Research Article Open Access
Scaffold Based Reconstruction of Focal Full Thickness Talar Cartilage Defects
Markus Walther1* and Katja Martin21Center for Foot and Ankle Surgery, Schön Klinik München Harlaching, Harlachingerstr. 51, 81547 Munich / Germany
2clinTEXTpert, Rosenweg 10a, 6340 Baar, Switzerland
- *Corresponding Author:
- Markus Walther
Center for Foot and Ankle Surgery
Schön Klinik München Harlaching
Harlachingerstr. 51, 81547 Munich, Germany
Tel: + 49 89 6211 2041
Fax: + 49 89 6211 2042
E-mail: MWalther@schoen-kliniken.de
Received Date: April 10, 2013; Accepted Date: July 27, 2013; Published Date: July 30, 2013
Citation: Walther M, Martin K (2013) Scaffold Based Reconstruction of Focal Full Thickness Talar Cartilage Defects. Clin Res Foot Ankle 1:115. doi: 10.4172/2329-910X.1000115
Copyright: © 2013 Walther M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Research on Foot & Ankle
Abstract
Most talar cartilage injuries have a traumatic pathogenesis. They occur as a result of a single or repeated traumatic event. Initially only the cartilage may be damaged by shear stress and may heal, remaining asymptomatic. Often the trauma causes microfractures in the subchondral plate and bone and upon loading, water is forced into the damaged subchondral area by the compressed cartilage, leading to a localized increased flow and fluid pressure. This can cause osteolysis and lead to the formation of subchondral cysts. Deep ankle pain on weight bearing, most probably caused by repetitive high fluid pressure will limit the patients mobility and hence quality of life and needs to be treated.
We describe an emerging and especially cost-effective scaffold-based reconstruction technique for osteochondral defects of the talus-Autologous Matrix-Induced Chondrogenesis (AMIC®)–a promising alternative to Matrix-induced Autologous Chondrocyte Implantation (MACI) and other techniques.
Keywords
Cartilage defect; Talus; AMIC; MACI; Chondro-Gide; Microfracture ; osteochondral defect OD; Osteochondral lesion OCL; Osteochondritis dissecans; OATS
Introduction
Osteochondral lesion (OCL) is a broad term used to describe an injury or abnormality of talar articular cartilage and adjacent bone and includes osteochondritis dissecans (OD) as well as osteochondral fractures and local degenerative lesions [1]. Trauma is reported to be an important etiologic factor of OCL of the talus with an incidence of 93-98% for lateral defects and 61-70% for medial defects [2,3]. In the acute setting, an OCL is often not recognized due to the prevailing symptoms of other injuries, e.g. ligaments. If symptoms like swelling or locking persist and do not resolve after 4-6 weeks, or intermittent deep ankle pain during or after activity presents, an OCL may be suspected [4], even if there is no trauma in history.
Treatment should be tailored to individual patient’s needs based on clinical findings and medical history. Besides addressing the OCL as such it is mandatory to verify and correct concomitant problems (e.g. malalignment, instability). Treatment decision should also take into consideration patient specifics like age and level of activity expected and will range from conservative, non-surgical to surgical options or a combination of all with the goal to reestablish functionality through restoring talar integrity and to preserve the joint.
In young patients with open growth plate, osteochondral defects can be managed conservatively with a high rate of complete remission [5,6]. Acute OCLs can be treated non operative. Acute lesions (Stage I and II) require 3 weeks of immobilization. Stage III and IV should be treated with a walker and partial weight bearing of 20 kg for 6 weeks [7]. Silent OCLs in the adult should be managed nonoperatively with regular follow ups. Especially if the lesion was detected by accident, there is no evidence that any treatment is necessary [4,6,8].
Symptomatic treatment with an ankle brace, physiotherapy, pain medication or treatment with hyaluronic acid helps to relieve symptoms; however, it cannot heal the lesion. Hyaline cartilage is a tissue containing very few cells, with chondrocytes making up only about 1-3% of its total volume. The ability to regenerate itself is very limited. At this stage of medical science a causative treatment cannot be expected by nonoperative management of chronic OCLs [9].
Surgical intervention is indicated after failed conservative attempts to treat symptomatic OCLs or OCLs larger than 1.5cm2. Options are arthroscopic debridement and bone marrow stimulation, defect reconstruction through fragment fixation, retrograde drilling and cancellous bone grafting as well as cartilage restoration procedures.
Surgical Treatment Options
The standard procedure for treatment of symptomatic Grade IIIIV° cartilage defects of the talus according to the International Cartilage Repair Society (ICRS) classification is microfracture (MFx). Through the perforated subchondral bone plate progenitor cells, mesenchymal stem cells, growth factors, and other healing proteins are released into the defect area and form a superclot that provides an enriched environment for fibrous cartilage formation [10]. MFx provides the highest level of evidence for its good clinical outcome compared to all other cartilage repair techniques [11]. However, the good initial clinical results are reported to start to worsen after 5 years [12,13]. Significantly inferior results have been found in defects exceeding 1.5 cm2 [14,15].
Autologous Chondrocyte Implantation (ACI) has been tried in the past decades for the treatment of larger talar defects, those associated with subchondral bone cysts and those who have failed arthroscopic debridement. It is an established 2-step procedure with seemingly good clinical results in which in a first step harvested cartilage cells are expanded in vitro and in a second step are re-implanted into the joint [16-18]. Drawbacks of the technique reported are a challenging time-consuming surgical technique involving periosteal flap placement in an open approach. Other difficulties faced are non-homogenous distribution of chondrocytes with the risk of leakage if the flap is not adequately sealed, complications at the periosteal harvest site and graft hypertrophy [19].
For some years now, Matrix-induced Autologous Chondrocyte Implantation (MACI) has gained increasing popularity. It differs from the original ACI mainly in handling properties, since the in vitro expanded cells are cultured onto a scaffold, forming a unit which is easier to implant [20]. Similar results as in ACI are reported [21].
Nam et al. [22] published data on 11 patients and found an AOFAS score of 84.3 and a mean Tegner score of 4.0 at a mean follow-up of 3 years. Baums et al. [23] followed up 12 patients and found a mean AOFAS score of 88.4 at 5 years. Neither study had any failures. Schneider et al. reported on 20 patients with a mean age of 36 (19 to 61) years and an AOFAS score of 87 with 2 failures at 21 months [19]. A mean AOFAS score of 89.5 at 3 years in 46 patients with a mean age of 31 was found following arthroscopic MACI of the talus by Giannini et al. [18]. In a very recent publication by Anders et al. [20] 22 patients showed stable improvement of pain and function at 5 years follow-up.
Depending on the location of the OCL an arthroscopic MACI as described by Cherubino et al. [24] or MACI via mini-arthrotomy might be suitable and is described by Giza et al. [25].
ACI and MACI are both very cost- and labor-intensive operative options, with the need for 2 interventions. The superiority of ACI/ MACI techniques over that of microfracturing could not be conclusively established so far [26-28]. In a systematic review Niemeyer et al. acknowledged that current evidence concerning the use of ACI in the talus is still elusive and a superiority or inferiority to other techniques could not be proven [28].
Most authors report that they still prefer an open approach with ACI/MACI necessitating an osteotomy [29,30]. Complications associated with osteotomies include the risk of nonunion, symptomatic hardware and injury to the previously intact articular surface.
Besides MFx, mosaicplasty, also called osteochondral autograft system (OATS) was and still is popular in some countries when attempting to reconstruct OCLs of the talus. It has proven to be delicate because of the incongruency of the donor- and recipient site and is associated with high donor-site morbidity. In more than 50% of the cases, complaints in the knee joint from where the grafts were harvested have been reported [31]. Furthermore, an autograft cannot be implanted in the ankle without an osteotomy of the medial or lateral malleolus, which can lead to future complications and late adverse effects already described above.
Current research is focusing on one-step procedures, e.g. scaffold enhanced microfracture with the goal to provide a simple, cost-effective clinical solution without the challenges associated with cell culture and a second surgical intervention [29,32].
Pre-requisite for the development of adequate repair cartilage is the stabilization of the progenitor cells-containing blood clot formed after microfraturing [33]. Stabilizing this superclot with a scaffold facilitates colonization, proliferation and chondrogenesis of mesenchymal stem cells [34,35].
Autologous Matrix-Induced Chondrogenesis (AMIC) is a scaffold enhanced cartilage repair technique. A collagen type-I/III bilayer matrix (Chondro-Gide, Geistlich Pharma AG, Switzerland) is added to the superclot and fixed with commercially available fibrin glue. Mesenchymal progenitor cells migrate towards and adhere to the porous layer of the matrix. In vitro studies have shown that the collagen matrix prevents shrinkage of the super clot and that its use in combination with commercially available fibrin glue (Tissucol or Tisseel from Baxter) supports chondrogenic differentiation of human mesenchymal stem cells and significantly enhance proteoglycan deposition [36-40].
As in MFx, in defects with subchondral cyst formation, AMIC can be combined with cyst resection and filling of the defect with bone graft, either from the iliac crest, the tibia or the calcaneus.
The Authors’ AMIC Technique
Access
The majority of OCLs require access to the medial talar shoulder. Entry is between the medial malleolus and the anterior tibial tendon. The skin incision is marked accordingly.
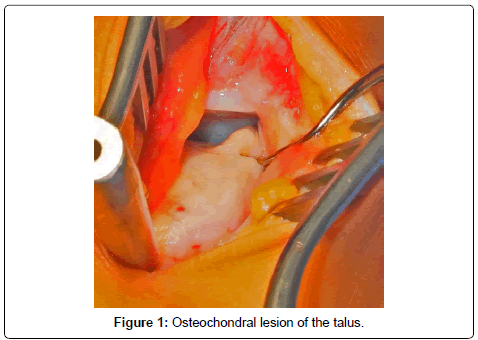
A mini-arthrotomy is performed to open the joint by dissection down to the level of the joint capsule. With the help of a K-wire distractor as guide, K-wires are drilled into the distal tibia and parallel into the neck of the talus, joint distraction is achieved with the foot in maximum plantar flexion (Figure 1).
Cartilage reconstruction
Prior to drilling or MFx, the OCL is cleaned of all unstable cartilage, necrotic bone and any cysts present are curetted. A healthy cartilage rim is created for matrix implantation. If the subchondral bone displays sclerosis, drilling under cooling may be required instead of MFx to penetrate below this layer.
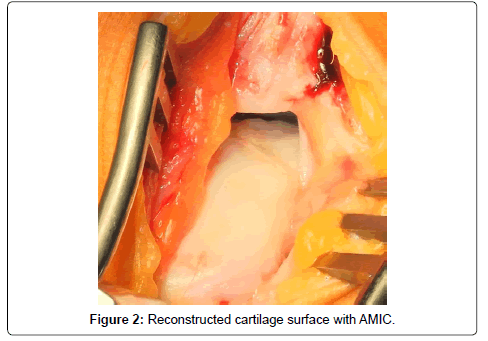
Bone graft is used to reconstruct osseous defects larger than 2 mm. To avoid delamination of the matrix, the defect should be filled only to the level of the subchondral bone lamella. Sealing of the graft with fibrin glue ensures adequate stability for talar cartilage reconstruction (Figure 2).
Then, the collagen matrix, comprising of a cell occlusive and a porous layer, is prepared by cutting it to fit the defect. For this purpose, a special template is pressed into the defect with a forceps so that the borders of the cartilage are clearly depicted. Using commercially available fibrin glue the matrix is glued onto the bone graft, carefully avoiding matrix overlap over adjacent cartilage. Overlaps can lead to delamination of the matrix on joint movement.
After hardening of the glue, the distractor is removed and the joint moved throughout the range of motion several times to verify stability of the matrix and appropriate level of bone graft. In case delamination occurs, bone graft level and matrix overlap should be checked and corrected as needed. The joint is then closed with resorbable sutures, and if needed, a drain is inserted. Elastic compression bandage is applied and the tourniquet released. The ankle is immobilized with a dorsal plaster splint for the first 48 h post-operative.
Special surgical considerations
Defects in the medial and lateral talar shoulder and centrally located defects require a ventrocentral approach between the anterior tibial and the extensor hallucis longus tendon that enables visualization of the entire ankle joint. If a dorsolateral approach is used, patient positioning must ensure adequate dorsal extension of the knee. A dorsomedial approach between the medial malleolus and the posterior tibial tendon may also be used as found necessary. To ensure success of talar articular cartilage reconstruction, any other concomitant pathology present such as axial deviations or hindfoot deformities must be corrected. Capsule and ligament issues, if any, must be addressed as appropriate.
Postoperative management
Meticulous attention to managing the patient immediately after surgery and ensuring patient compliance for 12 months thereafter are prerequisites for success of the surgical intervention. Premature loading of the joint, which is likely to interfere with the healing process and the formation of repair tissue is to be avoided. However, the joint must be subject to adequate motion to prevent adhesions and prevent stiffness. Thus, the recovery process comprises a sequence of steps of gradually increasing the load on the joint. As load bearing improves with growth of repair tissue, weight bearing, exercise and impact are added at stated intervals.
Initially the joint must be immobilized for 48 h following surgery at which time the drain can be removed. Limited continuous passive motion (20-0-20) lessens the risk of matrix delamination. In defects located very ventral or dorsal of the joint surface, the recommended range of motion is modified accordingly. For the next 2 weeks, the joint is protected with dorsal splints, at which time, healing should be completed. Thereafter, weight bearing is gradually increased; starting with 10 kg in the first 6 weeks, loading is increased by 20 kg every 2 weeks in the succeeding 6 weeks. At the end of 12 weeks, the patient is encouraged to perform activities of daily living. Swimming and cycling can also be added as desired by the patient. In general, the patient should avoid all high-impact sports as also those demanding movement with rapid directional change. Cartilage reconstruction can help return patients to activities of normal life including gentle sports activities.
The Authors’ Results
Post-operative results of 36 and more months are available for 20 patients with cartilage defects larger than 2,0 cm2, Grade III and IV according to the ICRS classification. They were treated with AMIC as described before. No intraoperative complications were observed. The average age at the time of surgery was 39 (Range 19 to 60). In 15 cases the cartilage reconstruction was possible without performing a medial malleolar osteotomy. In 13 cases an anteriormedial approach was used, in 2 cases an anterorlateral approach. The Chondro-Gide® matrix was fixed with fibrin glue in all cases. In 16 cases a cancellous bone graft from the ipsilateral calcaneus was performed. The postoperative care included a non weight bearing period of 6 weeks with continuous passive motion (CPM), followed by 6 weeks of increasing weight bearing.
Foot Function Index (FFI) [41] (0 - best result, 100 - worst result) improved from 55.0 (SD 19.6) to 24.5 (SD 14.5) in the pain subscale, the function subscale changed from 60.1 (SD 13.7) to 28.1 (SD 21.0). The total FFI showed a significant improvement from 57.9 (SD 13.7) to 26.5 (SD 17.4). The AOFAS Score [42] (0-worst result; 100- best result) increased from 50.8 (SD 17.9) to 81.7 (SD 12.8). There was no correlation between outcome and the age of the patient at the time of surgery. Patients who sustained a medial malleolar osteotomy had inferior results compared to patients without additional osteotomy: 76.5 (SD 9.8) vs. 85.4 (SD 13.6). In 2 cases, MRI showed new cyst formation. In 1 case, residual complaints necessitated repeat arthroscopy, which showed an unstable graft and hypertrophic repair tissue with impingement. In all other cases, MRI showed good defect filling without increased effusion (Figure 3).
Comparison AMIC to MACI
Scaffold based reconstruction techniques have emerged as valid treatment alternative for focal chondral and osteochondral cartilage defects of the talus (ICRS Grade III and IV) >1.5 cm2 resulting from traumatic events and for osteochondrosis dissecans. It is important to respect, that those techniques are not indicated for degenerative changes in the ankle joint, kissing lesions, inflammatory joint disease, crystal arthropathy and neuroarthopathy. There is no clear age limit for those procedures, however inferior results in older patients are more likely. Instability and axis malalignment can and should be addressed at the same session.
AMIC and MACI are both clinically established, longer follow-up results are published for the latter [20]. A comparative overview of the here described scaffold-based reconstruction techniques is presented (Table 1).
| AMIC | MACI | |
|---|---|---|
| Indication | chondral and osteochondral defects > 1.5cm2 | |
| Surgical Technique Access | 1-step arthroscopic, mainly mini-open or open | 2-step mainly mini-open or open (osteotomy) |
| Intraoperative Handling | easy | medium |
| Time | analog to conventional surgical intervention | time-consuming 2 surgical procedures |
| Post-OP | immobilization for 48h limited CPM and dorsal splints for 2 weeks gradual increase weight bearing for 12 weeks starting with 10kg first 2 weeks, then 20kg every 2 weeks | |
| Complications | normal surgery associated risks revision possible | harvest side morbidity normal surgery associated risks revision possible |
| Outcome and Prognosis | pain reduction and improvement functionality for daily activities durable mid-term | |
| Material and Infrastructure | Chondro-Gide Matrix Fibrin glue | MACI graft (expensive due to laboratory, transport and quality requirements) 2 surgical procedures Fibrin glue |
| Logistics | off-the-shelf | high logistical demand |
| Regulatory Approval | medical device Europe CE Mark - EU and rest of world US: not approved | pharmaceutical product in Europe requires EMEA authorization US: not approved Germany: not approved (prohibition right) |
Table 1: Comparison of 2 scaffold-based cartilage reconstruction techniques - AMIC and MACI.
Perspectives
Sufficiently powered, randomized clinical trials with uniform methodology and most importantly validated and sensitive outcome measures are needed to compare surgical strategies for OCL of the talus. Our results suggest that AMIC may be an effective way to treat fullthickness lesions of the talus in patients who do not respond to initial curettage. This especially when compared to ACI/MACI which failed so far to generate truly convincing evidence that extra money spent generates substantially better outcome. There is a clear trend to simple, one-step approaches in the talar cartilage repair strategies. In Germany the scaffold used for AMIC is approved as medical device and available for a fraction of the cost of a MACI graft. MACI for talus is not approved. Furthermore, all cell-based cartilage repair techniques are regulated as of 2013 as advanced-therapy medicinal products (ATMPs) requiring marketing authorization by the EMEA. This has and will drastically increase development costs and timelines, rendering scaffold-cellbased reconstruction techniques relatively unattractive to the industry, reimbursement providers as well as hospital administrators.
Generally, more comparative effectiveness research-costs versus clinical results - would improve the transparency of health care expenditures. Of course, such work is additionally complicated though the fact that treatment effects can vary by inter- and intra-patient variability. It would also facilitate the decisions of reimbursement providers and help to ensure that innovative treatment strategies are focused on cost-effectiveness.
References
- McGahan PJ, Pinney SJ (2010) Current concept review: osteochondral lesions of the talus. Foot Ankle Int 31: 90-101.
- Flick AB, Gould N (1985) Osteochondritis dissecans of the talus (transchondral fractures of the talus): review of the literature and new surgical approach for medial dome lesions. Foot Ankle 5: 165-185.
- Verhagen RA, Struijs PA, Bossuyt PM, van Dijk CN (2003) Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin 8: 233-242.
- van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ (2010) Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc 18: 570-580.
- Benthien RA, Sullivan RJ, Aronow MS (2002) Adolescent osteochondral lesion of the talus. Ankle arthroscopy in pediatric patients. Foot Ankle Clin 7: 651-667.
- Wester JU, Jensen IE, Rasmussen F, Lindequist S, Schantz K (1994) Osteochondral lesions of the talar dome in children. A 24 (7-36) year follow-up of 13 cases. Acta Orthop Scand 65: 110-112.
- van Bergen CJ, de Leeuw PA, van Dijk CN (2008) Treatment of osteochondral defects of the talus. Rev Chir Orthop Reparatrice Appar Mot 94: 398-408.
- Elias I, Jung JW, Raikin SM, Schweitzer MW, Carrino JA, et al. (2006) Osteochondral lesions of the talus: change in MRI findings over time in talar lesions without operative intervention and implications for staging systems. Foot Ankle Int 27: 157-166.
- van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ (2010) The natural history of osteochondral lesions in the ankle. Instr Course Lect 59: 375-386.
- Steadman JR, Rodkey WG, Briggs KK (2002) Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg 15: 170-176.
- Loveday D, Clifton R, Robinson A (2010) Interventions for treating osteochondral defects of the talus in adults. Cochrane Database Syst Rev : CD008104.
- Gobbi A, Nunag P, Malinowski K (2005) Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc 13: 213-221.
- Mithoefer K, Scopp JM, Mandelbaum BR (2007) Articular cartilage repair in athletes. Instr Course Lect 56: 457-468.
- Choi WJ, Park KK, Kim BS, Lee JW (2009) Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med 37: 1974-1980.
- Chuckpaiwong B, Berkson EM, Theodore GH (2008) Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy 24: 106-112.
- Petersen L, Brittberg M, Lindahl A (2003) Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin 8: 291-303.
- Lee KT, Lee YK, Young KW, Park SY, Kim JS (2012) Factors influencing result of autologous chondrocyte implantation in osteochondral lesion of the talus using second look arthroscopy. Scand J Med Sci Sports 22: 510-515.
- Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B (2008) Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med 36: 873-880.
- Schneider TE, Karaikudi S (2009) Matrix-Induced Autologous Chondrocyte Implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int 30: 810-814.
- Anders S, Goetz J, Schubert T, Grifka J, Schaumburger J (2012) Treatment of deep articular talus lesions by matrix associated autologous chondrocyte implantation--results at five years. Int Orthop 36: 2279-2285.
- Aurich M, Venbrocks RA, Fuhrmann RA (2008) [Autologous chondrocyte transplantation in the ankle joint. Rational or irrational?]. Orthopade 37: 188, 190-195.
- Nam EK, Ferkel RD, Applegate GR (2009) Autologous chondrocyte implantation of the ankle: a 2- to 5-year follow-up. Am J Sports Med 37: 274-284.
- Baums MH, Heidrich G, Schultz W, Steckel H, Kahl E, et al. (2006) Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am 88: 303-308.
- Cherubino P, Grassi FA, Bulgheroni P, Ronga M (2003) Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg (Hong Kong) 11: 10-15.
- Giza E, Sullivan M, Ocel D, Lundeen G, Mitchell ME, et al. (2010) Matrix-induced autologous chondrocyte implantation of talus articular defects. Foot Ankle Int 31: 747-753.
- Gobbi A (2008) Error in level of evidence. Arthroscopy 24: 247.
- Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G (2006) Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy 22: 1085-1092.
- Niemeyer P, Salzmann G, Schmal H, Mayr H, Südkamp NP (2012) Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc 20: 1696-1703.
- Wiewiorski M, Leumann A, Buettner O, Pagenstert G, Horisberger M, et al. (2011) Autologous matrix-induced chondrogenesis aided reconstruction of a large focal osteochondral lesion of the talus. Arch Orthop Trauma Surg 131: 293-296.
- Giza E (2008) Operative techniques for osteochondral lesions of the talus. Foot Ankle Spec 1: 250-252.
- Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, et al. (2009) Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med 37 Suppl 1: 105S-111S.
- Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B (2009) One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res 467: 3307-3320.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR (2009) Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med 37: 2053-2063.
- Chen H, Chevrier A, Hoemann CD, Sun J, Ouyang W, et al. (2011) Characterization of subchondral bone repair for marrow-stimulated chondral defects and its relationship to articular cartilage resurfacing. Am J Sports Med 39: 1731-1740.
- Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, et al. (2005) Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am 87: 2671-2686.
- Dickhut A, Dexheimer V, Martin K, Lauinger R, Heisel C, et al. (2010) Chondrogenesis of human mesenchymal stem cells by local transforming growth factor-beta delivery in a biphasic resorbable carrier. Tissue Eng Part A 16: 453-464.
- Kirilak Y, Pavlos NJ, Willers CR, Han R, Feng H, et al. (2006) Fibrin sealant promotes migration and proliferation of human articular chondrocytes: possible involvement of thrombin and protease-activated receptors. Int J Mol Med 17: 551-558.
- Sage A, Chang AA, Schumacher BL, Sah RL, Watson D (2009) Cartilage outgrowth in fibrin scaffolds. Am J Rhinol Allergy 23: 486-491.
- Richter W (2009) Mesenchymal stem cells and cartilage in situ regeneration. J Intern Med 266: 390-405.
- Homminga GN, Buma P, Koot HW, van der Kraan PM, van den Berg WB (1993) Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand 64: 441-445.
- Naal FD, Impellizzeri FM, Huber M, Rippstein PF (2008) Cross-cultural adaptation and validation of the Foot Function Index for use in German-speaking patients with foot complaints. Foot Ankle Int 29: 1222-1228.
- Kitaoka HB, Patzer GL (1997) Analysis of clinical grading scales for the foot and ankle. Foot Ankle Int 18: 443-446.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15137
- [From(publication date):
July-2013 - Apr 14, 2025] - Breakdown by view type
- HTML page views : 10547
- PDF downloads : 4590