Salivary Amylase: A Monitoring Index for Respiratory Infectious Virus Contamination
Received: 28-Apr-2023 / Manuscript No. JIDT-23-97306 / Editor assigned: 01-May-2023 / PreQC No. JIDT-23-97306 / Reviewed: 15-May-2023 / QC No. JIDT-23-97306 / Revised: 22-May-2023 / Manuscript No. JIDT-23-97306 / Accepted Date: 22-May-2023 / Published Date: 29-May-2023 DOI: 10.4172/2332-0877.1000547
Abstract
Contact infection is a common route of respiratory infections, including that caused by severe acute respiratory syndrome coronavirus 2. Monitoring of viral contamination of environmental surfaces is critical for implementing appropriate hygiene measures and reducing the risk of viral transmission. We assessed the novel utility of the salivary amylase test on environmental surfaces for monitoring the viral contamination risk. An assay based on the principle of immunochromatography was used to detect amylase. Contamination of plastic substrates with amylase and viral genes over time showed similar patterns under laboratory conditions. Moreover, amylase was detected on the surfaces surrounding individuals who performed behaviors (e.g., coughing and sneezing) that spread droplets. Accordingly, detection of amylase might indicate the presence of viral genes in cases where droplets from infected individuals were retained on surfaces. Environmental surfaces (n=186) located in public facilities were investigated and amylase was highly detected in the food courts (66.7-75.0%) and washbasins of hotel guest rooms (100%). However, no correlation was observed between the adenosine triphosphate level (a marker of hygiene control) and the sites positive for amylase. Our research provide a method revealing sites of viral contamination and lead to the establishment of an appropriate infection control system.
Keywords: Infection, SARS-CoV-2, World Health Organization (WHO), Salivary amylase
Introduction
The novel coronavirus disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly worldwide since the detection of its first case in Wuhan, China, in December 2019 [1,2]. The World Health Organization (WHO) has declared the spread of COVID-19 as a “public health emergency of international concern” [3]. COVID-19 is still raging around the world and causing significant health and economic damage.
Studies have suggested that causative viruses of respiratory infections, including SARS-CoV-2, may be transmitted via respiratory droplets and droplet nuclei expelled during coughing or talking, which directly reach the mucosal surface in susceptible individuals [4,5]. In addition, environmental transmission via indirect contact (e.g., materials and surfaces contaminated with droplets/droplet nuclei containing saliva and nasal mucus from infected individuals has been suggested [6,7]. SARS-CoV-2 has been reported to retain infectivity on environmental or finger surfaces for longer periods than influenza A virus [8,9]. Environmental contamination has been highlighted by several investigations reporting contamination of a wide range of environmental surfaces around patients infected with SARS-CoV-2, as well as various environmental surfaces in the community. The concern over environmental contamination with this virus, indicates the importance of adopting countermeasures to reduce the risk of infection [10-12]. Therefore, monitoring of environmental viral contamination and the implementation of appropriate hygiene measures are critical in reducing the risk of viral infection.
Real-time Polymerase Chain Reaction (PCR) for detecting viral genes is commonly used for monitoring viral contamination of environmental surfaces [7,10-12]. However, real-time PCR requires expensive devices and highly specialized procedures, which limits its application in the frequent monitoring of viral contamination in terms of time and expenses. By contrast, the detection of viruses in the saliva instead of nasal mucus samples is used for diagnosing upper respiratory tract infections, including that caused by SARS-CoV-2 [13,14]. In particular, salivary PCR tests are highly accurate in determining positivity for SARS-CoV-2 [15]. Thus, the detection of biological components present in droplets expelled by an infected individual during coughing or talking and retained on environmental surfaces is useful for obtaining an index of the risk of viral contamination on these surfaces.
Aiming at establishing a method for monitoring sites with a potential risk of viral contamination, the present study evaluated the feasibility of using amylase, one of the major protein components of saliva, as a detection index of droplets on environmental surfaces. Amylase is commonly used to assess stress conditions or in forensic research, and test kits are available, but there have been no studies of its applicability to environmental investigations aimed at infection control. Our study proposes amylase as an indicator of viral contamination and will contribute to effective tools used to control COVID-19 and other infectious diseases.
Materials And Methods
Amylase detection
Human salivary alpha-amylase was detected using RSID™ saliva lab kits, according to the manufacturer’s protocol, with some modifications to improve detection sensitivity [16]. Briefly, a cotton swab (J.C.B. Industry Limited, Tokyo, Japan) moistened with 130 μL extraction buffer (provided in the kit) was used to wipe the target surface (approximately 25 cm2 in area). The cotton swab used to wipe the target surface was immersed in a 1.5 mL sampling tube containing 130 μL running buffer provided in the kit. The cotton swab was pressed against the tube wall and moved along the wall surface in clockwise and counterclockwise rotations, 10 rotations in either direction. A 100-μL aliquot of the reaction mixture obtained by this process was added in drops to the cassette provided with the kit, and the presence/absence of a detection line (positive/negative) was visually assessed after standing for 10 min. The amount of amylase was evaluated on a 3-point scale (+, + +, +++) based on the intensity of the color of the detection line (Figures 1A and 1B).
Analysis of amylase and virus detection
Saliva was collected from three healthy participants 30 min after lunch. After the saliva was diluted 10-, 50-, and 100-fold with distilled water, a 1-μL droplet of diluted saliva was dropped onto each polypropylene plastic substrate. The part where the diluted saliva was dropped was swabbed with a cotton swab at 30 min, 3 h, 12 h, 24 h, 48 h, 120 h, and 2 weeks after drying to detect salivary amylase.
A suspension of human coronavirus OC43 strain (OC43, VR-1558; ATCC, Manassas, VA, USA), adjusted to a titer of 4 × 108 TCID 50/mL, was diluted 10-fold with the saliva collected above, and a 1-μL drop of the resulting solution was dropped onto each plastic substrate. At 30 min, 3 h, 12 h, 24 h, 48 h, and 120 h after drying, the virus retained on the plastic substrate was collected with 500 μL 0.05% Tween. The collected liquid was used for quantitation of the copies of the viral RNA genome and evaluation of the infectious titer. The mean temperature and humidity of the experimental environment were 22°C and 40%, respectively.
Viral RNA was extracted from the collected liquid using the QIAamp viral RNA mini kit (QIAGEN, Venlo, The Netherlands). The probe and primer sequences were designed (Supplementary Table 1) and the target viral gene was detected using real-time PCR, as previously described [17]. The infectious titer was determined using HCT-8 cells (CCL-244; ATCC), a human ileocecal adenocarcinoma cell line. HCT-8 cells confluent in a 96-well plate were washed with phosphate buffered saline (PBS, Fujifilm Wako Pure Chemical, Osaka, Japan). The collected liquid containing the virus was diluted 10-fold with Roswell Park Memorial Institute (RPMI) medium (Fujifilm Wako Pure Chemical) containing 2% inactivated horse serum (HS; Gibco, Grand Island, NY, USA) and 50 mg/L gentamicin to make six serial dilutions. Each dilution (100 μL/well) was added to HCT-8 cells, and the cells were incubated at 33°C for 1 h. Then 100 μL RPMI medium containing 2% HS, 50 mg/L gentamicin, and 2 mg/L acetyltrypsin (AT; Sigma-Aldrich, St. Louis, MO, USA) was added, and the mixture was further incubated for 4 days. After washing with PBS, 50 μL methanol (Fujifilm Wako Pure Chemical) was added and the mixture was allowed to stand for 1 min, following which the cells were washed with PBS. The virus was then detected using immunostaining with a monoclonal antibody against human coronavirus OC43 nucleoprotein (mouse anti- coronavirus group antigen antibody, nucleoprotein of OC-43, clone 542- 7D; Sigma Aldrich) diluted 2,000-fold. Horseradish Peroxidase (HRP)- linked goat anti-mouse IgG+IgM antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), diluted 2,000-fold, was used as the secondary antibody. The immune complexes generated were visualized using an HRP detection technique involving reaction with an N,N-Diethyl-P-Phenylenediamine (DEPDA) solution. TCID50 was measured in quadruplicate, and the infectious titer (TCID50/mL) was calculated using the method of Reed and Muench based on the dilution factor of the detected virus [18].
Model behaviors that generate droplets
Coughing, sneezing, meal with speech, and conference with speech were considered as model behaviors that generated droplets. A microplate-type polystyrene Petri dish was placed near an individual performing any of the model behaviors, and a salivary amylase test was performed by swabbing the plate surface (approximately 25 cm2 in area) using a cotton swab after the end of the behavior (Figures 2A-2D).
Detection of Atp
ATP was detected using LuciPac Pen and Lumitester Smart (Kikkoman Biochemifa, Tokyo, Japan) according to the A3 method [19]. The target surface (approximately 25 cm2 in area) was swabbed with a swab of LuciPac Pen moistened with distilled water, and the swab was treated according to the protocol provided by the manufacturer. The LuciPac Pen was inserted into a measurement chamber of the Lumitester Smart to obtain measurements in Relative Light Units (RLU). The surface to be assessed for ATP was located near the sampling point for amylase testing.
Detection of SARS-CoV-2 RNA
The target surface (approximately 25 cm2 in area) was swabbed with a BD sterile cotton swab CA (Nippon Becton Dickinson Company, Ltd., Tokyo, Japan) moistened with 0.05% Tween (Fujifilm Wako Pure Chemical). Each cotton swab was immersed in a tube containing 750 μL 0.05% Tween and transported and stored at ˗20°C. Within 48 h of sampling, the swab tube was vortexed and the supernatant was collected. Virus RNA was extracted using 140 μL of the supernatant according to the instructions of the QIAamp Viral RNA mini kit (QIAGEN). The SARS-CoV-2 genes were detected using reverse transcription-quantitative PCR (RT-qPCR) (NIID_2019-nCOV_N), as previously reported [20].
Amylase detection on environmental surfaces in public facilities
The test was performed at shopping mall A located in Saitama, Japan, shopping mall B located in Chiba, Japan, and a hotel located in Hyogo, Japan. The test at shopping mall A was conducted on March 15, 2021, during office hours (between 2:00 p.m. and 4:00 p.m.). A total of 21 environmental surfaces were examined: eight located in the food court area; four located on chairs, sofas, and tables provided for visitors’ rest in passageways within the facility (hereinafter referred to as “rest area”); and nine located in the area other than the food court area and the rest area (Supplementary Table 2). The test at shopping mall B was conducted on July 29, 2021 during office hours between 1:00 p.m. and 4:00 p.m. A total of 66 environmental surfaces were examined: 26 located in two food court areas, eight located in the rest area, and 32 located in the other area (Supplementary Table 3). The hotel test was conducted on July 8, 2021 between 11:00 a.m. and 1:00 p.m. for 92 surfaces located in guest rooms and eight surfaces located in the lobby floor (Supplementary Tables 4 and 5). A total of eight guest rooms, including six twin rooms (each equipped with an extra bed) and two double rooms, were examined for 12 environmental surfaces per room. Sampling was performed before cleaning immediately after the guests had checked out. Three guests stayed in each twin room, while one guest stayed in each double room.
Statistical analysis
A commercial statistical software program (IBM SPSS Statistics Version 24; IBM Corp., Armonk, NY, USA) was used for statistical analysis. To assess the relationship between the amount of amylase detected and ATP levels, statistical significance of differences between groups was analyzed using one-way analysis of variance, followed by Tukey’s multiple comparison test. P values <0.05 were considered statistically significant.
Results
Sensitivity of detecting amylase on polypropylene plastic surfaces
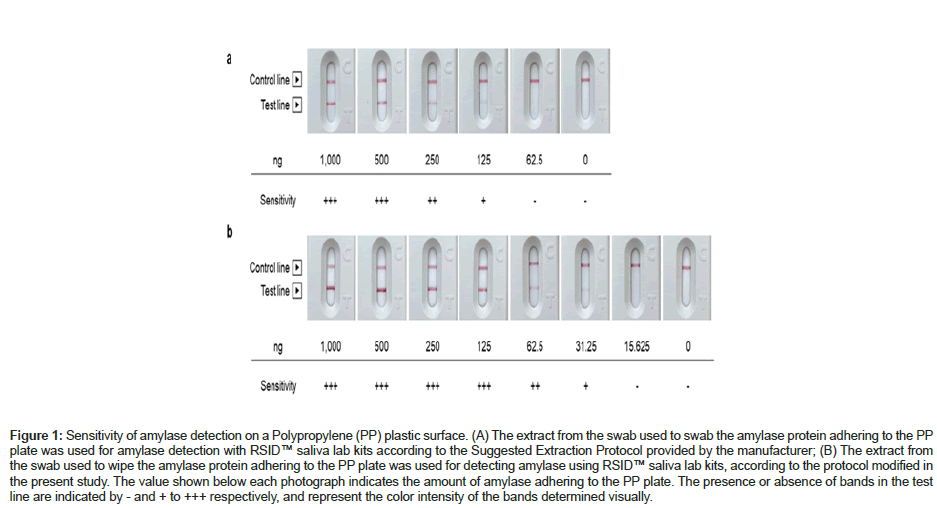
Originally, the lower detection limit for amylase on an environmental surface using the RSID™ saliva lab kits (Independent Forensics, Lombard, IL, USA was 125 ng) (Figure 1A). However, as a result of the improvements described in the Materials and methods section to increase the detection sensitivity, the lower detection limit for amylase changed to 31.25 ng (Figure 1B). As the thickness of the test line correlated with the amount of amylase, the latter was visually determined based on the test line thickness on a 3-point scale: the test line thickness levels equivalent to 31.25 ng, 62.5 ng, and ≥ 125 ng were expressed as +, ++, and +++, respectively.
Figure 1: Sensitivity of amylase detection on a Polypropylene (PP) plastic surface. (A) The extract from the swab used to swab the amylase protein adhering to the PP plate was used for amylase detection with RSID™ saliva lab kits according to the Suggested Extraction Protocol provided by the manufacturer; (B) The extract from the swab used to wipe the amylase protein adhering to the PP plate was used for detecting amylase using RSID™ saliva lab kits, according to the protocol modified in the present study. The value shown below each photograph indicates the amount of amylase adhering to the PP plate. The presence or absence of bands in the test line are indicated by - and + to +++ respectively, and represent the color intensity of the bands determined visually.
Stability of the virus genome, virus titer, and amylase
The stability of amylase and human coronavirus (OC43) RNA genome copy number/infectious titer on the surface of polypropylene plastic substrates was assessed. Amylase dropped onto plastic substrates was detected for up to 5 days at all the dilutions tested (Table 1). After 2 weeks, no amylase was detected on the surface where 100-fold diluted saliva was dropped (Table 1).
| Subject | Dilution | 0.5 h | 3 h | 12 h | 24 h | 48 h | 120 h | 2 weeks |
|---|---|---|---|---|---|---|---|---|
| A | 1/10 | +++ | +++ | +++ | +++ | +++ | +++ | ++ |
| 1/50 | ++ | ++ | ++ | ++ | ++ | ++ | + | |
| 1/100 | + | + | + | + | + | + | - | |
| B | 1/10 | +++ | +++ | +++ | +++ | +++ | +++ | ++ |
| 1/50 | ++ | ++ | ++ | ++ | ++ | ++ | + | |
| 1/100 | + | + | + | + | + | + | - | |
| C | 1/10 | +++ | +++ | +++ | +++ | +++ | +++ | ++ |
| 1/50 | ++ | ++ | ++ | ++ | ++ | ++ | - | |
| 1/100 | + | + | + | + | + | + | - | |
| Note: Saliva from three subjects was diluted 10-, 50-, and 100-folds with DW, and a 1-μL droplet of the solution was dropped onto each plastic substrate. The part where the diluted saliva was dropped was wiped with a cotton swab at 30 min, 3 h, 12 h, 24 h, 48 h, 120 h, and 2 weeks after drying to test salivary amylase. “+/-”: Hour. | ||||||||
Table 1: Stability of amylase detection on plastic substrate under experimental conditions.
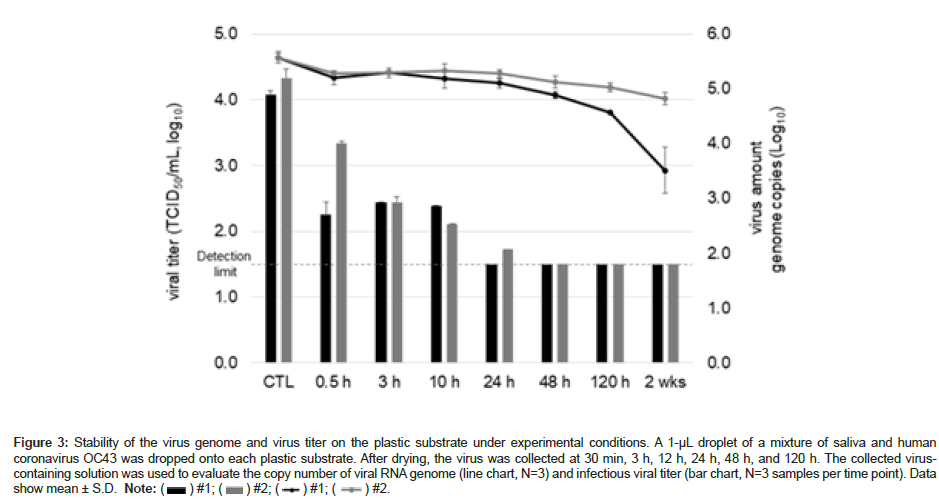
Although the copy number of the OC43 genome adhering to the environmental surface (Figure 3) decreased in a time-dependent manner, approximately 50% of the viral RNA was still detectable even after 5 days. However, the infectious titer of OC43 were observed up to 10 and 24 h, each sample. No infectious titer was observed after 48 h (Figure 3).
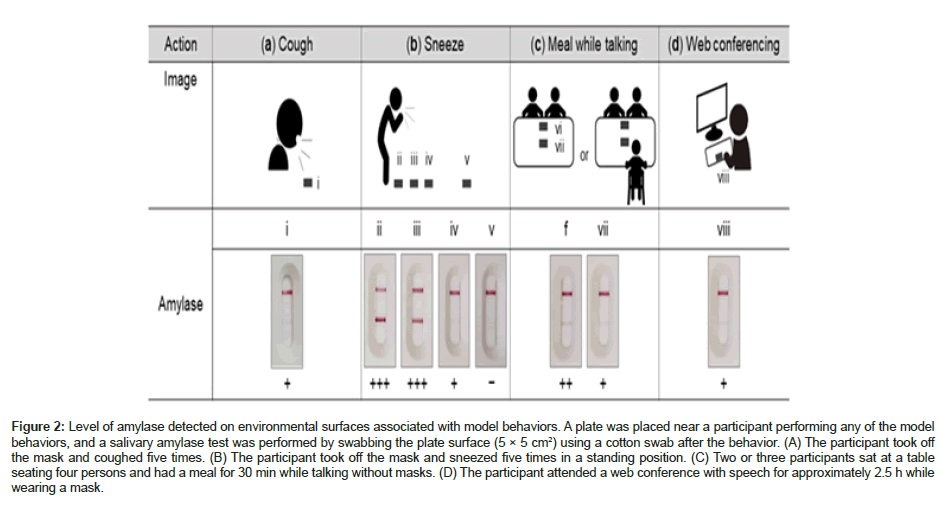
Figure 2: Level of amylase detected on environmental surfaces associated with model behaviors. A plate was placed near a participant performing any of the model behaviors, and a salivary amylase test was performed by swabbing the plate surface (5 × 5 cm2) using a cotton swab after the behavior. (A) The participant took off the mask and coughed five times. (B) The participant took off the mask and sneezed five times in a standing position. (C) Two or three participants sat at a table seating four persons and had a meal for 30 min while talking without masks. (D) The participant attended a web conference with speech for approximately 2.5 h while wearing a mask.
Figure 3: Stability of the virus genome and virus titer on the plastic substrate under experimental conditions. A 1-µL droplet of a mixture of saliva and human coronavirus OC43 was dropped onto each plastic substrate. After drying, the virus was collected at 30 min, 3 h, 12 h, 24 h, 48 h, and 120 h. The collected viruscontaining solution was used to evaluate the copy number of viral RNA genome (line chart, N=3) and infectious viral titer (bar chart, N=3 samples per time point). Data show mean ± S.D. Note: ( ) #1; (
) #1; ( ) #2; (
) #2; ( ) #1; (
) #1; ( ) #2.
) #2.
Amylase detection levels on environmental surface associated with model behaviors
After coughing, sneezing, meal with speech, and attending a conference with speech (all of which are known to spread of droplets), amylase was detected on the environmental surfaces near each participant who performed the behavior. Salivary amylase was detected on the surface of all plates, except for a plate placed on the floor 200 cm from the participant and swabbed after the participant sneezed.
Detection of amylase on environmental surfaces of public facilities
The positive rate for amylase in the food court area of shopping mall A was 75.0% (Supplementary Table 2). The positive rate was particularly high on the surfaces of tables and chairs. Similarly, the positive rate for amylase was high in the rest area (75.0%). By contrast, in the other areas, amylase was detected only on one out of nine surfaces (carrying handle of a shopping basket). High ATP levels (several thousands to several tens of thousands in Relative Light Unit (RLU)) were detected on almost all surfaces tested. SARS-CoV-2 was not detected in the PCR (data not shown).
Similar to the results for shopping mall A, the positive rate for amylase was high in the two food court areas of shopping mall B (66.7% and 75.0%), and amylase was detected on tables and chairs at high frequencies (Supplementary Table 3). Amylase was also detected on all environmental surfaces investigated in the rest area. The positive rate for amylase in the other areas was 37.5%. Furthermore, the positive rate for amylase was high on escalator handrail belts and places frequently touched by children (e.g., playground for children, child cart, and the capsule toy machine). The range of ATP levels tested in shopping mall B varied from 11 to 63,647 RLU. The presence of SARS-CoV-2 was assessed only in the food court and rest areas, and all the results were negative (data not shown).
Amylase was detected in more than half of the surfaces assessed in hotel guest rooms (Supplementary Table 4). The positive rate for amylase (number of positive rooms/number of examined rooms . 100) by sampling point was 100% for the edge of the washbasin sink, 87.5% for the water faucet handle, and 80.0% for glass, indicating that the positive rate was high around the washroom. In addition, a high positive rate of 87.5% was observed for electric appliances in the bedroom, including the light switch and remote controller. High levels of ATP (3,000 RLU or higher on average) were detected on the doorknob, water faucet handle, and refrigerator handle in the guest room (Supplementary Table 4). Amylase was detected in four out of eight surfaces located in the lobby floor (Supplementary Table 5). SARS-CoV-2 was not detected in the hotel (data not shown).
Correlation between amylase and Atp detection
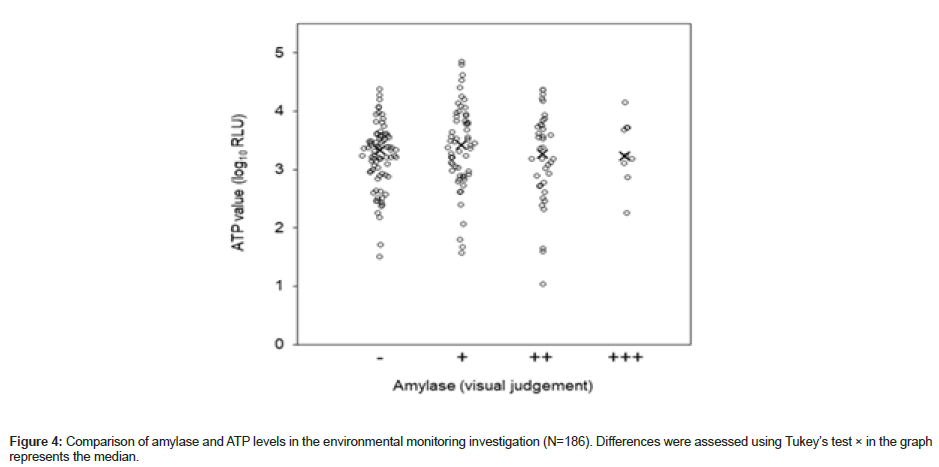
ATP levels determined in shopping malls A and B and the hotel were grouped by the level of amylase detected (-, +, ++, +++) for between-group comparison of ATP levels. No significant difference was observed between any of the group pairs (p = 0.454) (Figure 4).
Discussion
To establish a method for monitoring sites with potential risk of viral contamination, we investigated the utility of salivary amylase testing, which provided a detection index of droplets on environmental surfaces. Detection of amylase on polypropylene plastic plates was not consistent with the infectivity of OC43 (expressed as infectious titer). This indicated that amylase testing does not provide an index of infection risk and that detection of amylase suggests the presence of viral genes (i.e., viral contamination) in cases where virus-laden droplets expelled from an infected individual are retained on the surfaces of materials.
We detected amylase on the surface of all plates placed near individuals performing any of the four model behaviors that spread droplets, which were wiped after the end of the model behavior. In addition, in a field investigation performed in public facilities, the amylase detection rate was high at locations where droplets are assumed to be dispersed frequently (e.g., food courts, washbasins in hotel guest rooms). These results suggested that the amylase assay used in the present study can detect sites of droplet adhesion in the real environment.
Droplets expelled from humans are divided into large droplets (>10 μm) and small particles (<10 μm); the large droplets rapidly settle on environmental surfaces surrounding the humans expelling them [21]. As most of the viral particles shed from infected individuals are present in large droplets, methodologies capable of detecting any contamination due to large droplets are considered important in assessing the risk of viral contamination on environmental surfaces [22]. Zhang et al. reported that conversation lasting for 1 min and a single cough can generate approximately 3.09 μL and 6.15 μL large droplets, respectively [22]. Based on a report that the mean α-amylase concentration in human saliva was 2.64 mg/mL, the average lower detection limit for droplet volume of the amylase test established in this study was estimated to be 12 nL [23]. Consequently, the detection sensitivity of the present method was sufficient for detecting contamination of large droplets expelled in association with the above-mentioned behaviors, which is supported by the high frequency of amylase detected in the investigation in shopping malls and a hotel in this study. A previous study demonstrated the presence of bacteria derived from human saliva on the surface contaminated with SARS-CoV-2, suggesting the usefulness of biological substances derived from saliva as an index of contamination with SARS-CoV-2 [24]. An amylase test kit based on the principle of immunochromatography may be a useful tool for on-site assessment of the risk of viral contamination, as it allows simple and rapid testing.
ATP testing (an existing hygiene index) was performed concomitantly in public facilities during this investigation. ATP present in organic substances (e.g., microorganisms, feces, and dirt) can easily be detected using a luciferase bioluminescence assay, and the ATP level is used as a marker for hygiene management in hospitals to prevent nosocomial infections [25]. However, amylase was also detected in multiple environmental surfaces that were considered “clean” because of low levels of detected ATP (only several tens in RLU), with no correlation between the detection of amylase and ATP levels. Based on these findings, amylase was considered an index for assessing minute contamination with droplets that cannot be detected using ATP testing.
The three fields investigated in the present study were negative for SARS-CoV-2 genes, and we could not consistently demonstrate any relation between the amylase-positive sites and the sites of viral contamination, which is a limitation of the study. As SARS-CoV-2 is more stable than influenza viruses on environmental surfaces, we expected to detect it in field investigations [8,9,26]. The numbers of COVID-19-positive cases reported in prefectures where the individual investigation sites are located (Saitama, Chiba, and Hyogo) on the date of investigation were 11.62, 47.61, and 3.9 per 100,000 individuals, respectively. One possible explanation for the inability to detect SARSCoV- 2 in the present study is that individuals who were COVID- 19-positive might not have used the facility investigated. Therefore, environments such as hospital rooms which actually accommodate individuals with COVID-19 should be studied in future.
Amylase was detected at many assessment sites in the shopping malls investigated in the present study, despite the regular cleaning conducted in the wake of the pandemic. In addition, amylase was also detected at high rates from places where droplets expelled are unlikely to adhere directly, such as the upper surface of the backrest of food court chairs (the part that comes in contact with hands when people move the chair), as well as the surfaces of light switches and the back of the remote controller in the hotel guest room. These facts suggest that in addition to the direct adhesion of droplets, transmission of droplets via contact with hands might have occurred, indicating a risk of transmission of the virus via contact with sites contaminated with droplets in public facilities. Understanding the actual situation regarding contamination with droplets and implementation of countermeasures will be effective for controlling viral transmission.
Conclusion
For controlling the transmission of upper respiratory tract infections, sites potentially contaminated with virus-laden droplets should be monitored and countermeasures should be implemented. Our study demonstrated that amylase testing is a useful tool for detecting sites contaminated with droplets. In the future, an investigation of hospital rooms accommodating patients with COVID-19 will be necessary to clarify the correlation between the amount of virus retained and the level of amylase on environment surfaces. This will also be necessary to further clarify the risk of viral contamination associated with the detection of amylase, as well as the significance of amylase as an index of viral infection risk. Overall, this will contribute to the establishment of appropriate infection control systems based on amylase testing and result in the identification of sites with a high risk of viral contamination.
Declarations
Ethics declarations
This study protocol was approved by the institutional review broad of the Kao Corporation (K0141-2209). These studies were performed in accordance with the Declaration of Helsinki.
Acknowledgments
We thank Aeon Delight Co., Ltd. for providing the facilities for the field tests. We also thank Honyaku Center Inc. (https://www. honyakuctren.com) for English language editing.
Authors Contributions
K.H., K.M., T.Y., A.H., and T.M. designed and performed the study. K.H., K.M., T.Y., and A.H. analyzed the data. T.M. and I.S. supervised the study. K.H., K.M., and T.M. wrote the manuscript. K.H., T.Y., T.M., and I.S. edited the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
This study was fully funded by the Kao Corporation, and all authors were permanent employees of the Kao Corporation. Kao Corporation had no involvement in preparing this article in terms of study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit the article for publication.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
References
- Song F, Shi N, Shan F, Zhang Z, Shen J, et al. (2020) Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295: 210-217.
[Crossref] [Google Scholar] [PubMed]
- The-nCoV Outbreak Joint Field Epidemiology Investigation Team, Li Q (2020) An outbreak of NCIP (2019-nCoV) infection in China-Wuhan, Hubei Province, 2019-2020. China C.D.C. Wkly 2: 79-80.
[Google Scholar] [PubMed]
- Eurosurveillance Editorial Team. (2020) Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Euro Surveill 25: 200131e.
[Crossref] [Google Scholar] [PubMed]
- Al Huraimel K, Alhosani M, Kunhabdulla S, Stietiya, MH (2020) SARS-CoV-2 in the environment: modes of transmission, early detection and potential role of pollutions. Sci Total Environ 744: 140946.
[Crossref] [Google Scholar] [PubMed]
- Wei L, Lin J, Duan X, Huang W, Lu X, et al. (2020) Asymptomatic COVID-19 patients can contaminate their surroundings: an environment sampling study. mSphere 5:e00442.
- Cheng VC, Wong SC, Chan VWM, So SYC, Chen JHK, et al. (2020) Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol 41: 1258-1265.
[Crossref] [Google Scholar] [PubMed]
- Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, et al. (2020) Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA 323: 1610-1612.
[Crossref] [Google Scholar] [PubMed]
- Hirose R, Ikegaya H, Naito Y, Watanabe N, Yoshida T, et al. (2021) Survival of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza virus on human skin: importance of hand hygiene in coronavirus disease 2019 (COVID-19). Clin Infect Dis 73: e4329-4335.
[Crossref] [Google Scholar] [PubMed]
- Hung IFN, Cheng VCC, Li X, Tam AR, Hung DLL, et al. (2020) SARS-CoV-2 shedding and seroconversion among passengers quarantined after disembarking a cruise ship: a case series. Lancet Infect Dis 20: 1051-1060.
[Crossref] [Google Scholar] [PubMed]
- Harvey AP, Fuhrmeister ER, Cantrell M, Pitol AK, Swarthout JM, et al. (2021) Longitudinal monitoring of SARS-CoV-2 RNA on high-touch surfaces in a community setting. Environ Sci Technol Lett 8: 168-175.
[Crossref] [Google Scholar] [PubMed]
- Lee SE, Lee DY, Lee WG, Kang BH, Jang YS, et al. (2020) Detection of novel coronavirus on the surface of environmental materials contaminated by COVID-19 patients in the Republic of Korea. Osong Public Health Res Perspect 11: 128-132.
[Crossref] [Google Scholar] [PubMed]
- Mouchtouri VA, Koureas M, Kyritsi M, Vontas A, Kourentis L, et al. (2020) Environmental contamination of SARS-CoV-2 on surfaces, air-conditioner and ventilation systems. Int J Hyg Environ Health 230: 113599.
[Crossref] [Google Scholar] [PubMed]
- Eom G, Hwang A, Kim H, Yang S, Lee DK, et al. (2019) Diagnosis of tamiflu-resistant influenza virus in human nasal fluid and saliva using surface-enhanced Raman scattering. A C S Sens 4: 2282-2287.
[Crossref] [Google Scholar] [PubMed]
- Yokota I, Shane PY, Okada K, Unoki Y, Yang Y, et al. (2021) Mass screening of asymptomatic persons for SARS-CoV-2 using saliva. Clin Infect Dis 73: e559-e565.
[Crossref] [Google Scholar] [PubMed]
- Lee RA, Herigon JC, Benedetti A, Pollock NR, Denkinger CM (2021) Performance of saliva, oropharyngeal swabs, and nasal swabs for SARS-CoV-2 molecular detection: a systematic review and meta-analysis. J Clin Microbiol 59: e02881-20.
[Crossref] [Google Scholar] [PubMed]
- Casey DG, Price J (2010) The sensitivity and specificity of the RSID-saliva kit for the detection of human salivary amylase in the Forensic Science Laboratory, Dublin, Ireland. Forensic Sci Int 194: 67-71.
[Crossref] [Google Scholar] [PubMed]
- Lieberman D, Lieberman D, Shimoni A, Keren-Naus A, Steinberg R, et al. (2009) Identification of respiratory viruses in adults: nasopharyngeal versus oropharyngeal sampling. J Clin Microbiol 47: 3439-3443.
[Crossref] [Google Scholar] [PubMed]
- Ramakrishnan MA (2016) Determination of 50% endpoint titer using a simple formula. World J Virol 5: 85-86.
[Crossref] [Google Scholar] [PubMed]
- Bakke M, Suzuki S (2018) Development of a novel hygiene monitoring system based on the detection of total adenylate (ATP + ADP + AMP). J Food Prot 81: 729-737.
[Crossref] [Google Scholar] [PubMed]
- Shirato K, Nao N, Katano H, Takayama I, Saito S, et al. (2020) Development of genetic diagnostic methods for detection for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis 73: 304-307.
[Crossref] [Google Scholar] [PubMed]
- Marr LC, Tang JW, van Mullekom JV, Lakdawala S (2019) Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J R Soc Interface 16: 20180298.
[Crossref] [Google Scholar] [PubMed]
- Zhang N, Li Y (2018) Transmission of influenza A in a student office based on realistic person-to-person contact and surface touch behavior. Int J Environ Res Public Health 15: 1699.
[Crossref] [Google Scholar] [PubMed]
- Mandel AL, des Gachons CP, Plank KL, Alarcon S, Breslin PAS (2010) Individual differences in AMY1 gene copy number, salivary α-amylase levels, and the perception of oral starch. PLoS One 5: e13352.
[Crossref] [Google Scholar] [PubMed]
- Piana A, Colucci ME, Valeriani F, Marcolongo A, Sotgiu G, et al. (2021) Monitoring COVID-19 transmission risks by quantitative real-time PCR tracing of droplets in hospital and living environments. mSphere 6: e01070.
[Crossref] [Google Scholar] [PubMed]
- Boyce JM, Havill NL, Dumigan DG, Golebiewski M, Balogun O, et al. (2009) Monitoring the effectiveness of hospital cleaning practices by use of an adenosine triphosphate bioluminescence assay. Infect Control Hosp Epidemiol 30: 678-684.
[Crossref] [Google Scholar] [PubMed]
- Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen HL, et al. (2020) Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 1: e10.
[Crossref] [Google Scholar] [PubMed]
Citation: Hosokawa K, Mizukoshi K, Yamamoto T, Hayase A, Mori T, et al. (2023) Salivary Amylase: A Monitoring Index for Respiratory Infectious Virus Contamination. J Infect Dis Ther 11: 547. DOI: 10.4172/2332-0877.1000547
Copyright: © 2023 Hosokawa K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2223
- [From(publication date): 0-2023 - Nov 22, 2025]
- Breakdown by view type
- HTML page views: 1863
- PDF downloads: 360