Sclerosing Encapsulating Peritonitis: A Mysterious Cause of Small Bowel Obstruction
Received: 27-Feb-2023 / Manuscript No. JGDS-23-90176 / Editor assigned: 01-Mar-2023 / PreQC No. JGDS-23-90176 (PQ) / Reviewed: 15-Mar-2023 / QC No. JGDS-23-90176 / Revised: 03-May-2023 / Manuscript No. JGDS-23-90176 (R) / Published Date: 10-May-2023
Abstract
Sclerosing encapsulating peritonitis, also known as abdominal cocoon, is a rare condition of unknown etiology which results in intestinal obstruction due to total or partial encapsulation of the small bowel by a fibrocollagenous membrane. A high index of clinical suspicion is required for preoperative diagnosis. The early clinical features are nonspecific and not recognized, which makes a definite preoperative diagnosis difficult. The radiological diagnosis of abdominal cocoon is now made on computed tomography scan. Surgery plays main role in the management of this disease. Careful dissection and excision of the thick sac with the release of the small bowel loops will lead to complete recovery in the vast majority of cases. Here, we are reporting a case of sclerosing encapsulating peritonitis presented with delayed intestinal obstruction.
Keywords
Sclerosing encapsulating peritonitis; Encapsulation; Intestinal obstruction; Computed tomography; Fibrocollagenous membrane
Introduction
SEP incidence and prevalence has not been studied. However, older reports indicate a prevalence between 0.54%-7.3%. Additionally, 10%-20% of patients receiving peritoneal dialysis have been seen to develop this rare condition. Although not increasingly prevalent, literature suggests a similar pattern of modalities towards diagnosis of SEP [1]. In fact, diagnosis is usually confirmed by direct visualization of abdominal contents since imaging findings have a low specificity. Diagnosis should be considered in patients with unexplained recurrent episodes of small bowel obstruction and sac like encasing of intestines with interloop adhesions found on imaging. The purpose of this report is to create awareness of this condition by contributing to the proper elaboration of diagnostic and treatment guidelines. In response to the increasing mortality caused by recurrent inflammation and fibrotic nature of this disease, this report can assist in development of appropriate conservative and/or surgical intervention [2-4].
Case Presentation
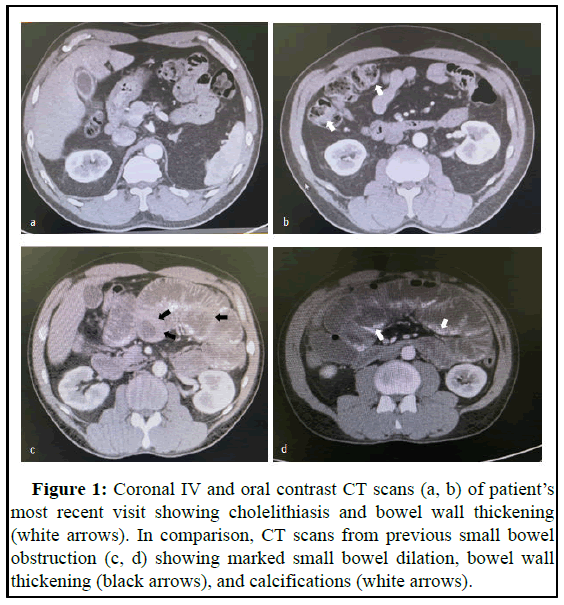
Case of a 45-year-old male with previous medical history of multiple small bowel obstruction comes to the ER biliary colic symptoms. Patient describes postprandial abdominal pain lasting for several hours before resolving on its own accompanied by 6 episodes of vomiting [5]. Upon further questioning, he referred previous admissions due to nonspecific abdominal pain and diagnosis of small bowel obstruction. Bowel movements were unaffected and he denied malignancy, peritoneal dialysis and tuberculosis. Upon physical examination, abdomen was soft and depressible, non-distended but moderately tender to palpation especially in the right upper quadrant. Bowel sounds were positive. There was no rebound tenderness, guarding or additional peritoneal signs present. Abdominal CT scan with oral and IV contrast findings showed evidence of cholelithiasis and bowel wall thickening (Figures 1a and 1b). Our patient was then scheduled for laparoscopic cholecystectomy to ameliorate symptoms [6-8]. Upon gas insufflation of the abdominal cavity poor visualization was achieved due to extensive adhesions and tethering of intestinal loops to adjacent structures. After arduously identifying the relevant anatomy, the procedure was converted to an open approach which was when the abnormal anatomy was directly visualized.
Bowel loops were organized in a clumped fashion surrounded by extensive adhesions and fibro collagenous membranes.
Patient was evaluated 2 weeks post operatively at clinic. He endorsed improvement of symptoms had been tolerating diet, passing flatus and bowel movements consistently. He was lost to follow-up since then [9].
This case was studied retrospectively while analysing the abdominal CT scans from previous hospital admissions, the first one occurring nine years ago. During this stay, an abdominopelvic CT scan was performed which showed findings consistent longstanding small bowel obstruction evidenced by small bowel dilation, bowel wall thickening, and calcifications (Figures 1c and 1d). Conservative management with intestinal rest and nasogastric tube decompression was initiated and resulted in clinical improvement. Patient then visited different hospitals for similar presentations (Figure 1).
Treatment guidelines include the removal of the inciting factor, for which was not clear in our case, bowel rest and TPN. The latter two however are not sufficient to combat the chronic nature of this condition. Many trials have been reported indicating use of immunosuppressive therapy including corticosteroids, colchicine, azathioprine and cyclosporine [10]. These interventions however, are indicated for patients who have a clear and active focus of inflammation. One medical therapy that has proved to be effective in reducing the mortality rate of SEP is tamoxifen. The latter contains anti-fibrotic properties that contributed to a significant decrease in mortality rate from 74.4% to 45.8%. The exact dosage indicated for this case is not clearly defined. When exploratory laparotomy is indicated in a deteriorating patient, the most common procedures performed are the ablation of fibrotic tissue, adhesiolysis, and resection of perforated or necrotic bowel. To reduce risk of recurrence, it is advised to continue steroid or tamoxifen therapy [11].
Figure 1: Coronal IV and oral contrast CT scans (a, b) of patient’s most recent visit showing cholelithiasis and bowel wall thickening (white arrows). In comparison, CT scans from previous small bowel obstruction (c, d) showing marked small bowel dilation, bowel wall thickening (black arrows), and calcifications (white arrows).
Results and Discussion
Sclerosing encapsulating peritonitis has previously been described in literature as a rare condition causing the small intestine to be wrapped in a fibrocollagenous membrane. Several risk factors have been identified including prolonged intraperitoneal dialysis, abdominal surgery, use of beta blockers, TB, systemic lupus erythematosus and retrograde menstruation but can be idiopathic. These inciting events cause the release of pro-inflammatory (TGF-β, IL-6), angiogenic (VEGF) cytokines and the subsequent expression of profibrotic genes (COL1A1, fibronectin 1, sulfatase 1) which contribute to peritoneal fibrosis, thickening and adhesion formation. This patient’s case seems to be primarily idiopathic as history taking did not reveal exposure to any risk factors associated with development of SEP. Although, our patient’s recurrent obstructive symptoms might have exposed the abdominal contents to prolonged inflammation and start of fibrinous cascade towards development of this disease.
The diagnostic strategy can be tailored to each particular case. SEP can be part of the differential diagnosis when evaluating the history and physical findings which present recurrent intestinal obstruction, anorexia, abdominal pain, nausea and vomiting and the presence of an inciting factor such as previous surgeries or peritoneal dialysis. Imaging can be helpful when evaluating SEP. Small bowel dilation, tethering, bowel wall thickening, calcifications and a thickened peritoneum can be indicative of this condition. One case reported a patient with a CT scan demonstrating stomach and jejunal dilation and bowel wall thickening encased in a peritoneal sac indicating abdominal cocoon syndrome. Many reports indicate that SEP may show the “cauliflower sign” on CT scan which is highly suggestive of the anomaly. Because of this, imaging must be carefully studied especially in patients suffering from recurrent SBO. When surgical intervention is indicated, the diagnosis is confirmed during direct visualization of the bowel loops encased in a cocoon like cyst with extensive adhesions.
Conclusion
SEP is a rare clinical entity and is often encountered unexpectedly in patients with acute intestinal obstruction. A high index of clinical suspicion in susceptible patients is necessary to achieve a preoperative diagnosis. Radiological imaging, particularly CT scans, plays a major role in establishing the diagnosis. Conservative management is the ideal approach for patients who present with mild symptoms; however, those with severe intestinal obstruction are likely to require surgical intervention, usually comprising of the complete excision of the membrane, adhesiolysis and occasionally resection.
References
- Danford CJ, Lin SC, Smith MP, Wolf JL (2018) Encapsulating peritoneal sclerosis. World J Gastroenterol 24: 3101-3111.
[Crossref] [Google Scholar] [PubMed]
- Ulusoy C, Nikolovski A, Ozturk NN (2021) Difficult to diagnose the cause of intestinal obstruction due to abdominal cocoon syndrome. Eur J Case Rep Int Med 8: 002588.
[Crossref] [Google Scholar] [PubMed]
- Wetherell J, Woolley K, Chadha R, Kostka J, Adilovic E, et al. (2021) Idiopathic sclerosing encapsulating peritonitis in a patient with atypical symptoms and imaging findings. Case Rep Gastrointest Med 2021: 6695806.
[Crossref] [Google Scholar] [PubMed]
- Alshomimi S, Hassan A, Faisal Z, Mohammed A, Al Dandan O, et al. (2021) Sclerosing encapsulating carcinomatous peritonitis: A case report. Saudi J Med Med Sci 9: 63-66
[Crossref]
- Colak K, Bektas H (2019) Abdominal cocoon syndrome: A rare cause of acute abdomen syndrome. Turk J Trauma Emerg Surg 25: 575-579.
[Crossref] [Google Scholar] [PubMed]
- Phelan PJ, Walshe JJ, Al-Aradi A, Garvey JP, Finnegan K, et al. (2010) Encapsulating peritoneal sclerosis: Experience of a tertiary referral center. Ren Fail 32: 459-463
[Crossref] [Google Scholar] [PubMed]
- Brown EA, Bargman J, van Biesen W, Chang MY, Finkelstein FO, et al. (2017) Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis-position paper for ISPD: 2017 update. Perit Dial Int 37: 362-374
[Crossref] [Google Scholar] [PubMed]
- Acar T, Kokulu I, Acar N, Tavusbay C, Hacıyanlı M (2015) Idiopathic encapsulating sclerosing peritonitis. Ulus Cerrahi Derg 31: 241-243.
[Crossref] [Google Scholar] [PubMed]
- Korte MR, Fieren MW, Sampimon DE, Lingsma HF, Weimar W, et al. (2011) Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: Results of the Dutch multicentre EPS Study. Nephrol Dial Transplant 26: 691-697.
[Crossref] [Google Scholar] [PubMed]
- Ethiraj D, Indiran V (2020) Abdominal Cocoon: “Cauliflower sign” on contrast enhanced computed tomography scan. GE Port J Gastroenterol 28: 76-77.
[Crossref] [Google Scholar] [PubMed]
- Tannoury JN, Abboud BN (2012) Idiopathic sclerosing encapsulating peritonitis: Abdominal cocoon. World J Gastroenterol 18: 1999-2004.
[Crossref] [Google Scholar] [PubMed]
Citation: Fullana JLO, Mantilla C, Bellido OR, Rodriguez JR, Bernardini HMS (2023) Sclerosing Encapsulating Peritonitis: A Mysterious Cause of Small Bowel Obstruction. J Gastrointest Dig Syst 13: 738.
Copyright: © 2023 Fullana JLO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1101
- [From(publication date): 0-2023 - Apr 26, 2025]
- Breakdown by view type
- HTML page views: 855
- PDF downloads: 246