Mini Review Open Access
Scheduled Outpatient Intravenous Infusion per Headache Protocol and Effect on Migraine-related Quality of Life in Patients with Chronic Daily Headaches
1Department of Headache Medicine, Baylor University Medical Center, Baylor Scott & White Healthcare, Dallas, TX, USA
2Department of Pediatric Neurology, University of Illinois College of Medicine, Peoria, IL, USA
- *Corresponding Author:
- Asra Akbar
Department of Pediatric Neurology, University of Illinois College of Medicine
420 NE Glen Oak Avenue, Suite 401, Peoria, IL-61603, USA
Tel: 314-753-0079
E-mail: dr_aakbar@yahoo.com
Received date: February 21, 2017; Accepted date: July 24, 2017; Published date: July 28, 2017
Citation: Akbar A (2017) Scheduled Outpatient Intravenous Infusion per Headache Protocol and Effect on Migraine-related Quality of Life in Patients with Chronic Daily Headaches. J Pain Relief 6:298. doi:10.4172/2167-0846.1000298
Copyright: © 2017 Akbar A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pain & Relief
Abstract
Chronic Daily Headache (CDH) and Medication overuse headaches (MOH) are important public health problems. CDH is defined as a headache occurring on 15 days or more per month over three month duration with a worldwide prevalence of 4%. MOH is a daily or near daily headache over 15 days a month that results from overuse of migraine abortive medications and is one of the most common chronic headache disorders (worldwide prevalence of 1-2%).
A total of 56 patients were enrolled in the study. 51 patients completed a one month follow-up and 39 patients completed a two month follow-up. After informed consent, migraine specific quality of life questionnaire (MSQ 2.1) and patient rating of migraine specific measures were administered on the first day of treatment and at 5 and 10 weeks following completion of treatment. The protocol included IV DHE, IV magnesium, IV Decadron, IV Depacon based on standard factors identified by the headache specialist and as needed Toradol, Benadryl and anti-nausea medications headache frequency and severity was decreased. Migraine associated photophobia and phonophobia improved markedly (p<0.0001) and number of people needing abortive medication more than 10 days a month was reduced by 40%.
The data provide evidence for the efficacy of scheduled outpatient intravenous therapy per the headache center protocol for CDH and suggests that patients experience diminished frequency and intensity of headaches with improved quality of life one month and two months post therapy. Outpatient settings and timings provided a more comfortable and time effective setting to patients and families with decrease visits to the emergency room with a will prove to be a more cost effective method of treatment.
Keywords
Chronic daily headaches; Medication overuse headache; Intravenous infusions; outpatient treatment; Migraine-related quality of life
Abbreviations
ICHD-3 beta: International Classification of Headache Disorders-beta; CDH: Chronic Daily Headache; MOH: Medication Overuse Headache; TM: Transformed Migraine; NDPH: New Daily Persistent Headache; HC: Hemicrania Continua; IV DHE: Intravenous Dihydroergotamine
Background
CDH can be a serious health issues that can affect the quality of life across patient age. The chronification of migraine although not fully understood is thought to be related to central sensitization along with "neurogenic inflammation" [1-4]. CDH includes transformed migraine (TM), chronic tension-type headache new daily persistent headache (NDPH) and hemicrania continua (HC) and medication overuse headache (MOH). In 1986 Raskin published about the use of DHE for intractable migraines. 49 of the 55 DHE treated patients became headache free within 48 hours and 39 of them sustained benefits in a Mean follow-up of 16 months [5]. Silberstein in 1990 concluded that a regimen of repetitive intravenous DHE can provide rapid relief of chronic intractable headache and medication overuse headaches [6]. Silberstein revised the DHE study in 1992 showing 50 patients treated with IV DHE [7].
At 3 months follow-up 44% had an excellent or good result. At 24 months, 39 patients were analyzed 59% had a good or excellent result. Charles JA and Von DP in 2010 showed patients reported an average of 63.4% reduction in the intensity of migraine pain by the end of the 3 day infusion with IV DHE [8]. Long-term follow-up data from 3 months to 4 years indicated an average 86% reduction in headache frequency. The study showed that use of outpatient continuous IV DHE is an effective therapy. Freitag in 2001 used a retrospective chart review of 642 current patients under treatment with divalproex sodium for CDH [9].
The Mean improvement was 47% with an improvement in migraine of about 65%. A study by stillman in 2004 study response of CDH with IV Depakote and showed that one hundred thirty treatments were given to 89 women and 17 men aged 17 to 76 years; for first treatments only 61 patients (57.5%) responded to treatment whereas for all treatments 82 patients (63.1%) responded [10].
Saper and Silberstein in 2006 documented efficacy of IV DHE for management of CDH [11]. 214 patients with chronic daily headache treated with repetitive intravenous DHE Silberstein reported that 92% of these patients became headache free usually within 48 hours with continued improvement over 2 years. However, fewer studies have been carried out an outpatient basis looking at the long term effects and efficacy of the treatments. Thus the Long term effects of scheduled outpatient IV infusions are limited.
Methods
Patients diagnosed with chronic daily headache by provider’s board certified in headache medicine were referred for IV treatment as part of standard medical practice. Treatment was provided in a tertiary care outpatient headache center which is an outpatient department. Participants were recruited for the study using standard Informed Consent as part of an IRB approved protocol. The Inclusion Criteria included admission to the Outpatient Infusion Center at the Baylor Comprehensive Headache Center age of ≥ 18 years old, planned admission for 5 days of infusion treatment and a history of Chronic Daily headache and/or medication overuse headaches.
The Exclusion criteria included patients who refused the study were not between the ages of 18-70 years old, patients with planned admissions of less than 5 days of the infusion treatment. For inclusion, patients had to complete a standard protocol for IV infusions including IV DHE, IV Depacon, IV steroids and/or muscle relaxant based on standard factors identified by the physician. At the conclusion of treatment the patients were prescribed an individualized treatment plan of standard abortive and preventative medications. Patients also received a psychological evaluation and 3 hours of cognitive behavioral group therapy and individual discharge treatment planning by a neuropsychologist during their course of care.
Measures were administered within the first two days of infusion treatment (for the month prior to IV infusion therapy, 4-6 weeks after completion of infusion therapy and 8-10 weeks up post infusion therapy. If this follow-up visit could not be completed in the clinic using the migraine specific quality of life questionnaire v2.1 (MSQ) looking at change in headache was conducted over the phone by the primary investigator.
Patients completed two questions for the time period of “In the last month- On how many days did you have a headache?”; On a scale of 0-10 on average how painful were these headaches?; Patients completed rating scales for frequency and subjective distress related to photophobia and phonophobia in the last month. Measurement of functioning was assessed using the migraine-specific quality of life questionnaire v2.1 (MSQ).
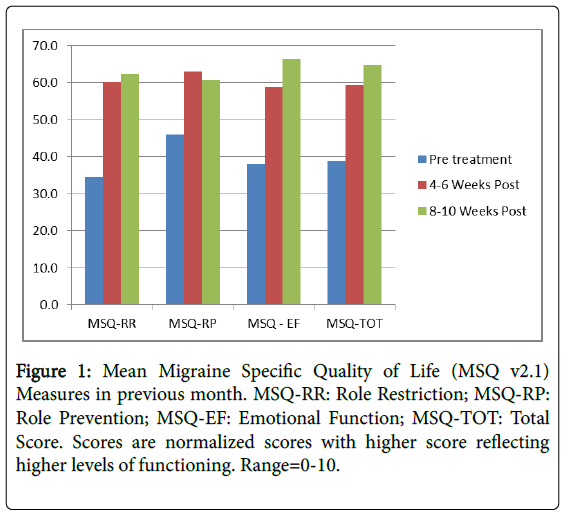
This widely used tool have has been found to be a valid and reliable measure of functioning for persons with chronic migraine [12]. It contains three subscales: Role Function-Preventive (RP); Role Function-Restrictive (RR) and Emotional Function (EF) with a total score. We used a normalized rescales from 0-10 where higher scores indicate better Headache related Quality of life HRQL (Figure 1).
Data was analyzed using Open Stat [13]. Descriptive data included age, gender and primary diagnosis. Two sets of comparisons were made to determine differences or changes in the groups between intervals- Pretreatment vs. 1st follow-up; Pretreatment vs. 2nd followup.
Preliminary analysis revealed that some of the variables were not meet assumptions for normal distribution. As a result all continuous variables were analyzed using an alternative T-test (Wilcoxin matched pairs signed-ranks T-test one taliled test). The variable that was categorical (i.e. patient’s response to item regarding medication use) was analyzed by a 2 × 2 chi square contingency table.
Results
A total of 59 patients met criteria for and were enrolled in the study with 51 being surveyed at 4-6 weeks post infusion and 39 at 8-10 weeks post infusion. The loss of participants at the second follow-up reflected an artificial end to the study when personnel needed to obtain that follow-up were no longer available. Mean age was 39.7 (SD=12.8); Gender was 93% female.
All patients were diagnosed with CDH with 76% also being diagnosed with MOH (including narcotics, medications containing butalbital, triptan overuse and over the counter medication overuse). Performance on outcome measures is summarized (Table 1).
| Measure | Pre Treatment | 4-6 Weeks Post | Pre vs. 4-6 Month Post P Value | 8-10 Weeks Post (n=39) | Pre vs. 8-10 Month Post P Value |
|---|---|---|---|---|---|
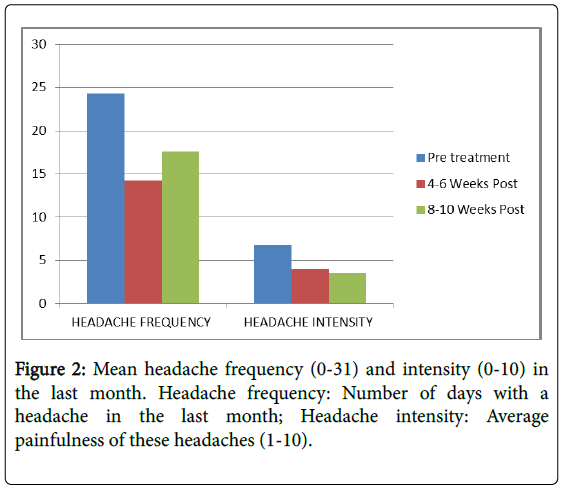
| Headache frequency (0-31) | 24.3 (7.6) | 14.2 (7.0) | <0.001 | 17.6 (6.2) | <0.001 |
| Headache Intensity (0-10) | 6.8 (1.8) | 4.0 (4.4) | <0.001 | 3.5 (1.2) | <0.001 |
| Photophobia Frequency (1-10) | 6.8 (3.4) | 3.3 (1.1) | <0.001 | 3.4 (0.8) | <0.001 |
| Photophobia Intensity (1-10) | 6.8 (3.4) | 3.3 (1.1) | <0.001 | 3.2 (0.8) | <0.001 |
| Phonophobia frequency (1-10) | 6.9 (3.3) | 3.1 (1.0) | <0.001 | 3.2 (0.7) | <0.001 |
| Phonophobia Intensity (1-10) | 6.8 (3.3) | 2.9 (0.9) | <0.001 | 3.2 (0.7) | <0.001 |
| MSQ-RR | 34.6 (21.6) | 60.2 (18.0) | <0.001 | 62.3 (9.6) | <0.001 |
| MSQ-RP | 46.0 (22.8) | 63.0 (19.2) | <0.001 | 60.6 (11.6) | <0.001 |
| MSQ-EF | 38.0 (23.3) | 58.8 (21.2) | <0.001 | 66.2 (10.2) | <0.001 |
| MSQ-TOT | 38.8 (20.0) | 59.3 (19.6) | <0.001 | 64.7 (10.6) | <0.001 |
| Using Meds >10 Days Month | 75% | 30% | 35% | ||
Table 1: Headache frequency and intensity; headache related symptoms; headache related quality of life; medication overuse: Prior to treatment; 4-6 weeks post treatment and 8-10 weeks post treatment.
Headache frequency was high (Mean=24.3; SD=7.6) with moderately high pain severity (Mean=6.8; SD=1.8) prior to treatment. Both headache frequency and pain severity declined markedly (p>0.0001) following treatment both at the first and second interval. Although headache frequency demonstrated some regression to the Mean at the second interval, pain severity continued to decline.
Photophobia and phonophobia associated with daily headache were initially rated as frequent (Mean=6.3; 6.9 respectively) and bothersome (Mean=6.8; 6.8 respectively). Both set of symptoms demonstrated marked reductions in frequency and interference at both sets of intervals (p>0.0001).
Ratings were approximately 50% lower at the first follow-up and were stable on the second interval. Consistent with the physician diagnosis of MOH for 76% of patients in the sample, 75% of patients endorsed the item associated with MOH prior to treatment. Only 30-35% continued to meet the criteria in the two follow-up intervals (p<0.0001).
Level of functioning improved at significant levels (p<0.0001) on both the total score on the MSQ v2.1. Improvements were noted both 4-6 weeks post infusion and were sustained at 8-10 weeks post infusion (Figure 2).
Discussion
We conducted a perspective observation study on patients diagnosed with CDH by headache certified providers who were between the ages of 18-70 years old. In our study 51 of the 59 enrolled patients were surveyed at 4-6 weeks post infusion and 39 patients were surveyed at 6-8 weeks post infusion. The numbers of headache days decreased by 41% in one month and by 27.5% in two month follow-up. Headache severity, post-infusion decreased by 41 % in one month and 48% in two months. Migraine-specific quality of life improved remarkably, migraine related photophobia and phonophobia also improved (p<0.001).
Patients using as needed analgesic medications more than 10 days a month was 75% pretreatment and reduced to 30% at 4-6 weeks post treatment and remained at 35% 8 weeks post infusions. The protocol administered including IV fluids for hydration, IV DHE standard dose with antiemetic, IV muscle relaxant, magnesium, IV Depacon and IV steroids for all patients meeting criteria. This was scheduled over a period of 5 days at an outpatient scheduled basis.
Limitations of our study includes non-inclusion of the control group, lack of subjects randomization for comparison purpose, lack of treatment standardization, smaller sample sizes at week 8-10 at followup, shorter duration not evaluating beyond 8-10 weeks and variation of the home prophylactic medications that were allowed to be continued.
Most studies [8-11] have used one agent DHE and no other combination of studies medications as used in our study. To our knowledge out study is one of the few that has reported the long term efficacy of scheduled outpatient IV infusion protocol.
Conclusion
Out-patient scheduled infusion therapy is safe and generally welltolerated, effective treatment for withdrawal of MOH and management of CDH even at long term. Outpatient infusions are cost-effective by saving inpatient hospital admissions, time effective and convenient to patients and families.
References
- Sheikh HU (2015) Approach to chronic daily headache. Curr Neurol Neurosci Rep 15: 4
- Kristoffersen ES, Lundqvist C (2014) Medication-overuse headache: Epidemiology, diagnosis and treatment. Ther Adv Drug Saf 5: 87-99.
- Takeshima T (2010). Clinical features and mechanisms of chronic migraine and medication-overuse headache. Rinsho Shinkeigaku 50: 990-993.
- Tepper SJ (2012) Medication-overuse headache. Continuum (MinneapMinn) 18: 807-822.
- Raskin NH (1986) Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology 36: 995-997.
- Silberstein SD, Schulman EA, Hopkins MM (1990) Repetitive intravenous DHE in the treatment of refractory headache. Headache 30: 334-339.
- Silberstein SD, Silberstein JR (1992) Chronic daily headache: Long-term prognosis following inpatient treatment with repetitive IV DHE. Headache 32: 439-445.
- Charles JA, Von DP (2010) Outpatient home-based continuous intravenous dihydroergotamine therapy for zintractable migraine. Headache 50: 852-860.
- Freitag FG, Diamond S, Diamond ML, Urban GJ (2001) Divalproex in the long-term treatment of chronic daily headache. Headache 41: 271-278.
- Stillman MJ, Zajac D, Rybicki LA (2004) Treatment of primary headache disorders with intravenous valproate: Initial outpatient experience. Headache 44: 65-69.
- Saper JR, Silberstein S, Dodick D, Rapoport A (2006) DHE in the pharmacotherapy of migraine: Potential for a larger role headache. Headache 46: S212-S220.
- Bagley CL, Rendas-Baum R, Maglinte GA, Yang M, Varon SF (2012) Validating migraine-specific quality of life questionnaire v2.1 in episodic and chronic migraine. Headache 52: 409-421.
- http://openstat.info/OpenStatMain.htm
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 8924
- [From(publication date):
July-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 8047
- PDF downloads : 877