Screening for Circulating Tumour Cells Allows Early Detection of Cancer and Monitoring of Treatment Effectiveness: An Observational Study
Received: 08-Jun-2017 / Accepted Date: 09-Jul-2017 / Published Date: 15-Jul-2017 DOI: 10.4172/2472-0429.1000123
Abstract
Background: Circulating-Tumour-Cells (CTC) provide a blood biomarker for early carcinogenesis, cancer progression and treatment effectiveness. An increase in CTCs is associated with cancer progression, a CTC decrease with cancer containment or remission. Several technologies have been developed to identify CTC, including the validated Isolation-by-Size-of-Epithelial-Tumour (ISET, Rarecells) technology, combining blood filtration and microscopy using standard histo-pathological criteria.
Methods: This study compared CTC count to cancer status and cancer risk, by monitoring treatment effectiveness in cancer patients and by screening for CTC in asymptomatic patients with risk factors, including family history of cancer.
Results: Between Sept-2014 and Dec-2016 we undertook 600 CTC tests (542 patients), including 50% screening requests of patients without cancer diagnosis but with risk factors. CTC were detected in all cancer patients (n=277, 100%), and in half of the asymptomatic patients screened (50%, 132 out of 265 patients). Follow-up tests including scans were scheduled within 1-6 months of CTC tests. In up to 50% of male patients with normal PSA (prostatespecific- antigen) levels but detected CTC, PET scans using PSMA (Ga-68-prostate-specific-membrane-antigens) revealed increased uptake in the prostate, indicative of early prostate cancer. Other types of cancer, including early breast, ovarian, lung, or renal cancer were detected in a small number of asymptomatic men or women with a positive CTC count.
A subgroup of patients with detected CTC underwent interventions, including nutritional therapy with immunestimulating and anti-carcinogenic nutrients. CTC repeat tests were available in 10% of patients with detected CTC (40 out of 409 patients, n=98 CTC tests) to assess treatment effectiveness.
Conclusion: CTC screening provided a highly sensitive biomarker for the early detection of cancer, with higher CTC counts being associated with higher risk of malignancy. CTC monitoring over time indicated treatment effectiveness. Nutrients with anti-carcinogenic properties could reduce CTC count, and included curcumin, garlic, green tea, grape seed, modified-citrus-pectin, and medicinal mushroom-extract.
Keywords: Circulating tumour cells (CTC); Cancer screening; Early detection; Treatment effectiveness; Prostate cancer; Breast cancer; Integrative nutritional therapy
127654List of Abbreviations
CTC: Circulating Tumour Cells; EDTA: Ethylene-Diamine-Tetra-Acetic-acid, EpCAM: Epithelial Cell Adhesion Molecule; ISET: Isolation by Size of Epithelial Tumours; PET: Positron Emission Tomography; PSA: Prostate Specific Antigen; PSMA: Prostate Specific Membrane Antigen
Introduction
Circulating Tumour Cells (CTC) provide a biomarker for cancer prognosis and treatment effectiveness, whereby an increase in CTC count is associated with cancer progression, shorter progression free survival, and shorter overall survival compared to a decrease in CTC count [1,2]. In a group of 177 women with metastatic breast cancer, CTC count was directly related to disease progression and survival, whereby a CTC count of less than 0.7 CTC/ml (5 CTC in 7.5 ml of whole blood) had a longer progression free survival and overall survival compared to a CTC count of more than 0.7 CTC/ml (median progression-free survival 2.7 months versus 7.0 months, p<0.001), and median overall survival (10.1 months versus >18 months, p<0.001) [1]. Furthermore, the type of CTC cells, either single cells or CTC clusters, are a prognostic predictor of metastasizing potential and overall survival, with a hazard ratio of 14.5 (p<0.001) for ≥ 3-cell CTC clusters compared to no CTC [3].
Presence of CTC has also been associated with early carcinogenesis and risk of cancer [4]. In a study of cancer-free patients with chronic obstructive pulmonary disease (COPD), CTC were detected in 3% of the patients, who developed lung cancer within 1-4 years after CTC screening.
Several technologies have been developed to identify CTC, including the Isolation-by-Size-of-Epithelial-Tumour (ISET) technique (Rarecells, France) [5], which involves blood filtration, and analysis by microscopy using standard histo-pathological/ cyto-morphological criteria [6,7]. Blood is treated to lyse red blood cells, and remaining rare cells, including CTC and inflammatory (white blood) cells, are then enriched on a filter, stained and analysed by standard cytological microscopy. The ISET technology allows direct identification of CTC, independent of the presence of tumour markers [7].
For example, the Cellsearch or Maintrac technologies use Epithelial-Cell-Adhesion Molecule (EpCAM) markers to detect CTC [8,9]. The ISET technology enables CTC to be detected in all types of cancer, including small-cell type cancers and blood type tumour cells. All CTC are larger than the filter holes of 8 microns, including solid tumour cells of 11.7-23.8 microns, small-cell type cancers (e.g. small cell lung carcinoma of 7.2-10 microns) and blood type cancers (e.g. leukemia cells of 8.9-15.3 microns) [10,11]. Furthermore, blood type cancer cells don’t express the EpCAM markers, and in cancer cells undergoing normal morphogenetic processes, also known as epithelial mesenchymal transition (EMT), which can lead to loss or gain of tumour markers including EpCAM markers [12].
In addition, the ISET technology allows observation of morphological changes of atypical cells, and therefore allows distinction between CTC with malignant features (3-4 criteria out of 4 for malignancy), ‘CTC’ with uncertain malignant features (2-3 criteria), and benign circulating epithelial cells and cell clusters (CEC), as well as reactive inflammatory cells [13]. Changes of the normal morphology of cells into atypical cells are meaningful, and can be regarded as precursors in cancer development [14,15].
Because of the morphological changes of cells during carcinogenesis, the identification of (atypical cells or) CTC by ISET technology may be superior to other indirect tumour marker dependent methodologies. For example, the CTC count was more accurate on average with the ISET methodology compared to the Cell search methodology in metastatic prostate and lung cancer patients [16].
The ISET technology has been validated in several published studies, providing high specificity (1 CTC/ml), and high sensitivity (0 CTC/ml in 600 healthy donors) in cancer patients with various types of cancer including liver, lung, pancreatic cancer, soft-tissue sarcoma, and melanoma [4,14,17-24].
In this study we used the ISET technology for the detection of CTC in cancer patients and as screening tool in patients with higher risk of malignancy, e.g. family history, smoking, age (>50 years). Here we provide evidence that screening for CTC allows for early detection of cancer. We further summarise follow-up results by CTC repeat test of patients with detected CTC who undertook immune-stimulating nutritional therapy.
Materials and Methods
Aims
The study aimed to compare CTC count to cancer status and cancer risk, by monitoring treatment effectiveness in cancer patients and to screen for CTC in patients with a family history of cancer or clinical indication but no tumour mass.
Study design and patients
For this cohort study, patients were recruited from two medical clinics in Melbourne, Australia, the National Institute of Integrative Medicine ‘NIIM’ Clinic, and the Eng Medical Centre, between Sept 2014 and Dec 2016. CTC tests were performed to monitor treatment effectiveness in cancer patients, and for early detection screening in patients with an increased risk of cancer, including patients with a family history of cancer, smoking habits, long term oral contraceptive use or hormone replacement therapy in women, advanced age (>50 years) in men, or other medical indication.
The study was approved by the NHMRC-endorsed NIIM Human Research Ethics Committee. Participating patients provided written informed consent. No individual patient data is divulged in this article.
The authors confirm that all ongoing and related trials for this study are registered.
Circulating tumour cell (CTC) detection
In this study we used the Isolation-by-Size-of-Epithelial-Tumour (ISET) methodology (Rarecells, France), combining blood filtration and analysis by microscopy using standard histo-pathological criteria [13,17]. We followed standardised validated protocols described previously [6].
Briefly, the ISET method is a blood filtration-based approach, which enriches rare cells on a polycarbonate membrane with 8 micron holes. 10 mL of peripheral blood was collected in buffered EDTA, maintained at room temperature and processed within 2 hours of collection. Blood was then diluted 1:10 with buffer containing 0.175% saponin, 0.2% paraformaldehyde, 0.0372% EDTA, and 0.1% bovine serum albumin, shaken for 10 minutes at room temperature, and filtered with the ISET filtration blocks and device (Rarecells, France) [6].
The dried filter membrane was stained with May-Gruenwald- Giemsa for cytological analysis.
A trained and experienced cancer cytologist conducted the analysis using a Leica DMLB microscope with 63 × 10 magnification and standard histo-pathological criteria to identify the degree of malignancy.
Circulating malignant cells were defined by the presence of 4 of the following criteria: a) anisonucleosis (ratio >0.5), b) nuclei larger than 2-3 calibrated pore sizes (8 microns) of the membrane (i.e. >24 microns), c) irregular nuclear borders, d) high nuclei-cytoplasmic ratio, and/or e) presence of three-dimensional sheets. Cells displaying 1-3 criteria were defined as atypical cells with uncertain malignant potential. Circulating benign cells were characterized by the absence of these criteria [17].
Images of CTC and atypical cells were taken with a digital Leica EC3 camera, and all images were reviewed independently by a second cytologist and any discrepancies discussed. All images were added to a library of digital images for future cross-reference.
Patient follow-up
Patients with detected CTC were advised on follow-up tests including scans by the consulting doctor. Asymptomatic men with detected CTC, and Ki-67, PSA or androgen receptor (AR) expression [25], and PSA (prostate specific antigen) levels in the normal range, had a pelvic PET scan using Ga-68 PSMA (Gallium-68 Prostate- Specific-Membrane-Antigens) [26]. The Ga-PSMA-PET/CT scan is a highly sensitive test detecting lesions of ≥ 2.4 mm short axis diameter [27-29]. Asymptomatic women with detected CTC, and endocrine receptor (HER2) positive expression, had an MRI scan of the breast. Symptomatic patients had a scan relevant to the area of their symptoms. CTC testing was repeated within 3-6 months in patients with detected CTC.
All patients with or without cancer diagnosis but with detected CTC were advised about immune-stimulating therapy. Protocols included nutrients with evidence of anti-carcinogenic properties.
Analysis
Descriptive analysis was used to compare CTC count and cancer status or risk at baseline, the primary outcome and observational component of the study. Simple comparative analyses were conducted for the subgroups of patients who undertook a repeat CTC test after a variety of treatments as intervention.
Results
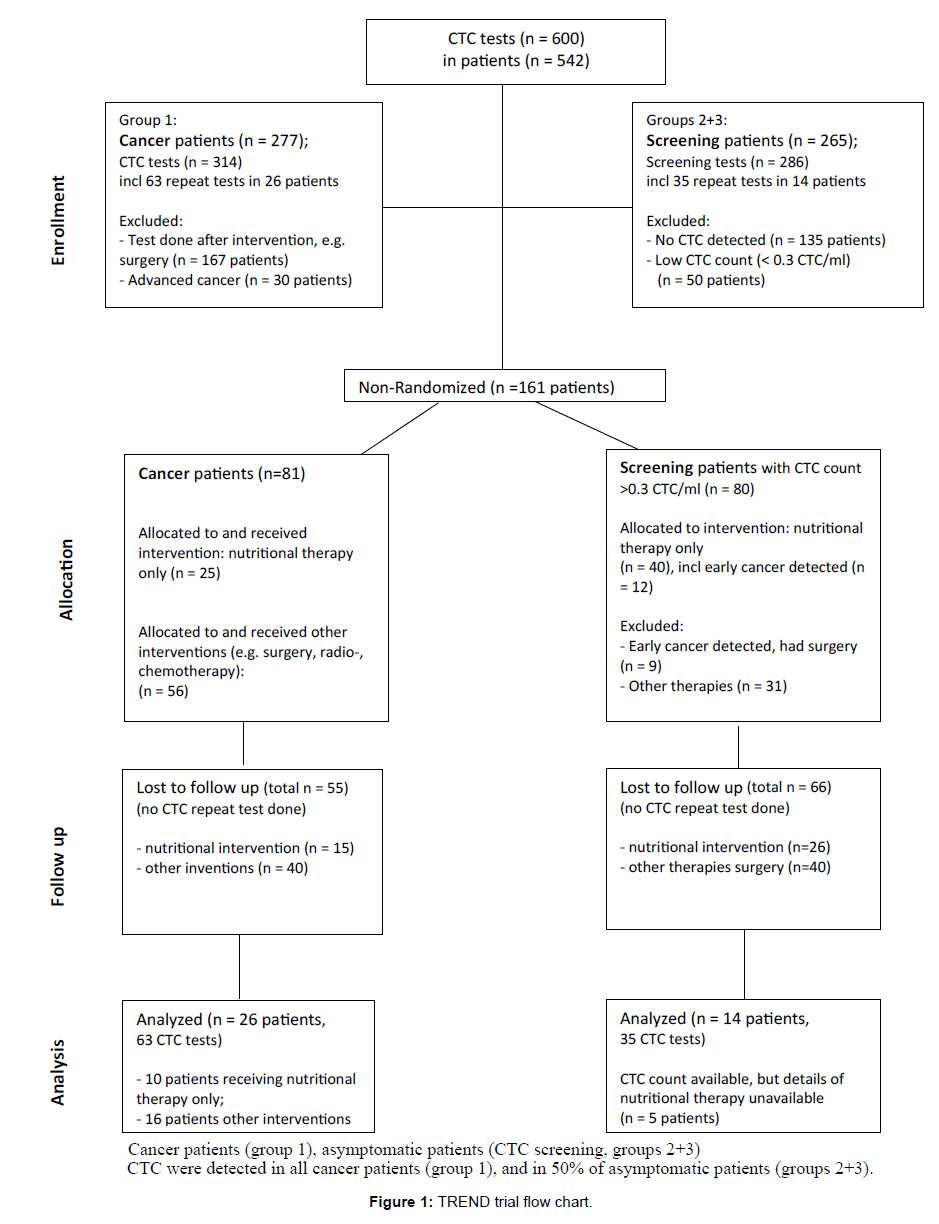
Between Sept-2014 and Dec-2016 we undertook 600 CTC tests in 542 patients, including 50% screening requests (n=286 tests) of patients without cancer diagnosis but with risk factors. CTC were detected in all cancer patients (n=277, 100%), and in half of the asymptomatic patients screened (50%, n=132 out of 265 patients). A subgroup of patients with detected CTC underwent interventions (n=161). CTC repeat tests were done for 10% of patients with detected CTC (40 out of 409 patients, n=98 CTC tests). Figure 1 summarises the trial flow.
Cancer Patients (group 1)
All patients with diagnosed cancer (group 1, Table 1) had a positive CTC count, detected with the ISET technology in patients with solid tumours and blood type tumours (e.g. non-Hodgkin’s lymphoma, multiple myeloma). The CTC count ranged from 0.2 CTC/ml to 65.4 CTC/ml including single CTC and CTC clusters. CTC baseline count usually correlated to patient’s cancer status and symptoms, with higher CTC counts presented in more advanced cases. Our data suggests a count of less than 0.3 CTC/ml to be usually associated with mild risk of malignancy, a count of 0.3-20 CTC/ml with moderate risk, and >20 CTC/ml with high risk of malignancy including metastasis, recurrence, and cancer progression. CTC count profile was similar in patients with other types of cancer. Follow-up is ongoing.
| CTC count1 | ||||
|---|---|---|---|---|
| Type of cancer | Number of patients | Stage 1 | Stage 2-3 | Stage 4 |
| <3 CTC/ml | 3-20 CTC/ml | >20 CTC/ml | ||
| N (% of type) | N (% of type) | N (% of type) | ||
| All | 277 | |||
| Breast | 81 | 52 (64) | 20 (25) | 9 (11) |
| Prostate | 69 | 54 (78) | 11(14) | 4 (5) |
| Colorectal, gastric | 37 | 26 (70) | 7 (19) | 4 (11) |
| Kidney, bladder | 19 | 11 | 6 | 2 |
| Blood type cancers: Lymphoma, NHL, HL, MCL, MM | 17 | 10 | 2 | 5 |
| Ovarian, endometrial, uterine, cervical | 15 | 10 | 4 | 1 |
| Lung | 6 | 2 | 1 | 3 |
| Melanoma | 9 | 9 | - | |
| Pancreatic | 3 | 2 | - | 1 |
| Thyroid | 5 | 5 | - | - |
| Other, e.g. tongue, brain, SCC | 16 | 9 | 6 | 1 |
1CTC baseline count, CTC repeat tests of same patient not included in this table.
Abbreviations: HL, Hodgkin’s lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; NHL, Non-Hodgkin’s lymphoma; SCC, squamous cell carcinoma
Table 1: CTC count by type of cancer (Group 1: Cancer patients).
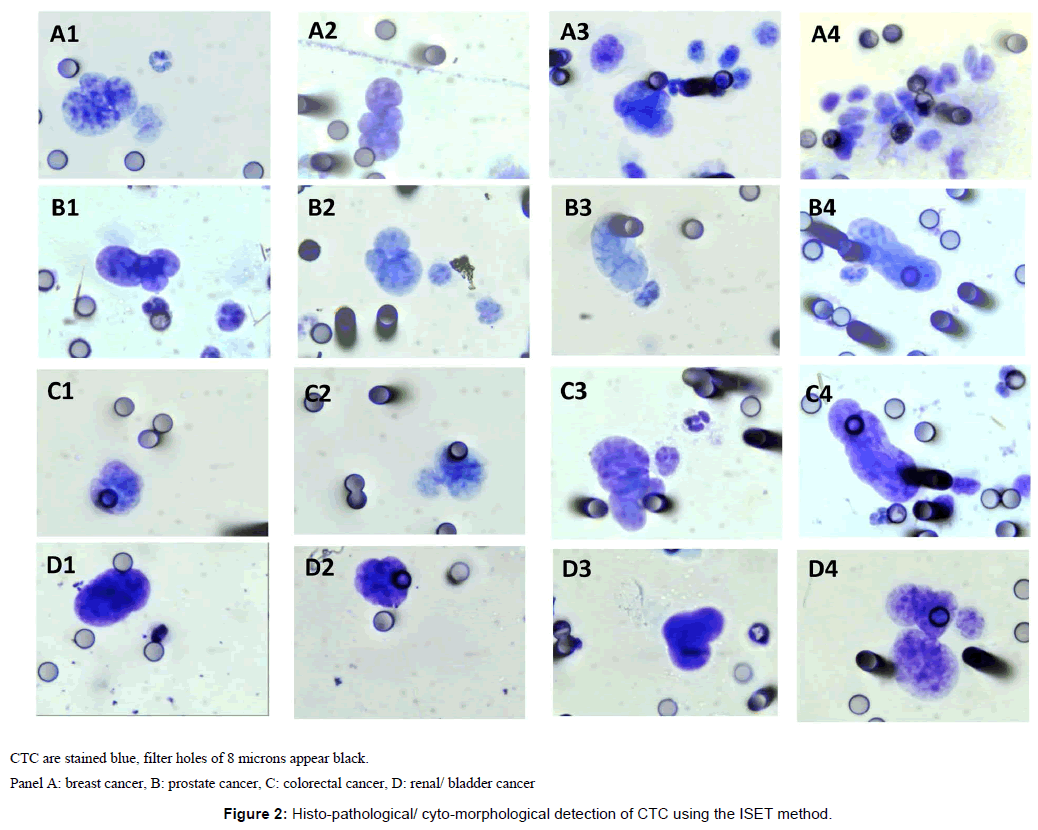
Figure 2 illustrates examples of CTC detected with the ISET method using cyto-morphological criteria.
To monitor treatment effectiveness, CTC testing was repeated 3-4 weeks after conclusion of a treatment cycle around 3 months in 10% of cancer patients (n=26). Treatment could include surgery, chemotherapy, radiotherapy, hyperthermia, and nutritional therapies. CTC count correlated to patient’s cancer status (Table 1), with an increase in CTC count over time indicating cancer progression or metastases, and a decrease in CTC count over time indicating cancer remission (Table 2).
| Patient ID, age | Cancer type | Test ID | CTC test time points (A-D) |
CTC count/ml | N months between CTC tests | Treatment details & comments |
|---|---|---|---|---|---|---|
| F1, 62 yrs | Colorectal | 292GL | A: Sep-15 | 0.4 | A: After surgery, radio, chemo | |
| 383GL | B: Jan-16 | 1.2 | 4 | |||
| 437GL | C: Mar-16 | 3.5 | 3 | C: Liver metastases detected | ||
| 595GL | D: Jul-16 | 1.9 | 4 | |||
| F2, 60 yrs | Colorectal | 338VD | A: Nov-15 | 0 | ||
| 405VD | B: Feb-16 | 0 | 3 | |||
| 592VD | C: Jul-16 | 21.1 | 5 | C: Lung metastases detected | ||
| F3, 41 yrs | Colorectal | 343NZ | A: Nov-15 | 2.0 | ||
| 609NZ | B: Jul-16 | 6.1 | 8 | B: Ongoing herbal therapy, details unknown | ||
| 782NZ | C: Dec-16 | 13.3 | 5 | |||
| F4, 33 yrs | Colorectal, sigmoid | 691GK | A: Oct-16 | 13.2 | ||
| 725GK | B: Nov-16 | 1.0 | 1 | B: After hyperthermia, IVC, IV-Curcumin | ||
| F5, 71 yrs | Breast | 171WS | A: May-15 | 0.6 | A: After surgery, radio | |
| 291WS | B: Sept-15 | 19.2 | 4 | B: Ongoing hormonal therapy, low Vit D level | ||
| 458WS | C: Apr-16 | 0.1 | 7 | C: After vitamin D, curcumin, relaxation | ||
| F6, 66 yrs | Breast | 296JWK | A: Sep-15 | 0.5 | A: After surgery | |
| 483JWK | B: Apr-16 | 2.5 | 7 | B: On chemo | ||
| F7, 65 yrs | Breast, bone, liver | 417SM | A: Feb-16 | 1.2 | A: Surgery 5 yrs ago | |
| 496SM | B:May-16 | 2.6 | 3 | B: Ongoing chemo | ||
| F8, 46 yrs | Breast | 255JB | A: Jul-15 | 0.1 | A: After surgery , chemo, radio a year earlier | |
| 497JB | B: May-16 | 6.6 | 10 | |||
| F9, 44 yrs | Breast | 153AB | A: May-15 | 2.6 | ||
| 390AB | B: Jan-16 | 0.7 | 8 | B: After surgery | ||
| F10, 42 yrs | Breast | 579DM | A: Jul-16 | 2.4 | ||
| 731DM | B: Nov-16 | 13.0 | 4 | B: After surgery, radio, chemo | ||
| F11, 63 yrs | Breast | 656DM | A: Aug-16 | 0.3 | ||
| 763DM | B: Nov-16 | 3.2 | 3 | B: After radio, chemo, supplements | ||
| M12, 35 yrs | Gastric | 460MM | A: Apr-16 | 4.7 | A: Ongoing chemo | |
| 690MM | B: Oct-16 | 0.1 | 6 | B: Chemo + immunotherapy drug | ||
| F13, 57 yrs | Melanoma | 27EN | A: Nov-14 | 7.2 | A: Melanoma detected | |
| 449EN | B: Mar-16 | 1.1 | 16 | B: After surgery | ||
| M14, 51 yrs | Lung | 64SW | A: Nov-15 | 1.2 | A: After CTC screening 4 mm tumour detected | |
| 427SW | B: Feb-16 | 0.9 | 3 | B: After surgery | ||
| F15, 48 yrs | Ovarian | 602GN | A: Jul-16 | 1.1 | ||
| 775GN | B: Dec-16 | 1.1 | 5 | B: Ongoing chemo | ||
| M16, 65 yrs | Prostate | 534NM | A: May-16 | 6.2 | ||
| 757NM | B: Nov-16 | 0.8 | 6 | B: Sonotherapy, supplements |
F, female; M, male
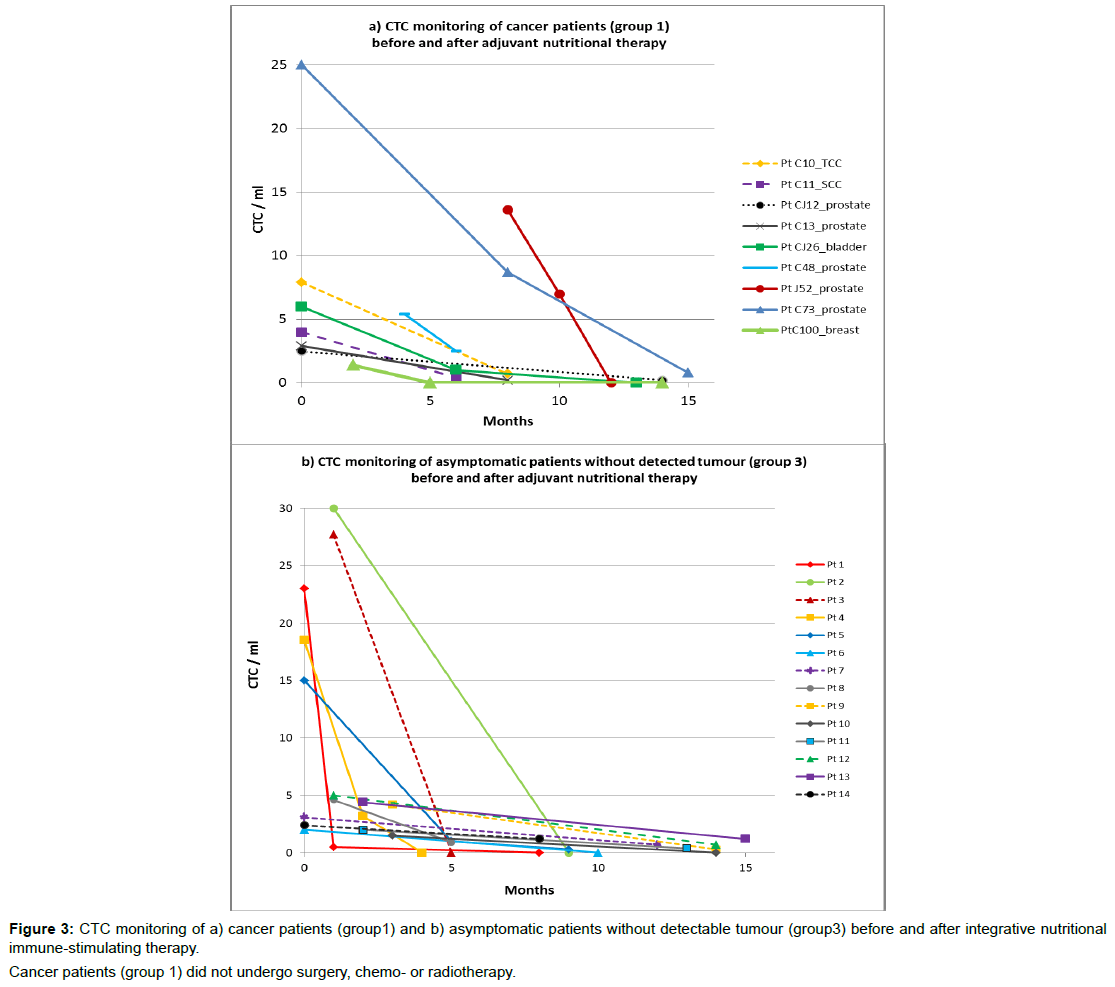
Figure3a illustrates CTC count over time available for 9 cancer patients with mild disease, who had not undergone surgery, chemo- or radiotherapy for a variety of reasons, but undertook evidence-based nutritional immune-stimulating and anti-carcinogenic therapy. In this group (group 1, n=9), CTC count ranged between 25 and 1.4 CTC/ml at baseline and decreased over time for all patients, down to no detected CTC for a third, after 3-12 months with adjuvant nutritional therapy.
Table 2: CTC repeat test results of cancer patients undergoing treatment incl. surgery, radio-, FDA-approved chemotherapy (group 1).
Table 2 summarises the CTC count over time in cancer patients who underwent treatment other than nutritional therapies. In this group of patients, surgery treatment generally resulted in a decrease of CTC, standard chemo- and radiotherapy treatment did not.
Early Detection Screening (groups 2 + 3)
CTC screening tests were undertaken in mostly asymptomatic patients without diagnosed cancer but with increased cancer risk, including family history of cancer or advanced age (>50 years). In CTC screening patients, baseline CTC count ranged from 0.2-50 CTC/ml (mean=16 CTC/ml). For those patients with detected CTC (group 2), follow-up tests including scans and repeat CTC tests were scheduled within 0.5-10 months (mean=3.5 months). Follow-up scans taken within 1-6 months revealed early cancerous lesions in about 20% of patients with detected CTC (Table 3).
| Patient ID, age | CTC test method | Date CTC test | CTC number/ml | Receptor expression (%) | PSA ug/L |
Date scan | N months between CTC and scan | Scan results/ Tumour detected | Results comments |
|---|---|---|---|---|---|---|---|---|---|
| F1, 37 yrs | Maintrac | Mar-15 | 2 | n/a | n/a | Apr-15 | 1 | Breast | MRI: 0.5 × 0.8 × 0.4 cm lesion right breast confirmed with FNA |
| F2, 37 yrs | ISET | May-15 | 0.8 | n/a | n/a | Jul-15 | 3 | Breast | CT scan: 0.7 × 0.6 × 0.7 cm tumour left breast, biopsy confirms neoplasm |
| F3, 44 yrs | ISET | May-16 | 101 | n/a | n/a | May-16 | 0.2 | Ovarian | Ultrasound: had ovarian cystectomy, ISET-CTC test after surgery: 0 CTC/ml |
| F4, 57 yrs | ISET | Nov-14 | 7.2 | n/a | n/a | Dec-14 | 1 | Melanoma | Biopsy, surgery |
| M5, 50 yrs | ISET | Dec-14 | 1.2 | n/a | n/a | Dec-14 | 0.5 | Lung | PET scan: 4mm right upper pulmonary tumour with radiotracer (FDG) uptake |
| M6, 54 yrs | ISET | Jun-16 | 7.2 | n/a | n/a | Jul-16 | 1 | Kidney | Nephrectomy in 12/16; CTC repeat after surgery 1/2017 1 CTC/ml |
| F7, 42 yrs | ISET | Jun-16 | 8.1 | n/a | n/a | Jun-16 | 0.5 | Lung, Mesothelioma | Symptoms at time of CTC test: Abdominal pain, pelvic fluid, bloating; Mesothelioma |
| M8a, 59 yrs | ISET; | Dec-14; | 2.6; | 1.44 | |||||
| M8b | Maintrac | Mar-15 | 33.5 | Ki67=19.3 | Jun-15 | 6 | Prostate | PSMA-PET: very mildly increased activity in the right side of the prostate | |
| M9, 55 yrs | Maintrac | Oct-15 | 0.5 | Ki67=78.9 AR=95.2; PSA=68.4 | 0.87 | Nov-15 | 1 | Prostate | PSMA-PET: low volume, low grade carcinoma |
| M10, 73 yrs | Maintrac | Sep-15 | 11 | Ki67=77.1; PSA=31.8 | 1.5 | Oct-15 | 1 | Prostate | PSMA-PET: Moderate uptake right lobe, low grade left lobe |
| M11, 58 yrs | Maintrac | Sep-15 | 10 | Ki67=85.7; PSA=50 | 4.4 | Oct-15 | 1 | Prostate | PSMA-PET: low volume low Gleason score prostatic malignancy; minimally increased uptake base of prostate right posterior, bilaterally mid-prostate anterior right, mid left, apex right |
| M12a, 71 yrs | ISET; | Feb-15; | 3.1; | 1.97 | |||||
| M12b | Maintrac | Oct-15 | 4.5 | Ki67=74.1 PSA=63.5 AR=51.8 | Oct-15 | 8 | Prostate | PSMA-PET: moderate grade prostate carcinoma, central aspect of the left lobe; linear low grade uptake in oesophagus most likely physiologic/salivary | |
| M13a, 66 yrs | ISET; | Sep-15; | 1.1; | 0.33 | |||||
| M13b | Maintrac | Oct-15 | 3.5 | Ki67=83.3; PSA=59; AR=71 | Oct-15 | 1 | Prostate | PSMA-PET: low grade prostate cancer | |
| M14a, 76 yrs | ISET; | Jan-15; | 4.9; | ||||||
| M14b | Maintrac | Sep-15 | 9 | Ki67=61.8; PSA=69 ; AR=65.2 | 2.19 | Oct-15 | 10 | Prostate | PSMA-PET: mild uptake in both lobes; likely to be true positive |
| M15, 65 yrs | Maintrac | Oct-15 | 5 | PSA=40 AR=40 | 2.74 | Nov-15 | 1 | Prostate | PSMA-PET: very low volume low grade prostate cancer |
| M16a, 53 yrs | ISET; | Feb-15; | |||||||
| M16b | Maintrac | Jun-15 | 4; 9 | Ki67=67.4 | 1.95 | Nov-15 | 10 | Prostate | MRI normal, but PSMA-PET abnormal |
| M17a, 69 yrs | ISET; | Sep-15; | 0.5 + inflammation; | 3.7 | PSMA-PET: no significant accumulation, no evidence of nodal or distant metastases; marked prostatomegaly, but no tumour; ISET-CTC: inflammation, atypical cells due to infection; |
||||
| M17b | Maintrac | Oct-15 | 3 | PSA=100; AR=46.2; Ki67=60 | Nov-15 | 2.5 | Prostate – no uptake | Maintrac-CTC does not distinguish between CTC and atypical inflammatory cells; | |
| M18, 65 yrs | Maintrac | Oct-15 | 12 | Ki67=53.8; PSA=66.7; AR=53.8 | 14.2 | Sep-15 | -1 (MRI before CTC) | Prostate | MRI prostate: multiple lesions (1.7 cm; 0.7 cm); had surgery, CTC count dropped to M: 4.7 CTC/ml |
| M19, 71 yrs | Maintrac | Feb-16 | 2.5 | 1.63 | Apr-16 | 2.5 | Prostate | PSMA-PET: low grade uptake right prostatic base | |
| M20a, 68 yrs | Maintrac | Dec-15; Feb-16 | 6.5; 7.5 | Ki67=72.6 | <0.01 | Jan-16 | 1 | Prostate | Had bladder cancer in 2014; prostectomy Jan 16; minimal uptake non-specific; NIIM CTC + lipoblast masses |
| M20b | ISET | May-16 | 2.8 | ||||||
| M21a, 67 yrs | ISET; | Aug-15; | 0.6; | ||||||
| M21b | Maintrac | Dec-15 | 3 | Ki67=50; AR=45.9 | 1.21 | Mar-16 | 7 | Prostate | PSMA-PET: possible low-grade prostate cancer in left posterior peripheral zone, more concerning uptake in right hepatic lobe |
| ISET | Apr-16 | 5.4 | |||||||
| M22a, 76 yrs | ISET; | Feb-16; | 0.7 atypical inflammatory cells; | normal | May-16 | 3 | Prostatitis | PSMA=PET CT: mild prostatitis; ISET-CTC identified inflammatory condition, no CTC detected; Maintrac-CTC does not distinguish between CTC and atypical inflammatory cells |
|
| M22b | Maintrac | Apr-16 | 2 | PSA=79; AR=88.6 | normal | ||||
| M23, 49 yrs | ISET; | May-16 | 65.4; | normal | April-16 | 1 | Prostate | PSMA-PET: moderate uptake | |
| Maintrac | May-16 | 13 | AR=62; PSA=0 |
||||||
| M24, 66 yrs | ISET; | May-16 | 10.7; | high normal | Jun-16 | 1 | Prostate | PSMA-PET: low to moderate uptake | |
| Maintrac | 11 | PSA=79; AR=73; |
ISET, ISET technology (Rarecells, France, www.rarecells.com) Maintrac technology (Germany, www.maintrac.com): Receptor expression and EpCAM marker based CTC testing. In our experience, the CTC count by Maintrac correlates to the ISET CTC count by a factor of 100. For comparison to ISET CTC counts Maintrac CTC counts have been divided by 100. F, female; M, male, AR, androgen receptor; Ki67, the Ki-67 protein is a cellular marker for cell proliferation; PSA, prostate specific antigen; PSMA, prostate specific membrane antigen; PET scan, positron emission tomography scan; n/a, not applicable
Table 3: Early detection CTC screening and follow-up scans of asymptomatic patients without detected tumour at time of CTC testing (group 2).
In up to 50% of male patients with normal PSA (prostate specific antigen) levels but with detected CTC, PET scans using PSMA (Ga-68 prostate-specific-membrane-antigens) revealed increased uptake in the prostate, which is indicative of early prostate cancer. In addition, early breast cancer, melanoma, ovarian, lung or renal cancer was detected during the study period in a small number of asymptomatic women and men (n=7) who had undergone the CTC screening test (Table 3).
Nutritional Therapies
A subgroup of patients with detected CTC was advised on evidencebased immune-boosting and anti-carcinogenic nutritional therapy by the consulting doctor. Treatment was tailored towards increasing natural killer cell count, inhibition of angiogenesis and metastasis. Supplements included curcumin, green tea, garlic extract, vitamin D, grape seed, lycopene, citrus pectin, medicinal mushroom extract, black cumin seed, artemisinin, and other immune stimulants with anticarcinogenic properties (Table 4).
| Patient ID; (gender, age) |
Group | Curcumin | Green tea | Garlic | Vit D | Grape Seed | Lycopene | Citrus Pectin | Mushroom extract |
Nigella sativa | Artemisinin | Others (immune stimulants) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1: Cancer patients with detected CTC, who did not undergo surgery, chemo- or radiotherapy during the intervention | ||||||||||||
| C10_TCC | 1 | √ | √ | √ | √ | √ | Vit E, Se, NK cell activator, reveratrol, astragalus | |||||
| C11_SCC (F, 68yrs) |
1 | √ | NK Cell activator, astragalus | |||||||||
| CJ12_prostate (M, 67 yrs) |
1 | √ | √ | √ | √ | √ | √ | √ | √ | Prostate formula: saw palmetto, lycopene, boswellia, pumpkin seed oil, boron, fish oil, Vit E, Se | ||
| C13_prostate (M, 71 yrs) |
1 | √ | √ | √ | √ | IVC, resveratrol, liver tonic, soy, Ca, Vit K2, phosphatidylserine, bromelain, salvestrol, p53, fish oil | ||||||
| CJ16_NHL & prostate (M, 65 yrs) |
1 | √ | √ | √ | √ | √ | Pomegranate, fish oil, Ca, Vit K2, | |||||
| CJ26_bladder (M, 53 yrs) |
1 | √ | √ | √ | √ | √ | Vit A | √ | √ | √ | NK cell activator, probiotic, salvestrol, astaxanthin, NAC | |
| J52_prostate, & bladder (M, 57 yrs) | 1 | √ | √ | √ | √ | √ | mistletoe, quercetin, bromelain, Se, soy, fucoidan (brown algae) | |||||
| C73_prostate (M, 49 yrs) | √ | Mg, Vit B12 | ||||||||||
| C100_breast (F, 56 yrs) | 1 | √ | √ | √ | √ | Fish oil, pomegranate, rosemary | ||||||
| Group 3: Asymptomatic patients without tumour but detected CTC | ||||||||||||
| Pt1 (F 51 yrs) | 3 | √ | √ | √ | √ | √ | √ | astragalus, probiotic, Vit C, boswellia, soy, liver tonics, NAC, Vit E, Se, Ca, Vit K2 | ||||
| Pt 2 (M, 50 yrs) | √ | Mg, Vit B12 | ||||||||||
| Pt3 (F 63yrs) | 3 | √ | √ | √ | √ | √ | √ | resveratrol, Vit C, NAC, Vit E, Se, Ca, Vit K2 | ||||
| Pt4 (F 56 yrs) | 3 | √ | √ | √ | √ | √ | broccoli, Vit A, CoQ10, NAC | |||||
| Pt5 (F 55 yrs) | 3 | √ | √ | √ | NK cell activator, astragalus, Se, Vit E, Se, Ca, Vit K2 | |||||||
| Pt7 (M 71 yrs) | 3 | √ | √ | √ | √ | √ | √ | √ | √ | Vit K2, reveratrol, broccoli, NAC, milk thistle, Vit C, Vit B12 | ||
| Pt9 (M, 66 yrs) | 3 | √ | √ | √ | √ | √ | √ | NK cell activator, salvestrol, glutathione, chlorophyll, broccoli, NAC, fish oil, Vit E, Se | ||||
| Pt10 (F, 63 yrs) | 3 | √ | √ | √ | √ | √ | √ | Nk cell activator | ||||
| Pt14 (F, 49 yrs) | 3 | √ | √ | √ | √ | resveratrol, salvestrol, broccoli, pomegranate, Vit B12, NAC, fish oil, Vit E, Se | ||||||
Abbreviations: C, cancer; S, screening; F, female; M, male; Ca, Calcium; NAC, N-acetylcysteine; NK cell, natural killer cell; NK cell activator contains enzymatically modified rice bran; Se, Selenium; Vit, vitamin
Table 4: Adjuvant nutritional treatment of patients with detected CTC (group 1 and group 3).
Treatment effectiveness of nutritional immune-boosting therapy was assessed by repeat CTC testing. CTC counts, available for cancer patients who did not undergo other therapies, (group 1, n=10, Figure 3a), and asymptomatic patients without detected tumour but positive CTC count (n=14, group 3), decreased over time (1-15 months) with nutritional therapy in all patients (Figure 3b). No adverse effects were reported by the patients who underwent integrative nutritional therapy.
Discussion
Our study suggests testing for Circulating Tumour Cells (CTC) to be a useful prognostic tool to screen for cancer risk and to monitor treatment effectiveness in cancer patients. A positive CTC count was associated with cancer risk, whereby a low CTC count (<0.3 CTC/ml) was correlated with mild malignant potential, 0.3-20 CTC/ml with moderate malignant potential, and a higher CTC count (>20 CTC/ml) with higher risk of malignancy, recurrence and metastasis, consistent with previous reports [1,2,4]. In addition to the CTC number, the type of cells, single cells or clusters, provide valuable insights into the cancer prognosis [3,4]. In this study we employed the ISET technology (Rarecells, France) [5] for CTC detection, which provides the advantage of a direct identification of malignant cells by cyto-morphological criteria [6], permitting distinction between precursor and malignant single cells and clusters, as well as reactive inflammatory atypical cells [9,16,17].
In our study, screening for CTC in asymptomatic individuals allowed the detection of early cancer, in about 20% of patients presenting with CTC. Importantly, in up to half of the men with detected CTC (25% of all men screened), but with normal PSA levels, subsequent positive PSMA-PET scans revealed early prostate cancer. This suggests CTC screening to be a more reliable measure for the detection of early prostate cancer than standard PSA testing [30]. In addition, early breast cancer, melanoma, ovarian, lung and renal cancer was detected in a small number of asymptomatic women and men with a positive CTC count. Early detection of cancer is associated with a greater range of treatment options and better prognosis [1,2,31].
A strength of our study was to compare the CTC count to cancer status and cancer risk in a large cohort of 542 patients. While CTC repeat test results after treatment were available in only a small subgroup of patients (40 out of 409 patients, 10%, with detected CTC), early results provide a trend towards treatment effectiveness of different types of interventions. However, statistical analysis in this patient cohort was not feasible due the small sample size and variety of treatments, therefore limiting generalisability about effectiveness of interventions.
Our study provided early evidence for integrative nutritional therapy to have the potential to lower CTC count, which in turn is associated with a lower risk of malignancy. Nutritional therapy was highly tolerable, and tailored towards increasing natural killer cell count, enhancing apoptosis of cancer cells, inhibition of angiogenesis and metastasis.
Natural Killer (NK) cells are an important gatekeeper stalling the growth of atypical cells, including cancer cells. Low NK cell levels have been associated with an increased risk of death in breast cancer [32]. Additionally, reduced NK cell activity increased the risk of metastasis by 350% during a 31-month period [33].
Garlic, available in form of garlic extract or garlic powder, has shown to increase natural killer cells [34]. Other anti-carcinogenic properties of garlic include reduced infection-induced carcinogenesis, and the induction of apoptosis [35,36].
Other nutrients with anti-carcinogenic properties include curcumin, green tea, grape seed extract, black cumin seed, artemisinin, modified citrus pectin, and mushroom extract.
Curcumin enhances apoptotic death, inhibits deregulated cellular proliferation, dedifferentiation and progression towards the neoplastic phenotype by altering key signaling molecules required for cell cycle progression, in addition to inhibiting H-Ras oncogene expression [37-39] .
Green tea with its polyphenols has been shown to inhibit several pathways and enzymes engaged in carcinogenesis, including the nuclear factor-κB (NF-κB), epidermal growth factor receptor (EGFR), insulinlike growth factor (IGF)-I, urokinase-plasminogen activator (uPA), matrix metalloproteinases (MMPs) involved in oncogene expression, and proteasome activities, and contributing to apoptosis and cell cycle arrest [40,41].
Grape seed extract inhibits advanced tumour growth and angiogenesis and upregulates insulin-like growth factor binding protein [42], and can induce apoptosis and cell cycle arrest [43].
Black cumin seed (Nigella sativa), with its main active ingredient thymoquinone, has shown promise in inducing tumour cell death, and inhibiting proliferation, angiogenesis, invasion and metastasis [44]. Artemisinin triggers apoptosis in human cancer cells [45].
Modified citrus pectin, containing the main active ingredient galectin-3, has numerous anti-metastatic properties through antiadhesion and apoptosis-promotion, and has shown promise in several clinical studies by halting cancer progression [46,47].
Medicinal mushroom extracts, including species of Auricularia, Flammulina, Ganoderma, Grifola, Hericium, Lentinus (Lentinula), Pleurotus, Trametes (Coriolus), Schizophyllum, and Tremella mushrooms, contain polysaccharides or polysaccharide–protein complexes, which enhance innate and cell-mediated immune responses, and inhibit proteins and enzymes involved in carcinogenesis, including NF-κB, protein-kinases, aromatase and sulfatase, and cyclooxygenase [48].
Additionally, a number of nutrients are essential for an active healthy immune system, including vitamin D, which has also been shown to play a role in anti-carcinogenesis.
Calcitriol derived from Vitamin D decreases the expression of aromatase, the enzyme that catalyses estrogen synthesis in breast cancer, both by a direct transcriptional repression and indirectly by reducing inflammatory prostaglandins [49].
Vitamin D, in addition to calcium, magnesium, Vitamin K, and boron, is also important for bone integrity [50], with bone always being affected in advanced breast and prostate cancer [51,52].
Lycopene, abundant particularly in tomatoes, has shown promise particularly in prostate cancer [53].
Conclusion
Here we provide evidence that screening for Circulating Tumour Cells (CTC) allows detection of early cancer, while CTC monitoring over time allows assessment of treatment effectiveness, with higher CTC counts being associated with higher risk of malignancy. Our study suggests CTC count to be a more reliable predictor of early prostate cancer than standard testing of PSA levels, identifying early prostate cancer confirmed by PSMA-PET scan in 50% of asymptomatic men with detected CTC. Furthermore, our study provides evidence that a combination of immune-stimulating nutritional supplements can reduce CTC count, and therefore risk of malignancy. Nutrients with anti-carcinogenic properties include curcumin, garlic, green tea, grape seed, black cumin seed, artemisinin, modified citrus pectin, and medicinal mushroom extract.
Authors Contribution
All authors conceived and designed the study. NIIM Director AS introduced CTC testing to the institute, and doctors PE and AS provided patients, patient data, and treatment plans for the study. KR established and oversaw ISET-CTC testing at the NIIM lab, collated and analysed the data, and wrote the manuscript, with contributions from co-authors. All authors read and approved the final version.
Acknowledgement
We are grateful to the team of technical and research assistants who made CTC testing at NIIM possible, including Nikolaj Travica, phlebotomist and research assistant, Viktor Svarcs, chief cytologist, Renee DeBoer and Anna Cabrera, technical assistants. We would also like to thank the cytologist Adela Cretoiu, who helped establish the ISETCTC technology at NIIM. We gratefully acknowledge Dr John Piesse, who also provided patients and patient data, and Dr Peter Fakler for valuable feedback on the manuscript.
References
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, et al. (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351: 781-791.
- Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, et al. (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12: 4218-4224.
- Wang C, Mu Z, Chervoneva I, Austin L, Ye Z, et al. (2016) Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat p: 1-12.
- Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud J-M, et al. (2014) “Sentinel” Circulating Tumor Cells Allow Early Diagnosis of Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. Plos One 9: e111597.
- Vona G, Sabile A, Louha M, Sitruk V, Romana S, et al. (2000) Isolation by size of epithelial tumor cells : a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 156: 57-63.
- Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, et al. (2012) Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 7: 306-315.
- Pachmann UA, Hekimian K, Carl S, Ruediger N, Rabenstein C, et al. (2011) Comparing sequential steps for detection of circulating tumor cells: more specific or just less sensitive?
- Paterlini-Brechot P, Benali NL (2007) Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett 253: 180-204.
- Paterlini-Bréchot P (2014) Circulating tumor cells: Who is the killer? Cancer Microenviron 7: 161-176.
- Harouaka RA, Nisic M, Zheng S-Y (2013) Circulating tumor cell enrichment based on physical properties. J of Lab Autom 18: 455-468.
- Barriere G, Fici P, Gallerani G, Fabbri F, Zoli W, et al. (2014) Circulating tumor cells and epithelial, mesenchymal and stemness markers: characterization of cell subpopulations. Annals of translational medicine 2: 109.
- Hofman VJ, Ilie MI, Bonnetaud C, Selva E, Long E, et al. (2011) Cytopathologic detection of circulating tumor cells using the isolation by size of epithelial tumor cell method: promises and pitfalls. Am J Clin Pathol 135: 146-156.
- Hofman V, Ilie MI, Long E, Selva E, Bonnetaud C, et al. (2011) Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay and the isolation by size of epithelial tumor cell method. Int J Cancer 129: 1651-1660.
- Garcia JA, Rosenberg JE, Weinberg V, Scott J, Frohlich M, et al. (2007) Evaluation and significance of circulating epithelial cells in patients with hormone‐refractory prostate cancer. BJU international 99: 519-524.
- Farace F, Massard C, Vimond N, Drusch F, Jacques N, et al. (2011) A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer 105: 847-853.
- Hofman V, Long E, Ilie M, Bonnetaud C, Vignaud J, et al. (2012) Morphological analysis of circulating tumour cells in patients undergoing surgery for non‐small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology 23: 30-38.
- Vona G, Estepa L, Béroud C, Damotte D, Capron F, et al. (2004) Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology 39: 792-797.
- Khoja L, Shenjere P, Hodgson C, Hodgetts J, Clack G, et al. (2014) Prevalence and heterogeneity of circulating tumour cells in metastatic cutaneous melanoma. Melanoma Res 24: 40-46.
- Khoja L, Backen A, Sloane R, Menasce L, Ryder D, et al. (2012) A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer 106: 508-516.
- Chinen L, Mello C, Abdallah E, Ocea L, Buim M, et al. (2014) Isolation, detection, and immunomorphological characterization of circulating tumor cells (CTCs) from patients with different types of sarcoma using isolation by size of tumor cells: a window on sarcoma-cell invasion. OncoTargets Ther 7: 1609-1617.
- De Giorgi V, Pinzani P, Salvianti F, Grazzini M, Orlando C, et al. (2010) Circulating benign nevus cells detected by ISET technique: warning for melanoma molecular diagnosis. Arch Dermatol 146: 1120-1124.
- Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria J, et al. (2011) Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer 105: 1338-1341.
- Hofman V, Ilie M, Long E, Guibert N, Selva E, et al. (2014) Detection of circulating tumor cells from lung cancer patients in the era of targeted therapy: promises, drawbacks and pitfalls. Curr Mol Med 14: 440-456.
- Buhmeida A, Pyrhönen S, Laato M, Collan Y (2006) Prognostic factors in prostate cancer. Diagn Pathol 1: 4-4.
- Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart H, et al. (2013) PET imaging with a [68Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. European journal of nuclear medicine and molecular imaging 40: 486-495.
- Mottaghy FM, Behrendt FF, Verburg FA (2016) 68-Ga-PSMA-HBED-CC Pet/CT: where molecular imaging has an edge over morphological imaging. Eur J Nucl Med Mol Imaging 43: 394-396.
- Verburg FA, Pfister D, Heidenreich A, Vogg A, Drude NI, et al. (2016) Extent of disease in recurrent prostate cancer determined by [68Ga] PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. European journal of nuclear medicine and molecular imaging 43: 397-403.
- Giesel FL, Fiedler H, Stefanova M, Sterzing F, Rius M, et al. (2015) PSMA PET/CT with Glu-urea-Lys-(Ahx)-[68Ga (HBED-CC)] versus 3D CT volumetric lymph node assessment in recurrent prostate cancer. European journal of nuclear medicine and molecular imaging 42: 1794-1800.
- Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, et al. (2004) Prevalence of prostate cancer among men with a prostate-specific antigen level≤ 4.0 ng per milliliter. New England Journal of Medicine 350: 2239-2246.
- Tol J, Koopman M, Miller M, Tibbe A, Cats A, et al. (2010) Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Annals of Oncology 21: 1006-1012.
- Eichbaum MH, Kaltwasser M, Bruckner T, de Rossi TM, Schneeweiss A, et al. (2006) Prognostic factors for patients with liver metastases from breast cancer. Breast cancer res treartm 96: 53-62.
- Koda K, Saito N, Takiguchi N, Oda K, Nunomura M, et al. (1996) Preoperative natural killer cell activity: correlation with distant metastases in curatively research colorectal carcinomas. International surgery 82: 190-193.
- Lamm DL, Riggs DR (2001) Enhanced immunocompetence by garlic: role in bladder cancer and other malignancies. Nutrition J 131: 1067S-1070S.
- Kyo E, Uda N, Suzuki A, Kakimoto M, Ushijima M, et al. (1998) Immunomodulation and antitumor activities of aged garlic extract. Phytomedicine 5: 259-267.
- Thomson M, Ali M (2003) Garlic [Allium sativum]: A review of its potential use as an anti-cancer agent. Current cancer drug targets 3: 67-81.
- Sa G, Das T (2008) Anti cancer effects of curcumin: cycle of life and death. Cell Division 3: 1.
- Limtrakul P-n, Anuchapreeda S, Lipigorngoson S, Dunn FW (2001) Inhibition of carcinogen induced c-Ha-ras and c-fos proto-oncogenes expression by dietary curcumin. BMC Cancer 1: 1.
- Kim M-S, Kang H-J, Moon A (2001) Inhibition of invasion and induction of apoptosis by curcumin in H-ras-transformed MCF10A human breast epithelial cells. Archives of pharmacal research 24: 349-354.
- Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E (1997) Why drinking green tea could prevent cancer. Nature 387: 561-561.
- Khan N, Mukhtar H (2008) Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett 269: 269-280.
- Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C (2004) Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin‐like growth factor binding protein‐3. Intern Cancer J 108: 733-740.
- Kaur M, Agarwal C, Agarwal R (2009) Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. Nutrition J 139: 1806S-1812S.
- Randhawa MA, Alghamdi MS (2011) Anticancer activity of Nigella sativa (black seed)—a review. The American j of Chinese medicine 39: 1075-1091.
- Singh NP, Lai HC (2004) Artemisinin induces apoptosis in human cancer cells. Anticancer Research 24: 2277-2280.
- Glinsky VV, Raz A (2009) Modified citrus pectin anti-metastatic properties: one bullet, multiple targets. Carbohydrate research 344: 1788-1791.
- Azémar M, Hildenbrand B, Haering B, Heim ME, Unger C (2007) Clinical benefit in patients with advanced solid tumors treated with modified citrus pectin: a prospective pilot study. Clinical Medicine Insights Oncology 1: 73.
- Zaidman B-Z, Yassin M, Mahajna J, Wasser SP (2005) Medicinal mushroom modulators of molecular targets as cancer therapeutics. Applied Microbiol and Biotechnol 67: 453-468.
- Krishnan AV, Feldman D (2011) Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annual review of pharmacology and toxicology 51: 311-336.
- Schwarz EC, Qu B, Hoth M (2013) Calcium, cancer and killing: the role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1833: 1603-1611.
- Mundy GR (2002) Metastasis: Metastasis to bone: causes, consequences and therapeutic opportunities. Nature Reviews Cancer 2: 584-593.
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP (2007) Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. clinical nutr 85: 1586-1591.
- van Breemen RB, Pajkovic N (2008) Multitargeted therapy of cancer by lycopene. Cancer Lett 269: 339-351.
- Hadley CW, Miller EC, Schwartz SJ, Clinton SK (2002) Tomatoes, lycopene, and prostate cancer: progress and promise. Experimental Biology and Medicine 227: 869-880.
Citation: Ried K, Eng P, Sali A (2017) Screening for Circulating Tumour Cells Allows Early Detection of Cancer and Monitoring of Treatment Effectiveness: An Observational Study. Adv Cancer Prev 2: 123. DOI: 10.4172/2472-0429.1000123
Copyright: © 2017 Ried K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4531
- [From(publication date): 0-2017 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 3637
- PDF downloads: 894