Research Article Open Access
Sandfly and Leishmaniasis: A Review
Mobeen Ghazanfar* and Muhammad Faheem Malik
Department of Zoology, University of Gujrat, Pakistan
- *Corresponding Author:
- Ghazanfar M
Department of Zoology
University of Gujarat, Pakistan
Tel: +92 53 3643112
E-mail: mobimubeen56@yahoo.com
Received Date: July 18, 2016; Accepted Date: August 24, 2016; Published Date: August 31, 2016
Citation: Ghazanfar M, Malik MF (2016) Sandfly and Leishmaniasis: A Review. J Ecosys Ecograph 6:207. doi:10.4172/2157-7625.1000207
Copyright: © 2016 Ghazanfar M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
Sandfly has a long history of association with humans, which still are suffering from its harmful impacts. It parasites humans and other animals and acts as a source of nuisance and annoyance to them. The present bibliographical study explains the role of Sandfly as a disease vector of Leishmaniasis. This article highlights various aspects of the life of the vector which includes its general description of morphology, biology, life cycle, and major control strategies. Regarding to control strategies of the vector, it was reviewed that chemical control is the most common and efficient technique but sensitivity to is decreasing due to increased insecticide resistance and environmental constraints. This article also highlights the different types of Leishmaniasis and symptoms, treatment, mortality rate and species diversity of Sandfly in Pakistan.
Keywords
Sandfly; Phlebotomine flies; Leishmaniasis; Sandfly diversity in Pakistan
Introduction (Leishmaniasis and Sandfly as Vector)
Leishmaniasis is found every continent except for Antarctica and Australia. Sandflies are the carrier of lashmaniasis ; affecting human health in more than 90 countries in the subtropics, tropics, and southern Europe [1]. It is more common in rural areas as compare to urban ones. This disease is more common in male adults [2]. Leishmaniasis disease is trasnmited by the Phlebotomine flies, genus phlebotomus in the wew world and Lutzomyio in the old world. The vector of this disease belongs to the order Diptera , class insecta , Family: Psychodidae [3]. Sandfly is about 3 mm in length, characterized as “hopping” flight. They have dark and large eyes, long antennae the mouthparts are oriented downward, dagger shaped and short, the legs are delicate and long [4].
This disease is cause by 20 Leishmania species by the bite of female sandflies. Thirty species of sandflies are the vector of disease. Their reservoir hosts are humans and wild or domestic animals. Female Sandflies feed on reservoir hosts and get infected [5]. This parasite is transmitted through the use of infected syringes from infected individuals. The common species of Leishmania are L. chagasi , L.infantum and L. donovani [6]. There has been chance of increase in Leishmaniasis in the last two decades due to the migration of people towards urban area [7]. Through travelling, Leishmaniasis spread in people which are living in non-endemic areas.
Habitat and flight range
Sand flies are nocturnal and sensitive to dehydration. They shelter in caves, rocks, animal burrows, tree holes and human rooms or accommodation. They fly close to the ground in short hops (jumps) therefore they are called weak flyers. Their flying range is 300 m but in deserted environments some species can travel up to 2300 m [8]. Due to short range of flight, adult stay near to the larval developmental site. Flies in the New World are found near tree holes and caves [9]. Lutzomyia shannoni in the United States was found in hardwood forest and meadow. In the Old World sand flies are found associated with contaminated soils of animal shelters, rodent burrows and termite mounds, also in the earthen floors of human habitations [10].
Feeding behavior
Male and female sandfly feed on nectar from fruits, flowers and plant juices. Carbohydrates are the source of energy. Female flies suck a blood meal to complete the development of egg batches. Some species of sandfly are autogenous, these species lay butch of eggs without first feeding on blood, female species of sandfly are the disease causing agents [11]. Most anthropophilic (blood sucking arthropods) sand flies bite people outside their tents, houses and accommodation [12].
Growth and development of Sandfly
Sandfly requires 28 degree centigrade temperature and 40% humidity for growth and development. In laboratory, these flies take 20-40 days to complete the life cycle. The sandfly shows holometabolous metamorphosis (egg, larva, pupa and adult). They can laid between 30 to 70 eggs. Within 1-2 weeks, they hatch. Larvae feed on dead organic matter. Pupal development is completed in 5 to10 days. Before the sun rising adult emerge from pupa, during day time sandfly cannot survive in dry environment so, resting at humid sites. At night this fly drops the ambient temperature and become active by increasing the humidity [13].
Diagnosis
Due to high specificity, parasitological diagnosis remains the gold standard in leishmaniasis diagnosis. Conventional techniques like microscopy and culture still the standard approach for the diagnosis of leishmaniasis at primary health levels in areas of endemicity, because more sophisticated techniques like molecular diagnosis are currently expansive and rarely available [14].
Visceral leishmaniasis are commonly diagnose through serological approaches; identification of antibodies in the serum. Immunochromatographic dipstick tests and freeze dried antigen based direct agglutination test have high specifity, sensitivity and easy to use, require minimal laboratory setup [15].
Morphologically identical species can be differentiated with molecular techniques such as PCR, isoenzymes, etc. Sibling species of sandflies can be identified by polymerase restriction fragment length polymorphism (PCR-RFLP) of the 18S rRNA gene. PCR is a powerful tool for research on sandfly species and the relationship between Leishmania species and their vectors [16].
The infection of sandflies with Leishmania can also be examined by dissecting an individual under a microscope. Fresh specimen should be used. The expertise and skills are needed for the study of small size individuals. This process takes long time, large numbers of individuals have to be examined to obtain data for each area, and the rate of infection of sand flies with Leishmana is generally low 0.01-1% [17].
To identify Leishmaniasis infections in experimentally infected and field captured phlebotomine sandflies molecular techniques are used such as PCR-RFLP, KDNA-PCR, flurescent quantitative PCR and mini exon PCR. Real time PCR also used to detect the sandfly infection of Leishmania [18-20].
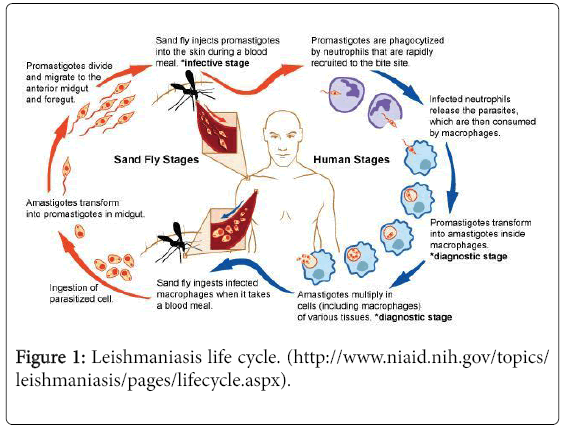
Sandfly and Leshmania Life Cycle
In infected host, the ingested amastigotes (protist cell, non-motile) transform into promastigotes (external flagellum) in the blood meal of different hematophagus arthropods. If the host is unsuitable, than parasites passed out with the feces [21] Leshmania species and other trypanosomatids attached to the parasites on the midgut epithelium [16] during digestion of the blood meal, theparasite is retained in the gut and starts a stage differentiation [22]. In next phase promastigotes move to the cuticle lined foregut of the sandfly. In the foregut some attach and some remain free for next transmission by bite [23] into the vertebrate host.
In the alimentary tract of the sandfly, promastigotes multiply by binary fusion. After 7 days promastigotes undergo metacyclogenesis and become infectious (metacyclic promastigotes). When the sandfly poke the skin with its proboscis during feeding, metacyclic promastigotes are released into the host together with saliva (Figure 1). In host macrophage, meatamorphose into the amastigote form. By binary fission, they increase in number within the phagolysosome until the cell bursts and infects other phagocytic cells. In this way the cycle continues.
Forms of Leishmaniasis Disease
There are four major forms of Leishmaniasis
Cutaneous leishmaniasis
Visceral leishmaniasis
Diffuse cutaneous leishmaniasis
Mucocutaneous leishmaniasis
Cutaneous Leishmaniasis: It is characterized by skin ulcers, lesions may be associated with sporotrichotic (fungus infection). After being bitten, people develop skin sores a few weeks to months. In the old world this disease is caused by L. tropica , L. aethiopica and L. infantum and in New world by L. guyanesis , L. Mexicana and L. braziliensis [16,23]. There are two epidemiological forms of cutaneous Leishmaniasis which are arthroponotic: cutaneous Leishmaniasis and zoonotic cutaneous Leishmaniasis. The main reservoir hosts are humans and rodents. The main vector in arthroponotic cutaneous leishmaniasis and zoonotic cutaneous leishmaniasis are P. sergenti and P. papatasi [23-25]. The infection manifests mainly on adults and young children [26].
Visceral Leishmaniasis: This is the most severe form of the disease also known as Kala azar (black fever) caused by L. donovani and if left untreated are always fatal and mortality rate is 100%. Symptoms of the disease are weight loss, fever (within several weeks or months), hepatomegaly, splenomegaly, pancytopenia, anaemia and lymphadeopathy. This disease is most common in developing countries [6,27]. It is distributed worldwide in both the old and new world. The occurrence of visceral leishmaniasis can also be influenced by environmental changes such as incursion of agricultural farms, urbanization and settlements into the forest areas. The distribution of parasites also is affected by humidity, atmospheric temperature, land degradation, global warming and rainfall [28]. Clinical diagnosis of visceral leishmaniasis is complex because its feature are shared by host of other disease such asthphoid, malaria and tuberculosis. Diagnosis of this disease is in vitro culture and DNA of parasites can be detected in tissue samples (blood or urine). Visceral leishmania caused by L. donovani complex and L. infantumespecially [29]. Visceral leishmaniasis is anthroponotic (disease causing agents carried by humans to other animals) in transmission [30].
Diffuse Cutaneous Leishmaniasis: This disease is characterized by many non-ulcerative skin lesions on the entire body. These lesions contain vacuolated, infected macrophages with only few lymphocytes present. It is wide spread, similar to lepromatous leprosy lesions [31]. The uncontrolled parasite growth results from lack of cell mediated immunity to leishmanial antigen [32].
Mucocutaneous Leishmaniasis: After the onset of cutaneous leishmaniasis, mucocutaneous leishmaniasis occurs and it is characterized by the destruction of pharyngeal and oral- nasal cavities Genetic factors are also important in the incidence of this disease. The initial symptoms of mucocutaneous leishmaniasis are not severe with stuffiness and nasal inflammation, but slowly perforation of the septum and ulceration may occur. The lesion expands to the larynx, gums, pharynx, soft palate and face [33]. Bones are not affected due to bacterial infections untreated disease may lead to diarrhea, pneumonia and tuberculosis [34]. Death may also occur due to malnutrition (difficulty in swallowing), lung infections, and suffocation (due to closure of laryngeal aperture) [35].
Mortality Rate
Leishmaniasis currently is threatening about 350 million people in endemic areas. Global there are 10 to 12 million cases [36-38]. A most recent study indicates that 50,000 deaths occur each year [36].
Sandfly in Pakistan
According Durrani et al. [13] conducted a study from May 2007 to June 2008 and recorded the sandfly fauna in four region of Pakistan. They collected 20,683 sandflies which belong to the genus Phlebotomus . No sand flies were recorded in Eastern Pakistan.
Durrani et al. [13] collected the following species:
P. papatasi
P. major
P. bergeroti
P. alexandri
P. orientalis
P. longipes
P. sergenti
P. pedifer
Endemic areas for the disease vector, described by Bhattacharia et al. [39] were Swat and Gilgit, Lasbela, Rawalpindi, Abbottabad, Chilas, Skardu, Mansehra, Chitral, Dir, D.G. Khan, Rajanpur, Quetta, QilaSaifullah, Qila Abdullah, Pishin, Larkana, AzadKashmir and Dadu. These areas are the North, West and South Western Pakistan. Ali et al. [40] confirmed that south-eastern areas of Pakistan are nonendemic.
Sandfly Control Techniques
Sandfly control is similar to that used for mosquito because both vector share similar characteristics. Bacillus sphaericus is use to control sandfly larvae. In this innovative technique, bait-fed adults were used to carry the bacterial control agent to larval habitats, resulting in larval mortality in burrows up to 10-30 m away from the baited solution [41].
Insecticides
Spray of insecticides on burrows, walls and humans accommodation. The first insecticides use to control sandfly was DDT (dichlorodiphenyltrichloroethane) and it was sprayed in India, Brazil, China, Soviet Union. It caused reduction in population of sandfly, but there was no direct impact on disease control [42]. From the Middle East, study of four species of sand flies indicates that malathion and DDT less toxic than the newer pyrethroid insecticides [43].
In 1958 and 1970 there was program to control visceral leishmaniasis in Bihar (India). No visceral leishmania disease was reported during these years but at the end of this control program, disease resurface. Antimalarial programs reduce the population of sandfly in Iran, Bangladesh, Peru and Italy. But this did not reduce leishmaniasis [42].
Following insecticides are currently use to control sandfly [44]
Talstar P® (bifenthrin, NSN 6840-01-525-6888)
Aqualure® 20+20 (permethrin, PBO; NSN 6840-01-606-8581)
Demon® WP (cypermethrin, NSN 6840-01-390-4822)
Demand® CS (λ-cyhalothrin, NSN 6840-01-428-6646)
Pestabs® (λ-cyhalothrin, NSN 6840-01-431-3357)
Habitat modification and pesticides
Habitat modification such as ploughing or flooding in the host ecosystem reduces the sandfly density. Pesticide application is effective to control the density of sandflies if target sites are known. But this application is not sustainable, limited by resources and time [21].
Treatment of Leishmaniasis
For all four major forms of Leishmaniasis chemotherapy is effective. Certain factors compromise chemotherapeutic options such as toxicity, costs, complicated and long term regimens “establishment” [45,46]. Many drugs are also use to get recovery from Leishmnaisis.
The eradication of this disease will be achieved through vaccination [47]. Bacon [48] describes that if population of Venezuela, Peru, Colombia, Ecuador, Mexico, Brazil and Bolivia were vaccinated through vaccine than it would provide ten years of protection, 41,000-144,000 cases of cutaneous leishmaniasis could be averted, and this would be lower cost than other treatments. Vaccine provides 5 years of protection and 50% efficacy [48]. In Bihar State similar study for visceral leishmaniasis also confirmed the cost effectiveness of vaccine [48]. The development of vaccine is under processing.
Conclusion
Leishmaniasis occurs due to sandfly species that feed on human and other reservoir hosts. Young children and male adults are mostly affected. Each year millions of deaths occur due to this disease. Waste openly disposed is the major factor for attracting the vector [49-52]. It is concluded that vector and parasites have adaptability to spread in new environments such as urban and suburban areas. Migration of people from rural to urban areas increases the risk of leishmaniasis. Slow progress has been made on the development of a vaccine. The development of vaccine has proven a challenging and difficult risk due to inadequate knowledge of the parasite pathogenesis and complexity of the immune response. Sandfly population should be controlled to prevent Leishmaniasis. There is lack of information and interest to evaluate the significance of Sandfly control in disease control. This is a neglected disease due to limit resources for diagnosis, treatment and control in developing countries.
Recommendations
Following are some suggestion to control the vector and Leishmaniasis:
• To get protection from sandflies keep the environment clean.
• Use bed net to protect yourself and use pyrethroid-containing insecticide.to spray the bed net
• Uses of insecticides to control sandfly.
• Do not expose the skin, wear long sleeved shirts.
• Use insect repellent on exposed skin.
• Waste should not be openly disposed.
• Drug treatments are expensive, poor people cannot afford. Drugs are also toxic and have numerous side effects.
• To control this disease efficacious vaccine is needed. Vaccines are well tolerated, safe and immunogenic. Vaccines effective in treatment and prevention of different form of leishmaniasis.
• If Leishmaniasis symptoms appear it is important to consult a physician immediately without wasting time.
• There should be need to develop public awareness about this vector.
• There should be need to aware people about this vector.
References
- Desjeux P (2000) Pyrethroid impregnated bed nets: an alternative vector control approach for leishmaniasis. Proceedings of 13th European SOVE Meeting.
- Terefe Y, Afera B, Bsrat A, Syoum Z (2015) Distribution of human leishmaniasis (VL) and its associated risk factors, in Metemma, Ethiopia. Epidemiol Res Int 2015: 630812.
- Kishore K, Kumar V, Kesari S, Dinesh DS, Kumar AJ, et al. (2006) Vector control in leishmaniasis. Indian J Med Res 123: 467-472.
- Claborn DM (2010) The biology and control of leishmaniasis vectors. J Global Infect Dis 2: 127-134
- Philippe D (1996) Leishmaniasis public health aspects and control. Elesevier science Inc 14: 417-423.
- Schonian G, Mauricio I, Cupolillo E (2010) Is it time to revise the nomenclature ofLeishmania? Trends Parasitol 26: 466-469.
- Desjeux P (2004) Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27: 305-318.
- Gomez SE, Doud CW, Maroli M (2005) Short report: surveillance of Leishmania sp. among sand flies in Sicily (Italy) using a fluorogenic real-time polymerase chain reaction. Am J Trop Med Hyg 72: 138-141
- Feliciangeli MD (2004) Natural breeding places of phlebotomine sandflies.Med Vet Entomol 18: 71-80.
- Claborn DM, Masuoka P, Morrow M, Keep L (2008) Habitat analysis of North American sand flies near veterans returning from leishmania-endemic war zones.Int J Health Geogr 7: 65.
- Hashiguchi Y, Gomez EAL (1991) A review of leishmaniasis in Ecuador. Bull Pan Am Health Org 25: 64-76.
- Colacicco-Mayhugh MG, Grieco JP, Putnam JL, Burkett DA, Coleman RE (2011) Impact of phlebotomine sand flies on United States military operations at Tallil Air Base, Iraq: 5. Impact of weather on sand fly activity. J Med Entomol 48: 488-545.
- Durrani ZA, Durrani ZR, Kamal N (2012) Prevalence of Leishmania in sand fly in Pakistan. Pak J Zool 44: 61-65
- Garcia AL, Kindt A, Quispe-Tintaya KW, Bermudez H, Llanos A, et al. (2005) American tegumentary leishmaniasis: antigen-gene polymorphism, taxonomy and clinical pleomorphism. Infect Genet Evol 5: 109-116.
- Chappuis F, Rijal S, Soto A, Menten J, Boelaert M (2006) A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ 333: 723-726.
- Mukhopadhyay J, Ghosh K, Braig HR (2000) Identification of cutaneous leishmaniasis vectors, Phlebotomuspapatasi and P. duboscqi using random amplified polymorphic DNA. Acta Trop 76: 277-283.
- Hendry K, Vickerman K (1988) The requirement for epimastigote attachment during division and metacyclogenesis in Trypanosoma congolense. Parasitol Res 74: 403-408.
- Kato H, Uezato H, Gomez EA (2007) Establishment of a mass screening method of sandfly vectors for Leishmania infection by molecular biological methods. Am J Trop Med Hyg 77: 324-329.
- Paiva BR, Secundino NF, Nascimento JC, Pimenta PF, Galati EA, et al. (2006) Detection and identification of Leishmania species in field-captured phlebotomine sandflies based on miniexon gene PCR. Acta Trop 99: 252-259.
- Harwood RF, James MT (1977) Entomolgy in human and animal health (7th edn.). Macmillan Publishing ColkInc
- Kitchen LW, Lawrence KL, Coleman RE (2009) The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets. J Vector Ecol 34: 50-61.
- Bonaldo MC, Souto-Padron T, de Souza W, Goldenberg S (1988) Cell-substrate adhesion during Trypanosoma cruzi differentiation. J Cell Biol 106: 1349-1358.
- Motazedian Mh, Mehrabani D, Oryan A, Asgari Q, Karamian M, et al. (2006) Life cycle of cutaneous leishmaniasis in Larestan, southern Iran. J Clin Infect Dis 1: 137-143.
- Shirian S, Oryan A, Hatam GR, Panahi S, Daneshbod Y (2014) Comparison of conventional, molecular, and immunohistochemical methods in diagnosis of typical and atypical cutaneous leishmaniasis. Arch Pathol Lab Med 138: 235-240.
- Mehrabani D, Motazedian MH, Oryan A, Asgari Q, Hatam GR, et al. (2007) A search for the rodent hosts of Leishmania major in the Larestan region of southern Iran: demonstration of the parasite in Tateraindica and Gerbillus sp., by microscopy, culture and PCR. Ann Trop Med Parasitol 101: 315-322.
- Rab Ma, Azmi Fa, Iqbal J, Hamid J, Ghafoor A, et al. (1986) Cutaneous leishmaniasis in Baluchistan: Reservoir host and sandfly vector in uthal, Lasbella. J Pak Med Assoc 134-138.
- Killick-Kendrick R (1979) Biology of the Kinetoplas-Tida. In: Lumsden WHR, Evans DA (eds.) Academic Press, The University of Michigan, New York. pp: 738.
- Bari UA, Rahman SB (2008) Cutaneous leishmaniasis: an overview of parasitology and host-parasite-vector inter relationship. J Pak Assoc Derma 18: 42-48.
- Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM (2003) Post kalaazar dermal leishmaniasis. Lancet Infect Dis 3: 87-98.
- Alval J, Aparicio P, Aseffa A, Boer MD, Canavate C, et al. (2008) The relationship between leishmaniasis and AIDS: the second 10 years.Clin Microbiol Rev 21: 334-359.
- Petersen EA, Neva FA, Oster CN, Bogaert Diaz H (1982) Specific inhibition of lymphocyte proliferation resposes by adherent suppressor cells in diffuse cutaneous leishmaniasis. N Engl J Med 306: 387-392.
- Barral A, Costa JM, Bittencourt AL, Barral- Netto M, Carvalho EM (1995) Polar and subpolar diffuse cutaneous leishmaniasis in Brazil: clinical and immune pathologic aspects. Int J Dermatol 34: 474-479.
- Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, et al. (2007) Cutaneous leishmaniasis. Lancet Infect Dis 7: 581-596.
- Wilson ME (1998) Leishmaniasis in public health and preventive medicine.In:Wallace RB, Boebbelin BN (eds.). New York: McGraw-Hill. pp: 334-336.
- Philip D, Marsden, Elmer A, Llanos-Cuentas, Edinaldo L, et al. (1984) Human Mucocutaneous Leishmaniasis in Tres Bracos, Bahia-Brazil. An area of Leishmania braziliensis transmission III mucosal disease presentation and initial evolution. Revista da Socied de Brasileira de Medicina tropical 17: 170-186.
- Hotez PA (2011) Handful of ‘antipoverty’ vaccines exists for neglected diseases, but the world's poorest billion people need more. Health Aff 30: 1080-1087.
- Hotez PJ (2012) The four horsemen of the apocalypse: tropical medicine in the fight against plague, death, famine, and war. Am J Trop Med Hyg 87: 3-10.
- WHO (2014) “Leishmaniasis”. Media Centre.
- Bhattacharya A, Ghosh TN (1983) A search for leishmania in vertebrates from kala azar affected areas of Bihar, India. Trans R Soc Trop Med Hyg 77: 874-875.
- Ali N, Afrin F (1997) Protection of mice against visceral leishmaniasis by immunization with promastigote antigen incorporated in Liposomes. J Parasitol 83: 70-75.
- Robert LL, Perich MJ, Schlein Y, Jacobson RL, Wirtz RA, et al. (1997) Phlebotomine sand fly control using bait-fed adults to carry the larvicideBacillus sphaericusto the larval habitat.J Am Mosq Control Assoc 13: 140-144.
- Alexander B, Maroli M (2003) Control of Phlebotomine Sandflies. Med Vet Entomol 17: 1-18.
- Tetreault GE, Zayed AE, Hanafi HA, Beavers GM, Zeichner BC (2001) Suseptibility of sand flies to selected insecticides in North Africa and the Middle East. J Am Mosq Cont Assoc 17: 23-27.
- Bowles EDC, Britch SC, Linthicum KJ, Johnson RN, Linton YM, et al. (2015) Sand flies (Diptera: Psychodidae: Phlebotomine) significane, surveillance, and control in contingency operations. Armed forces pest management board technical guide no. 49.
- Beaumier CM, Gillespie PM, Hotez PJ, Bottazzi ME (2013) New vaccines for neglected parasitic diseases and dengue. Transl Res 162: 144-155.
- Duthie MS, Raman VS, Piazza FM (2012) The development and clinical evaluation of second generation leishmaniasis vaccines. Vaccine 30: 134-141.
- Lee BY, Bacon KM, Shah M, Kitchen SB, Connor DL, et al. (2012) The economic value of a visceral leishmaniasis vaccine in Bihar state, India. Am J Trop Med Hyg 86: 417-425.
- Bacon KM, Hotez PH, Kruchten SD, Kamhawi S, Bottazzi ME, et al. (2013) The potential economic value of a cutaneous Leishmaniasis vaccine in seven endemic countries in the Americas. Vaccine 31: 480-486.
- Molyneux D, Killick-kendrick R (1987) The Leishmaniases in Biology and Medicine. In: Peters W, Killick-Kendrick R (eds.) Information Systems Division, National Agricultural Library. Academic Press, London 1: 121-176.
- Goddard J (1996) Physician's guide to arthropods of medical importance. Boca Raton: CRC Press
- Hide M, Bucheton B, Kamhawi S, Bras-Goncalves R, Sundar S (2007) Understanding human leishmaniasis: the need for an integrated approach. John Wiley and sons, Inc. pp: 88-89
- Wadhone P, Maiti M, Agarwal R, Kamat V, Martin S, et al. (2009) Miltefosine promotes IFN gamma dominated anti leishmanial immune response. J Immunol 182: 7146-7154.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 18464
- [From(publication date):
September-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 16883
- PDF downloads : 1581