were consistently resistant as their severity score was 1 and with a healthy plant survival percent ranging from 80 to 95%. Moreover, 14 genotypes responded within the resistant severity rating scale range of 2. Sixteen of the 54 genotypes were highly susceptible, with rating scale of 5 while the rest 13 and nine genotypes had intermediate rating scale of 3 and 4, respectively. The susceptible control Marko Fana and most other local genotypes had an average rating scale of 5 with <20% of surviving plants. Thus, from 54 genotypes, 14 genotypes (26%) had a mean wilt severity scale of 2 and were considered resistant, whereas 13 (24%) genotypes had severity scale of 3 and 9 genotypes (17%) with the rating scale of 4, whereas 16 genotypes (30%) showed rating scale of 5 (Figure 3).
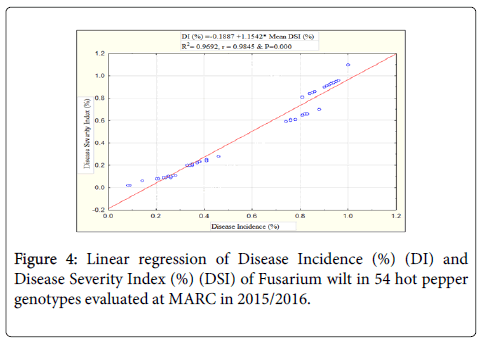
Relationship between disease incidence and severity index of Fusarium wilt: Results from linear regression analysis between disease incidence (%) and disease severity index (%) of Fusarium wilt in 54 hot pepper genotypes showed that the presence of highly significant (P<0.000) linear relationship between the two disease parameters. The coefficient of determination (R2) was 0.9692 or 96.92% with near to perfect correlation (r=0.9845) (Figure 4). This indicated that both methods are equally important and should be used in evaluation for resistance to wilt disease.
Capsaicin content: Among the whole genotypes compared, the capsaicin content ranged from 0.16-0.55% and 26,372 to 88,775 SHU (Table 2). While the minimum (26,372) SHU was recorded for Acc-32 and the maximum (88,775) SHU for Acc-24. The percent capsaicin content of 13 genotypes was less than 0.25%. Based on SHU, Weiss [9] classified pepper cultivars into five pungency levels, non-pungent (0-700 SHU), mildly pungent (700-3,000 SHU), moderately pungent (3,000-25,000 SHU), highly pungent (25,000-70,000 SHU) and very highly pungent (>80,000 SHU). Accordingly, all of the Ethiopian pepper genotypes considered in the current study could be categorized only into highly pungent and very highly pungent types. Having SHU of >70, 000, only five of the genotypes (Acc-24, Acc-39, Acc-4, Acc-7 and Acc-9) could be categorized under very highly pungent, whereas the remaining 44 genotypes with 25,000-70,000 SHU could be categorized under highly pungent types.
| Accessions | Fruit weight | Powder weight | F:P | International color unit | ASTA value | Percent of capsaicin | Scoville heat unit |
|---|---|---|---|---|---|---|---|
| Acc-1 | 3 | 2 | 1.4 | 59368 | 148 | 0.23 | 37288 |
| Acc-2 | 3 | 1.9 | 1.6 | 91184 | 228 | 0.4 | 63949 |
| Acc-3 | 3 | 1.8 | 1.7 | 70192 | 175 | 0.27 | 43663 |
| Acc-4 | 2.9 | 1.9 | 1.5 | 118408 | 296 | 0.46 | 73319 |
| Acc-5 | 3 | 1.7 | 1.7 | 110044 | 275 | 0.35 | 55642 |
| Acc-6 | 2.4 | 1.4 | 1.7 | 99548 | 250 | 0.4 | 64239 |
| Acc-7 | 2.6 | 1.5 | 1.7 | 101024 | 253 | 0.45 | 71967 |
| Acc-8 | 2.7 | 1.7 | 1.6 | 55268 | 138 | 0.37 | 58926 |
| Acc-9 | 3.2 | 2.1 | 1.5 | 98564 | 246 | 0.46 | 74479 |
| Acc-10 | 2.8 | 1.7 | 1.7 | 61172 | 153 | 0.21 | 33327 |
| Acc-11 | 3.1 | 2 | 1.6 | 73308 | 183 | 0.34 | 54193 |
| Acc-12 | 2.9 | 1.7 | 1.6 | 60352 | 151 | 0.29 | 47141 |
| Acc-13 | 3 | 1.9 | 1.6 | 65108 | 163 | 0.22 | 35645 |
| Acc-14 | 2.4 | 1.4 | 1.7 | 39688 | 99 | 0.22 | 34873 |
| Acc-15 | 2.7 | 1.6 | 1.7 | 72816 | 182 | 0.31 | 49459 |
| Acc-16 | 2.8 | 1.8 | 1.6 | 71996 | 180 | 0.34 | 55062 |
| Acc-17 | 2.6 | 1.2 | 2.1 | 32800 | 82 | 0.35 | 56221 |
| Acc-18 | 3 | 1.7 | 1.7 | 39032 | 98 | 0.23 | 37191 |
| Acc-19 | 2.9 | 2.2 | 1.3 | 73472 | 184 | 0.33 | 52550 |
| Acc-20 | 3.5 | 2.3 | 1.5 | 72652 | 182 | 0.33 | 52744 |
| Acc-21 | 3.1 | 2 | 1.5 | 82820 | 207 | 0.35 | 56608 |
| Acc-22 | 2.9 | 1.9 | 1.6 | 44772 | 112 | 0.2 | 32651 |
| Acc-23 | 3.4 | 2.2 | 1.6 | 40508 | 101 | 0.25 | 40379 |
| Acc-24 | 3.2 | 1.9 | 1.7 | 111848 | 280 | 0.55 | 88775 |
| Acc-25 | 2.8 | 1.8 | 1.6 | 93644 | 234 | 0.3 | 47914 |
| Acc-26 | 3.2 | 2.5 | 1.3 | 43624 | 109 | 0.26 | 41055 |
| Acc-27 | 2.9 | 1.8 | 1.6 | 85444 | 214 | 0.22 | 35066 |
| Acc-28 | 2.8 | 1.7 | 1.6 | 33128 | 83 | 0.22 | 35645 |
| Acc-29 | 2.4 | 1.6 | 1.4 | 76588 | 161 | 0.38 | 61148 |
| Acc-30 | 3.4 | 2.5 | 1.4 | 52972 | 132 | 0.3 | 47817 |
| Acc-31 | 3.2 | 2.2 | 1.5 | 101516 | 254 | 0.42 | 67330 |
| Acc-32 | 3.1 | 2.3 | 1.4 | 41984 | 105 | 0.16 | 26372 |
| Acc-33 | 3 | 2.3 | 1.3 | 117752 | 294 | 0.38 | 60568 |
| Acc-34 | 3.1 | 1.9 | 1.7 | 110864 | 277 | 0.38 | 61824 |
| Acc-35 | 2.9 | 1.7 | 1.7 | 66092 | 165 | 0.28 | 44533 |
| Acc-36 | 3.3 | 2.2 | 1.5 | 81344 | 203 | 0.36 | 57863 |
| Acc-37 | 2.7 | 1.7 | 1.6 | 55104 | 138 | 0.19 | 30719 |
| Acc-38 | 3.2 | 2 | 1.6 | 71012 | 178 | 0.26 | 41635 |
| Acc-39 | 2.9 | 2 | 1.5 | 118408 | 296 | 0.54 | 87423 |
| Acc-40 | 3.3 | 2 | 1.6 | 87904 | 220 | 0.36 | 57960 |
| Acc-41 | 3.2 | 2.1 | 1.5 | 58876 | 147 | 0.25 | 40186 |
| Acc-42 | 2.8 | 1.7 | 1.6 | 39852 | 100 | 0.22 | 36128 |
| Acc-43 | 2.9 | 1.7 | 1.8 | 33292 | 83 | 0.3 | 47914 |
| Acc-44 | 3 | 1.8 | 1.7 | 38376 | 96 | 0.29 | 46658 |
| Acc-45 | 2.8 | 1.8 | 1.5 | 53956 | 135 | 0.29 | 47044 |
| Acc-46 | 2.5 | 1.4 | 1.7 | 40344 | 101 | 0.2 | 32941 |
| Acc-47 | 2.8 | 1.4 | 2 | 56744 | 142 | 0.33 | 52357 |
| Acc-48 | 2.7 | 1.6 | 1.6 | 39688 | 99 | 0.23 | 37771 |
| Acc-49 | 2.6 | 1.2 | 2.2 | 40180 | 100 | 0.25 | 40765 |
| Mean | 2.9 | 1.8 | 1.6 | 69074 | 172 | 0.32 | 50141 |
Table 2: Capsaicin and oleoresin content of 49 local hot pepper genotypes evaluate at MARC, 2014/2015.
Oleoresin content: The oleoresin content in international color unit (ICU) and ASTA value ranged from 32,800-118408 ICU and 82-296 ASTA value with overall means of 69,074 ICU and 172 ASTA value (Table 2). Accordingly, genotypes Acc-39 and Acc-4 had maximum international color unit of 118,408 ICU and 296 ASTA value, followed by Acc-24, Acc-33, and, Acc-5 and Acc-34.
Based on the classification method of ASTA [10], all the 49 genotypes were grouped into four categories. Thus, seven genotypes gave poor quality of <100 ASTA value (Acc-28, Acc-43, Acc-44, Acc-48, Acc-14, Acc-18, and Acc-17). Eight genotypes (Acc-2, Acc-6, Acc-9, Acc-21, Acc-25, Acc-27, Acc-40 and Acc-36) gave very good quality color within the range of 200 - 250 ASTA value. However, the highest quality colors of greater than 250 ASTA value was obtained from ten genotypes (Acc- 4, Acc-5, Acc-6, Acc-7, Acc-24, Acc-27, Acc-33, Acc-34, Acc-39, and Acc-31), and 24 genotypes gave intermediate quality colors of within 101-200 ASTA value.
Discussion
The study was undertaken to identify wilt resistant local collections and exotic materials of hot pepper at MARC green house. Significant differences among genotypes were observed and some local materials equally performed with the exotic genotypes in wilt disease incidences and severity indices. From the total of 54 hot pepper genotypes screened, only two genotypes PBC 731 and Acc-39 were found resistant <10 DI, followed by moderately resistant Acc-46, 52 and 53 with (11-20% DI), whereas, 25 local accessions were rated highly susceptible (>50% wilting).
Similarly, wide variation in resistance scores rating scale was observed both between accessions as well as between plants within genotypes/accessions which is due to their genetic differences. The present study is in agreement with the reports of Mamta et al. [11] which indicated great disease incidence variation within the range of 0 to 78.7% and from thirty varieties screened, only two varieties were found 100% resistant, against wilt of chilli.
Evaluation of resistance was measured on the basis of total number of dead to live plants of each genotype, after inoculation. Grouping of genotypes on the basis of their reaction to Fusarium wilt diseases facilitated the identification of resistant and susceptible genotypes based on disease incidence and severity index percent and severity rating scale. Thus, based on incidence percent from the total of 54 hot pepper genotypes screened, 25 local genotypes were found highly susceptible with DI (>50% wilting), thirteen were moderately susceptible (31-50%), 11 moderately resistant (21-30% wilting), three resistant (11-20% wilting) and two genotypes were rated highly resistant (1-10% wilting).
The practical method to control Fusarium wilt is the use of resistant varieties but there was no exhaustive screening work has been done so far in local pepper breeding programs. However, this is the first widescale screening report in large number of local and exotic hot pepper accessions for resistance to Fusarium wilt throughout the country. Moreover, so far the screening methods have traditionally relied on field trials to differentiate resistance levels and hence this inoculation technique, when used in the green house as a screening method, reflect the level of resistance that may be expressed under field conditions.
The variable inoculums densities and uncontrolled environmental conditions may severely affect the outcome of field trials that lead to variable results [12]. However, this screening method demonstrated the availability of genotypes that are potential sources of resistance to Ethiopian isolates of F. oxisporium which could be incorporated into commercially acceptable pepper cultivars. From the result of this study majority of the local materials showed susceptibility response to wilt diseases and only 10 local genotypes Acc-4, 5, 6, 12, 40, 39, 32, 33, 46 and 49, and three exotic genotypes 54, 50, 53 and 51 scored better in disease resistance. However, these local genotypes that scored better disease resistance tend to produce high capsaicin and oleoresin yield. Moreover, six accessions (Acc-4, Acc-5, Acc-6, Acc-12, Acc-36 and Acc-40) which scored moderate resistance also gave higher dry pod yields with good quality fruits. Though all AVRDC genotypes give high green pod yield and scored better disease resistance but their pod characters are not acceptable for dry pod production and hence could be considered as potential parents for improving commercially acceptable local pepper cultivars in the future crossing program.
Color analysis, based on ASTA method [10] is the most widely used to measure the commercial quality of cultivars and quantifies the total carotenoid content which has been used as a parameter of quality in selection, breeding, and cultivar characterization work [13]. High quality and expensive pepper powders usually show minimum ASTA values of 100 and as a rule, when the ASTA color value is high the oleoresin is more expensive [14]. Thus, according to ASTA [10] classification theses 49 hot pepper Ethiopian accessions only, 11 accessions (Acc-20, Acc-28, Acc-43, Acc-44, Acc-42, Acc-48, Acc-14, acc-18, and Acc-17), gave ASTA value lower than 100, whereas, all the rest 38 accessions are within the acceptable range of (100-296 ASTA) value. However high color value of greater than 150 ASTA value are required to compete in the world market and thus accessions of Acc-4, Acc-5, Acc-6, Acc-7, Acc-24, Acc-27, Acc-33, Acc-34, Acc-39, and Acc-31 gave high color quality of greater than 250 ASTA unit. However, Acc-17, 28 and 43 gave the lowest values, in color content as well as in ASTA value as opposed to the highest values found in Acc-39, Acc-24, Acc-9, Acc-5, Acc-7 and Acc-4.
The color values recorded in this study are within the ranges reported by Kim et al., Topuz and Ozdemir and Howard et al. [15-17] for C. annum cultivars. Similar to this study, Zaki et al. [18] reported maximum color value of (116160 cu) and extractable color of 294.38 ASTA value from pepper. Moreover, Lannes et al. [19] showed a variation of extractable color from 173-213 ASTA value, Dhali et al. [20] reported the values within the range of this study 82-190 and 85-1780 ASTA respectively.
Based on heat unit all the peppers accessions classified as highly pungent 25-70000 SHU and very highly pungent as the Scoville Heat Unit (SHU) values exceed 70,000. However, two accessions with high capsaicin and oleoresin content “Acc-24 and 39” can serve as potential sources of gene for both capsaicin production and oleoresin production. In line with this study, Kumar et al. [21] reported a range of capsaicin 0.33-0.49% percent.
However, as opposed to the current study, Deepa et al. [22] reported the highest range of 12.04-17.06% capsaicin content. The two accessions which gave the highest color value Acc-24 and 39 also showed relatively higher capsaicin content and hence according to Dhalli and Hundai [20] further breeding program should be under taken in order to lower the capsaicin content of these high color varieties. As the above analysis indicated, all the accessions which gave high oleoresin were with the exception of Acc-24 and 39, are within the preferable pungency limit of (0.2-0.5%) and could directly be recommended for high oleoresin production content. In this study these local accessions express a wide range of fruit quality characteristics, in which all Assossa and West Harareghe and Gojam accessions had medium fruit size and pungency with low oleoresin content, whereas most accessions, from Southern region have relatively large pod size, high pungency and oleoresin yield and showed good levels of resistance. However, from this study there are, local accessions that showed high variability in their resistance to wilt, and quality traits and the best resistant ones could be a primary candidate to produce breeding lines from a commercial point of view for future crossing program [23-28].
Conclusion
Significant differences were observed among genotypes, with respect to severity index. When genotypes were partitioned with respect to their places of origin, local genotypes were more severely infected and exhibited a relatively higher overall mean severity index of 50% than exotic types (8%). Grouping of the 54 tested genotypes based on the severity index (SI) showed that 11 genotypes were found highly resistant (<10% SI), nine were moderately resistant (10-20%), 10 genotypes were moderately susceptible (21-40% SI), four were susceptible (41-60% SI), while all the rest 20 genotypes were highly susceptible (>60% SI). In general, from the 54 genotypes tested in the green house, potential materials were identified from both local collections and exotic materials. Acc-39 (local) and PBC-731 (exotic) were found to be the most resistant genotypes. Percent capsaicin content ranged from 0.16%-0.55%, whereas the capsaicin content in heat unit (ASHU) varied from 26,372 to 88,775 SHU The oleoresin content in international color unit (ICU) and ASTA value were ranged from 32840-118408 color unit and 82-296 ASTA value with an overall mean value of 69704 ICU and 172 ASTA. Based on the classification of ASTA (1999) all the 49 genotypes were grouped into four categories; low extractable color quality (7 genotypes), medium (24 genotypes), very good color quality (8 accessions) and highest color quality (10 genotypes). In this study, hot pepper genotypes identified with desirable capsaicin and oleoresin content as well as Fusarium wilt resistance could be used as source of gene in future hybridization program for improvement of the crop.
References
- Girma T, Lidet S, Shimeles A, Waga M, Shiferaw M, et al. (2011) Pepper production, and marketing. Amaharic Manual, SOS, Sahil, Ethiopia.
- Shimeles A (2015) Overview of pepper breeding research achievements and challenges in Ethiopia. African Journal of Agricultural Research.
- Shimeles A, Berhanu B, Bekele K (2007) Survey report on current pepper production constraints in major pepper growing areas of Ethiopia. EIAR, 2007, Addis Abeba, Ethiopia.
- Oelke LM, Bosland PW, Steiner R (2003) Differentiation of race specific resistance to Phytophthora root rot and foliar blight in Capsicum annuum. Journal of the American Society for Horticultural Science 128: 213-218.
- Nelson PE, Toussoun TA, Marasas WFO (1983) Fusarium species: an illustrated manual for identification.
- Black LL, Green SK, Hartman GL, Poulos M (1993)Â Pepper diseases: a field guide. CTA.
- Bayoumi TY, El-Bramawy MAS (2007) Genetic analyses of some quantitative characters and Fusarium wilt disease resistance in sesame. In African Crop Science Conference Proceedings 8: 2198-2204.
- Galanihe LD, Priyantha MGDL, Yapa DR, Bandara HMS, Ranasinghe JADAR (2004) Insect pest and disease incidences of exotic hybrids chilli pepper varieties grown in the low country dry zone of Sri Lanka. Annals of Sri Lanka 6: 99-106.
- Weiss EA (2002) Spice Crops. CABI Publishing International: New York, NY, USA, p: 411.
- American Spice Trade Association (1999) ASTA cleanliness specifications for spices, seeds, and herbs. Washington, DC.
- Joshi M, Srivastava R, Sharma AK, Prakash A (2015) Screening of resistant varieties and antagonistic Fusarium oxysporum for biocontrol of Fusarium wilt of chilli. Journal of Plant Pathology & Microbiology.
- Fokunang CN, Ikotun T, Dixon AGO, Akem CN (2000) Field reaction of cassava genotypes to anthracnose, bacterial blight, cassava mosaic disease and their effects on yield. African Crop Science Journal 8: 179-186.
- Conforti F, Statti GA, Menichini F (2007) Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chemistry 102: 1096-1104.
- Pérez-Gálvez A, MÃnguez-Mosquera MI, Garrido-Fernández J, Lozano-Ruiz MM, Montero de Espinosa V (2004) Correlación entre unidades asta-concentración carotenoide en pimentones. Predicción de la estabilidad del color durante el almacenamiento. Grasas Aceites 55: 213-218.
- Kim S, Park JB, Hwang IK (2002) Quality attributes of various varieties of Korean red pepper powders (Capsicum annuum L.) and color stability during sunlight exposure. Journal of Food Science 67: 2957-2961.
- Topuz A, Ozdemir F (2007) Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. Journal of Food Composition and Analysis 20: 596-602.
- Howard LR, Talcott ST, Brenes CH, Villalon B (2000) Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. Journal of Agricultural and Food Chemistry 48: 1713-1720.
- Zaki N, Hasib A, Hakmaoui A, Dehbi F, Ouatmane A (2013) Assessment of color, capsaicinoids, carotenoids and fatty acids composition of paprika produced from Moroccan pepper cultivars (Capsicum annuum L.). Assessment.
- Lannes SD, Finger FL, Schuelter AR, Casali VW (2007) Growth and quality of Brazilian accessions of Capsicum chinense fruits. Scientia Horticulturae 112: 266-270.
- Dhali RK, Hundai JS (2005) Gene action of yield and quality traits in chilli (Capsicum annuum L.). Indian Journal of Agricultural Research 39: 291-294.
- Kumar BK, Munshi AD, Joshi S, Kaur C (2003) Note on evaluation of chilli (Capsicum annuum L.) genotypes for biochemical constituents. Capsicum and Eggplant Newsletter 22: 41-42.
- Deepa N, Kaur C, George B, Singh B, Kapoor HC (2007) Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT-Food Science and Technology 40: 121-129.
- Gómez-Ladrón de Guevara R, Pardo-González JE, Varón-Castellanos R, Navarro-Albaladejo F (1996) Evolution of color during the ripening of selected varieties of paprika pepper (Capsicum annuum L.). Journal of Agricultural and Food Chemistry 44: 2049-2052.
- Deli J, Pfander H, Tóth G (2002) Investigation of carotenoid composition of paprika paste. Chromatographia 56: 177-179.
- Derera J, Tongoona P, Vivek BS, Laing MD (2008) Gene action controlling grain yield and secondary traits in southern African maize hybrids under drought and non-drought environments. Euphytica 162: 411-422.
- Pratt RC, Anderson RJ, Louie R, McMullen MD, Knoke JK (1994). Maize responses to a severe isolate of maize chlorotic dwarf virus. Crop Science 34: 635-641.
- Sanogo S (2003) Chile pepper and the threat of wilt diseases. Plant Health Progress.
Citation: Aklilu S, Ayana G, Abebie B, Abdissa T (2018) Screening for Resistance Sources in Local and Exotic Hot Pepper Genotypes to Fusarium Wilt (Fusarium oxysporium) and Associated Quality Traits in Ethiopia. Adv Crop Sci Tech 6: 367. DOI: 10.4172/2329-8863.1000367
Copyright: © 2018 Aklilu S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5764
- [From(publication date): 0-2018 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 4749
- PDF downloads: 1015