Safety Profile of Schedule III Buprenorphine and Schedule II Oral Opioids in Elderly with Chronic Low Back Pain: A Retrospective US Medicare Claims Analysis
Received: 01-Aug-2024 / Manuscript No. jpar-24-146199 / Editor assigned: 03-Aug-2024 / PreQC No. jpar-24-146199(PQ) / Reviewed: 17-Aug-2024 / QC No. jpar-24-146199 / Revised: 21-Aug-2024 / Manuscript No. jpar-24-146199(R) / Published Date: 28-Aug-2024 DOI: 10.4172/2167-0846.1000656
Abstract
Objective: This study aimed to evaluate and compare the safety of CIII buprenorphine and oral CII opioids among Medicare patients with chronic Low-Back Pain (cLBP).
Methods: The retrospective study was conducted in Merative Medicare MarketScan® database (2018-2021). The first date of CIII buprenorphine or oral CII opioid medication prescription was defined as the index date. Patients with cLBP were observed 6 months pre-index and until the end of index treatment or the end of continuous healthcare coverage. The main outcome was the incidence of serious Treatment-Emergent Adverse Events (TEAE). Primary analysis compared CIII buprenorphine (Belbuca® and transdermal patch) with oral CII opioids, while sub-analyses compared Belbuca® to CII opioids and buprenorphine patches. Incidence Rate Ratios (IRR) and Incidence Rate Differences (IRD) (per 1,000 person-years) were reported. Propensity-Score Matching (PSM) was performed to balance differences in patients’ characteristics.
Results: CIII buprenorphine treatment (n=545 patients) was associated with significantly lower rates (p<0.050) of serious confusion (IRR=0.07), syncope (IRR=0.08), headache (IRR=0.11), urinary discomfort (IRR=0.16), constipation (IRR=0.17), cerebrovascular accident (IRR=0.18), atrial fibrillation (IRR=0.19), osteoarthritis (IRR=0.28), cellulitis (IRR=0.29), pneumonia (IRR=0.34), abdominal pain (IRR=0.45), sleep disturbances (IRD=-91.85), and hypotension (IRD=-30.62). The buprenorphine cohort (n=951 patients) more frequently experienced serious bone fractures (IRR=5.90).
Belbuca® (n=124 patients) showed significantly lower rates of serious TEAEs including fatigue (IRR=0.20), constipation (IRR=0.12), osteoarthritis (IRD=-340.08), and urinary discomfort (IRD=-194.33) than CII opioids (n=297 patients). Belbuca® had significantly lower incidence of serious dehydration (IRR=0.08), pneumonia (IRR=0.12), opioid abuse/dependence (IRD=-806.84), abdominal pain (IRD=-496.52), and appetite loss (IRD=-372.39) than patches (n=62 patients per cohort), while patches had significantly lower rates of serious osteoarthritis (IRD=867.30) and confusion (IRD=462.56).
Conclusion: Based on this retrospective claims analysis, CIII buprenorphine may have a milder safety profile than oral CII opioids for cLBP treatment. Belbuca® seems to be better tolerated than CII opioids and buprenorphine patch, based on this study.
Keywords
Chronic pain; Insurance claims; Buprenorphine buccal film; Buprenorphine patch; Opioids; Safety; Adverse events
Introduction
According to the World Health Organization (WHO), chronic Low Back Pain (cLBP) affects over six hundred million people worldwide, representing one of the leading causes of disability globally [1]. Most people experience chronic back pain at least once over their course of life [1, 2]. The prevalence of cLBP increases with age, affecting 20-25% of the population aged 65 or older [1, 3, 4]. In older individuals, cLBP is characterized by more frequent and longer pain episodes that require continuous monitoring [4]. Persisting pain reduces the ability to participate in family, social, and routine daily activities, negatively impacting overall well-being and mental health [1, 4]. Clinical management of cLBP requires substantial resources, particularly in the geriatric population. The estimated economic burden of cLBP in the United States is over $100 billion annually and is expected to rise with the fast-growing aging population [4].
Management of cLBP in older adults is challenging due to the high prevalence of comorbidities, multiple medication regimens, slower metabolism, and increased fall risk [5]. The current WHO guideline for treating older adults with cLBP recommends a risk-stratified approach, predominantly based on multimodal non-pharmacological strategies, such as education programs, exercises, and physical and psychological therapies [6]. Still, pharmacological treatment remains an essential part of pain management, though it is important to consider medication pharmacokinetic, safety profiles, and individual comorbidity when selecting the appropriate treatment regimen. Pharmacotherapy for cLBP in older patients employs lower therapeutic doses considering the frequency of polypharmacy, co-morbid medical disorders, and potentially decreased renal and hepatic metabolism. Although used judiciously in elderly due to the increased morbidity and mortality risk, opioids remain a mainstay in persistent refractory severe cLBP treatment [5]. The rate of opioid prescribing in the elderly population is high; with one in four Americans aged 65 and older prescribed at least one opioid in 2017, according to the Centers for Disease Control and Prevention [7]. Common side effects of opioid use in the elderly population include constipation, urinary retention, and cardiovascular and endocrine disorders [8], while central nervous system adverse events and respiratory depression represent the most serious conditions that may lead to a higher risk of death in this population [9]. Older patients with chronic pain are at an increased risk of opioid use overdose and abuse. According to the Centers for Medicare and Medicaid Services, more than 6 of every 1,000 Medicare beneficiaries were diagnosed with opioid use disorder [9], with considerable opioid-related mortality [10].
Buprenorphine represents a valuable therapeutic alternative for elderly patients with cLBP due to the potent and sustained analgesia, resulting from a very high binding affinity and partial agonism at the µ-opioid receptor, as well as the prolonged pharmacokinetic characteristics of transdermal and transmucosal delivery platforms [11, 12]. Buprenorphine is also considered to potentially have a milder safety profile than full µ-opioids, including lower rates and severity of constipation and urinary retention, lower risk of respiratory depression, and limited abuse potential [13]. In 2022, the United States Departments of Defense and Veterans Affairs added buprenorphine to the clinical practice guideline for the use of opioids as a first-line treatment for chronic pain due to its relatively lower risk for overdose and misuse [14]. Importantly, the pharmacokinetic and safety profile of buprenorphine is not altered by patient age due to the rapid conversion to non-active conjugates [13]. The bioavailability of orally administered buprenorphine is low, approximately 10%. However, the issue has been resolved by using the alternative routes. Transdermal patches provide approximately 15% and buccal film 46–65% bioavailability, while both formulations bypass first-pass metabolism [15].
Studies directly comparing buprenorphine to oral opioids for chronic pain in terms of their safety in the elderly population are lacking, although the topic is particularly important given the current opioid public health emergency [9, 11]. This study aimed to explore and compare Treatment-Emergent Adverse Event (TEAE) rates while using Schedule III (CIII) buprenorphine (Belbuca® and buprenorphine patch) and Schedule II (CII) oral opioids among Medicare patients with cLBP using real-world claims data. Additional sub-analyses investigated safety outcomes between Belbuca® vs. CII oral opioids and separately Belbuca® vs. buprenorphine patches.
Methodology
Data source
This retrospective cohort study used US insurance claims data from the Merative MarketScan® Medicare Supplemental and Coordination of Benefits Database. The Merative MarketScan® databases consist of deidentified, longitudinal, patient-level closed claims and specialty data for patients in the US sourced directly from a diverse pool of payers. This study focuses on retirees with employer-sponsored Medicare Supplemental and Medicare Advantage plans and includes drug information and outcomes data for healthcare services performed in both inpatient and outpatient settings [16]. The study was performed in insurance data claimed in the period from January 1, 2018, to December 31, 2021.
Study population
The study observed Medicare beneficiaries (≥ 65 years of age) with cLBP defined as at least two diagnoses of low back pain on different dates during the six-month pre-index period. The diagnoses were identified using the International Classification of Diseases – Clinical Modification (ICD-10-CM) codes (Supplement Table A1). The population of interest considered for the study were patients prescribed oral CII opioids or CIII buprenorphine (Belbuca® or buprenorphine patch). The respective drug codes were identified in the database based on the National Drug Codes (NDCs) (Supplement Table A2,A3). Patients were treatment-naïve, with no CIII buprenorphine and CII oral opioids in the 6-month pre-index period. Exclusion criteria were a gap in the health plan or pharmaceutical coverage during the observational period and Belbuca® or buprenorphine patch prescriptions within the CII opioid cohort. To enable a fair comparison, CII opioid patients that received Belbuca® or buprenorphine patch prescriptions in the post-index period were excluded from the cohort. On the other hand, CIII buprenorphine patients were allowed to have concomitant CII opioid use during the post-index period. This way, the study ensured that potential selection bias is conservative, with possibly overestimated rates of adverse events in CIII buprenorphine cohort.
Study design
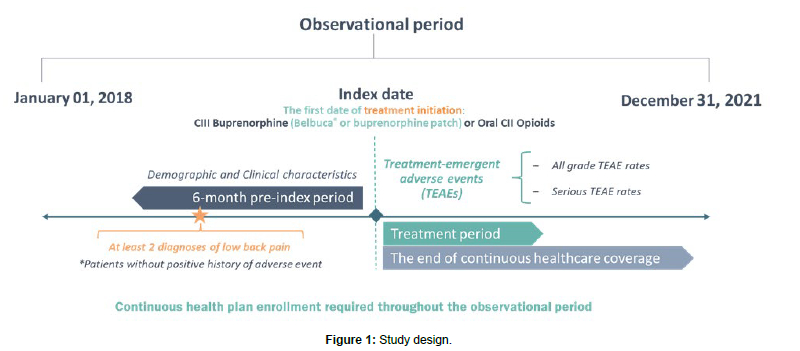
The index date was defined as the first date of buprenorphine or oral CII opioid treatment. The analysis observed 6-month pre-index period and the post-index period lasted until the end of continuous healthcare and pharmaceutical coverage. Clinical characteristics were evaluated in the pre-index period, while demographic characteristics were assessed on the index date. Patients were classified into cohorts based on the index prescription. The primary analysis compared CIII buprenorphine (prescribed Belbuca® or buprenorphine patch) and oral CII opioid patients (short-acting [SAO] and long-acting [LAO]), while sub-analyses considered the comparison of Belbuca® vs. CII oral opioids and Belbuca® vs. buprenorphine patch patients. The study design is presented in Figure 1.
Outcome measures
The main study outcome was the incidence of TEAEs. The list of relevant adverse events was comprised based on the published literature sources including the most common adverse events (≥5% rate), serious adverse events, adverse events leading to treatment discontinuation, and opioid-related adverse events [17-26]. Finally, the list comprised 44 relevant TEAEs reported in Table 1.
| Cardiovascular |
|
| Central Nervous System (CNS) |
|
| Opioid Use Disorder (OUD) |
|
| Hormonal |
|
| Musculoskeletal |
|
| Respiratory |
|
| General |
|
| Gastrointestinal (GIT) |
|
| Skin Toxicities |
|
| Urinary |
|
Table 1: List of captured adverse events of interest.
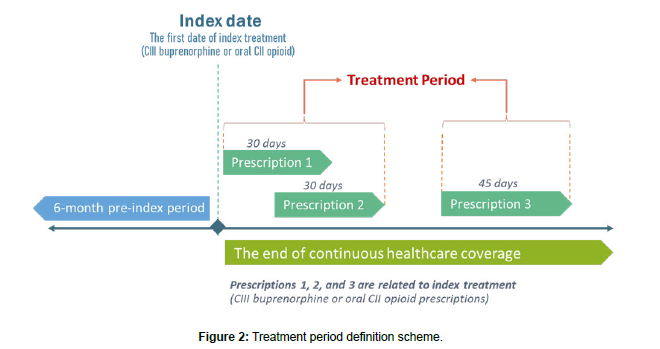
Diagnoses of TEAEs were identified in the database based on the ICD-10-CM codes (Supplement Table A4). The diagnosis was considered as TEAE if occurred during the treatment period with index medication and only among patients without a history of investigated TEAE during the pre-index period. The treatment period was calculated by summing up the drug supply periods for the index medication, excluding treatment gaps and extracting the days of prescription overlaps. In the CIII buprenorphine cohort, only buprenorphine prescription claims were considered when defining the treatment period although patients were allowed to use oral CII opioid concomitantly. The scheme of treatment period definition is presented in Figure 2. Repeated events experienced by a patient during the post-index period were counted as separate events. All-grade TEAEs were all relevant diagnoses observed within treatment periods, while serious TEAEs were defined as events claimed in inpatient or Emergency Department (ED) settings. TEAE rates were reported per 1,000 person-years. The comparison between the cohorts was performed using absolute Incidence Rate Difference (IRD) and Incidence Rate Ratio (IRR). The IRD is calculated as a crude difference of the observed incidence rates (i.e., cohort I incidence rate minus cohort II incidence rate), while IRR represents a relative difference measure calculated as a quotient of incidence rates (i.e., cohort I incidence rate divided by cohort II incidence rate).
Sub-analyses
The primary analysis compared study outcomes between CIII buprenorphine (Belbuca® and buprenorphine patch) vs. oral CII opioid (SAO and LAO) cohorts. Sub-analyses aimed to assess the outcomes in more granularly stratified populations. Sub-analysis #1 compared TEAE rates between Belbuca® vs. CII oral opioid cohorts, while sub-analysis #2 compared Belbuca® vs. buprenorphine patches.
Statistical analysis
Descriptive statistics was reported summarizing continuous variables as means with standard deviations and categorical variables as numbers with proportions of the sample. The independent t-test was performed to test the difference between the compared cohorts for continuous variables, while chi-square test of independence was used for categorical variables. P-values lower than 0.05 were considered statistically significant.
All TEAE rates and IRD values were reported per 1,000 person-years, while IRR was reported as a rate ratio with 95% confidence intervals (95% CI). The incidence rate ratio test computed IRD and IRR and explored the statistical significance of TEAE rate differences between study cohorts. If TEAE occurred in only one cohort, the P-value was reported for IRD, otherwise it refers to the statistical difference between the cohorts in IRR Negative IRD values and IRR less than 1 imply that the TEAE rate was lower in the referent cohort (CIII buprenorphine in primary analysis and Belbuca® in sub-analyses).
To control for confounders and minimize the selection bias, the Propensity-Score Matching (PSM) analysis was performed applying the nearest-neighbour matching algorithm. Demographic and clinical characteristics of patients were used as covariates in the matching process.
Statistical analyses were performed using the IBM Statistical Package for the Social Sciences (SPSS®) and MedCalc® statistical software.
Results
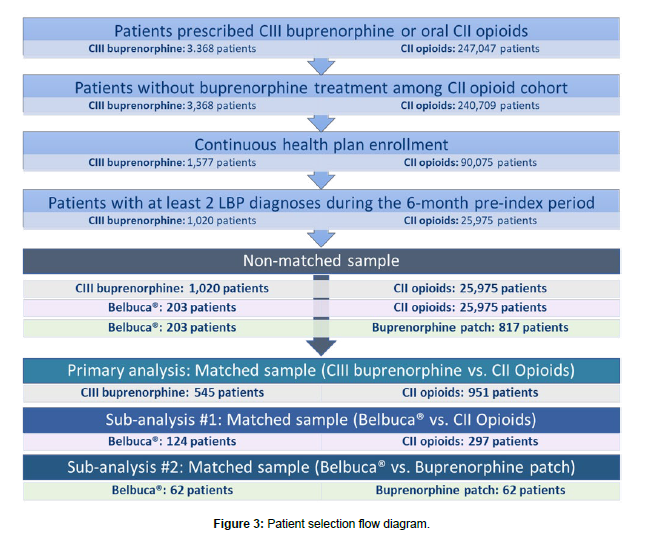
The final sample of patients before PSM consisted of 26,995 patients (1,020 in CIII buprenorphine cohort and 25,975 in CII opioid cohort). The CIII buprenorphine cohort was composed of 203 patients treated with Belbuca® and 817 patients on buprenorphine patch. In the sample of 1,496 matched patients, 545 patients were treated with CIII buprenorphine and 951 patients with CII opioids. The subgroup analyses considered 421 patients (124 Belbuca® matched to 297 CII opioid patients) and 124 patients (62 patients in both Belbuca® and buprenorphine patch cohorts). The flow diagram depicting patient selection process is shown in Figure 3.
Primary analysis: CIII buprenorphine vs. CII opioidss
Non-matched population: Demographic characteristics of the total sample of 26,995 patients with cLBP stratified in the treatment cohorts are presented in Table 2. Medicare beneficiaries with cLBP were approximately 75 years old and predominantly females. A higher proportion of males was observed in the CII opioid cohort (34.4% vs. 43.8%, p<0.001). The majority of patients were covered by the Preferred Provider Organization (43.4% vs. 47.5%, p=0.011 in CIII buprenorphine vs. CII opioids). A higher proportion of CIII buprenorphine patients were covered by the Comprehensive plan (33.3% vs. 24.5%, p<0.001), while CII opioid patients were more commonly covered by the Health Maintenance Organization plan (17.2% vs. 20.9%, p=0.004). CII opioid-treated patients mostly resided in the North East region, while the majority of CIII buprenorphine patients were located in the North Central and South regions (p<0.001, respectively). Observing the comorbidity burden, CIII buprenorphine patients had a higher Charlson Comorbidity Index (CCI) than CII opioid patients (2.3 vs. 1.9, p<0.001), with a significantly higher proportion of patients in CCI category 4+ (24.8% vs. 18.0%, p<0.001), while more patients in CII opioid cohort were noted in CCI=0 category (26.7% vs. 35.2%, p<0.001). There were significant differences between study cohorts in almost all CCI components (except moderate or severe liver disease, hemiplegia or paraplegia, metastatic solid tumors, and AIDS/HIV), as well as all mental health disorders and other chronic pain comorbidities (p<0.05), except spine disorders. The list of clinical characteristics is presented in Table 3.
| CIII Buprenorphine (N=1,020) |
CII Opioids (N=25,975) |
P-value* | |
|---|---|---|---|
| Age, mean (SD) | 74.9 (8.0) | 74.5 (7.2) | 0.154 |
| Gender, n (%) | |||
| Male | 351 (34.4) | 11,372 (43.8) | |
| Female | 669 (65.6) | 14,603 (56.2) | |
| Health Plan, n (%) | |||
| Basic/major medical | 0 (0.0) | 0 (0.0) | - |
| Comprehensive | 340 (33.3) | 6,372 (24.5) | |
| Exclusive Provider Organization | 4 (0.4) | 133 (0.5) | 0.821 |
| Health Maintenance Organization | 175 (17.2) | 5,418 (20.9) | 0.004 |
| Non-Capitated Point-of-Service | 4 (0.4) | 121 (0.5) | 1.000 |
| POS with capitation | 13 (1.3) | 433 (1.7) | 0.335 |
| Preferred Provider Organization | 443 (43.4) | 12,331 (47.5) | 0.011 |
| Consumer-Driven Health Plan | 3 (0.3) | 155 (0.6) | 0.293 |
| High Deductible Health Plan | 5 (0.5) | 178 (0.7) | 0.563 |
| Unknown | 33 (3.2) | 834 (3.2) | 0.965 |
| Region, n (%) | |||
| North East | 88 (8.6) | 4,691 (18.1) | |
| North Central | 468 (45.9) | 10,905 (42.0) | 0.013 |
| South | 360 (35.3) | 7,609 (29.3) | |
| West | 103 (10.1) | 2,742 (10.6) | 0.640 |
| Unknown | 1 (0.1) | 28 (0.1) | 1.000 |
| *Chi-square test was performed for categorical variables and independent T-test for continuous variables | |||
Table 2: Demographic characteristics of non-matched patients.
Table 3: Clinical characteristics of non-matched patients.
Matched population: The sample of matched patients consisted of 1,496 patients (545 patients in CIII buprenorphine cohort and 951 patients in CII opioid cohort). Patients were well-balanced between the study cohorts and there were no differences in demographic and clinical characteristics that could impact study outcome measures (Supplement Tables A5,A6).
The rate of serious hypertension was significantly higher (IRR 0.23, p=0.036) in CII opioid cohort, while other cardiovascular TEAEs were occurred with similar incidence in both cohorts (Table 4). Out of all-grade CNS-related TEAEs, the rates of all-grade confusion, syncope, cerebrovascular accident, and sleep disturbances more commonly occurred in the CII opioid cohort (IRR 0.15, 0.35, 0.42, and 0.31, p<0.050, respectively). The rate of all-grade headache, fatigue and dehydration was significantly higher among CII opioid patients (IRR 0.18, 0.71, and 0.52, p<0.050). The rate of all-grade constipation was significantly higher in the CII opioid cohort (IRR 0.41, p=0.041), while all-grade anorexia/loss of appetite was more frequent in CIII buprenorphine cohort (IRR 2.12, p=0.032). The rates of all-grade osteoarthritis (IRR 0.34, p<0.001), pneumonia (IRR 0.26, p<0.001), cellulitis (IRR 0.52, p=0.026), and urinary discomfort (IRR 0.45, p=0.002) were significantly higher in CII opioid patients.
| CIII Buprenorphine (N=545) |
CII Opioids (N=951) |
Incidence Rate Difference (95% CI) |
Incidence Rate Ratio (95% CI) | P-value* | |
|---|---|---|---|---|---|
| Cardiac Adverse Events | |||||
| QT prolongation | 6.95 | 0.00 | 6.95 (-13.26 - 27.16) | - | 0.501 |
| Hypotension | 13.89 | 30.62 | -16.73 (-57.14 - 23.70) | 0.45 (0.03 - 6.26) | 0.463 |
| Atrial Fibrillation | 173.67 | 290.85 | -117.18 (-251.20 - 16.90) | 0.60 (0.32 - 1.15) | 0.096 |
| Coronary Artery Disease | 173.67 | 122.46 | 51.21 (-64.89 - 167.32) | 1.42 (0.62 - 3.64) | 0.399 |
| Hypertension | 20.84 | 91.85 | -71.01 (-131.63 - (-10.37)) | 0.23 (0.04 - 1.06) | 0.036 |
| Central Nervous System-related Adverse Events | |||||
| Dizziness | 180.62 | 199.00 | -18.38 (-144.59 - 107.85) | 0.91 (0.45 - 1.92) | 0.764 |
| Somnolence | 6.95 | 0.00 | 6.95 (-13.26 - 27.16) | - | 0.501 |
| Confusion | 69.47 | 474.55 | -405.08 (-534.50 - (-275.60)) | 0.15 (0.06 - 0.31) | |
| Seizures | 20.84 | 45.92 | -25.08 (-74.59 - 24.43) | 0.45 (0.36 - 3.39) | 0.359 |
| Syncope | 145.89 | 413.32 | -267.43 (-407.40 - (-127.40)) | 0.35 (0.19 - 0.65) | |
| Cerebrovascular Accident | 90.31 | 214.31 | -124.00 (-229.00 - (-19.00)) | 0.42 (0.18 - 0.97) | 0.028 |
| Nervousness | 0.00 | 0.00 | - | - | - |
| Visual Discomfort | 41.68 | 30.62 | 11.06 (-46.10 - 68.23) | 1.36 (0.24 - 13.79) | 0.753 |
| Suicidal Ideation | 0.00 | 15.31 | -15.31 (-35.52 - 4.90) | 0.00 (0.00 - 17.70) | 0.138 |
| Sleep Disturbances | 187.57 | 597.01 | -409.44 (-573.60 - (245.20)) | 0.31 (0.19 - 0.53) | |
| Opioid Use Disorder-related Adverse Events | |||||
| OAD | 194.51 | 183.70 | 10.81 (-117.00 - 138.65) | 1.06 (0.52 - 2.29) | 0.886 |
| Opioid Poisoning | 48.63 | 15.31 | 33.32 (-23.84 - 90.49) | 3.18 (0.41 - 143.2) | 0.282 |
| General Adverse Events | |||||
| Headache | 55.58 | 306.16 | 250.58 (-357.50 - (-143.60)) | 0.18 (0.07 - 0.43) | |
| Fatigue | 639.12 | 903.17 | -264.05 (-512.40 - (-15.60)) | 0.71 (0.50 - 1.00) | 0.041 |
| Allergic Reactions | 13.89 | 45.92 | -32.03 (-77.22 - 13.17) | 0.30 (0.03 - 2.64) | 0.215 |
| Dehydration | 166.73 | 321.47 | -154.74 (-290.30 - (-19.10)) | 0.52 (0.28 - 0.98) | 0.031 |
| Dry Mouth | 0.00 | 15.31 | -15.31 (-35.52 - 4.90) | 0.00 (0.00 - 17.70) | 0.138 |
| Xerostomia | 6.95 | 0.00 | 6.95 (-13.26 - 27.16) | - | 0.501 |
| Sweating | 13.89 | 15.31 | -1.42 (-36.42 - 33.59) | 0.91 (0.05 - 53.55) | 0.906 |
| Hot Flushes | 0.00 | 0.00 | - | - | - |
| Sinusitis | 90.31 | 30.62 | 59.69 (-18.58 - 137.97) | 2.95 (0.67 - 26.93) | 0.136 |
| Gastrointestinal Adverse Events | |||||
| Nausea and Vomiting | 361.24 | 382.70 | -21.46 (-198.79 - 155.91) | 0.94 (0.58 - 1.59) | 0.803 |
| Constipation | 180.62 | 443.93 | -263.31 (-413.20 - (-113.40)) | 0.41 (0.23 - 0.72) | 0.001 |
| Hepatotoxicity | 0.00 | 0.00 | - | - | - |
| Cholecystitis | 55.58 | 61.23 | -5.65 (-75.67 - 64.36) | 0.91 (0.24 - 4.12) | 0.854 |
| Abdominal Pain | 527.97 | 612.32 | -84.35 (-301.99 - 133.36) | 0.86 (0.58 - 1.30) | 0.447 |
| Diarrhea | 145.89 | 91.85 | 54.04 (-50.98 - 159.06) | 1.59 (0.62 - 4.81) | 0.324 |
| Anorexia/Loss of Appetite | 291.77 | 137.77 | 154.00 (9.70 - 298.30) | 2.12 (1.02 - 4.95) | 0.032 |
| Hormonal Adverse Events | |||||
| Adrenal Insufficiency | 6.95 | 0.00 | 6.95 (-13.26 - 27.16) | - | 0.501 |
| Musculoskeletal Adverse Events | |||||
| Bone Fractures | 90.31 | 122.46 | -32.15 (-124.76 - 60.47) | 0.74 (0.28 - 2.05) | 0.499 |
| Osteoarthritis | 361.24 | 1,056.26 | -695.02 (-917.30 - (472.60)) | 0.34 (0.23 - 0.50) | |
| Respiratory Adverse Events | |||||
| Respiratory Depression | 312.61 | 489.86 | -177.25 (-354.60 - 0.10) | 0.64 (0.40 - 1.04) | 0.056 |
| Pneumonia | 159.78 | 612.32 | -452.54 (-612.90 - (-292.10)) | 0.26 (0.15 - 0.45) | |
| Skin-related Adverse Events | |||||
| Cellulitis | 173.67 | 336.78 | -163.11 (-301.60 - (-24.50)) | 0.52 (0.28 - 0.96) | 0.026 |
| Pruritus | 48.63 | 30.62 | 18.01 (-42.62 - 78.65) | 1.59 (0.30 - 15.67) | 0.606 |
| Erythema | 48.63 | 15.31 | 33.32 (-23.84 - 90.49) | 3.18 (0.41 - 143.18) | 0.282 |
| Rash | 34.73 | 45.92 | -11.19 (-68.35 - 45.98) | 0.76 (0.15 - 4.87) | 0.696 |
| Skin Irritation | 6.95 | 0.00 | 6.95 (-13.26 - 27.16) | - | 0.501 |
| Urinary Adverse Events | |||||
| Urinary Discomfort | 215.36 | 474.55 | -259.19 (-418.30 - (-100.00)) | 0.45 (0.27 - 0.77) | 0.002 |
| *Incidence rate ratio test was performed to assess statistical differences between study cohorts. If the rate of adverse event was zero in one of the cohorts, p-value reflects the difference between study cohorts in the absolute incidence rate difference Abbreviations: OAD: Opioid abuse/dependence; CI: Confidence interval |
|||||
Table 4: All-grade TEAE rates (per 1,000 person-years) during the CIII buprenorphine and CII opioid treatment among matched sample in the primary analysis.
The reported rates of serious hypotension (IRD -30.62 per 1,000 person-years, p=0.036) and atrial fibrillation (IRR 0.19, p=0.001) were significantly lower in the CIII buprenorphine cohort (Table 5). From all investigated serious CNS-related TEAEs, the rates of serous confusion (IRR 0.07, p<0.001), syncope (IRR 0.08, p<0.001), cerebrovascular accident (IRR 0.18, p=0.003), and sleep disturbances (IRD -91.85 per 1,000 person-years, p<0.001) were significantly higher among CII opioid-treated patients. The rate of serious headache was also significantly more frequent among CII opioid patients (IRR 0.11, p=0.002). Out of GIT-related TEAEs, serious constipation and abdominal pain more commonly occurred among CII opioid patients compared to the CIII buprenorphine cohort (IRR 0.17 and 0.45, respectively, p<0.05). Serious bone fractures were more frequent among CIII buprenorphine patients (IRR 5.90, p=0.044), while serious osteoarthritis occurred more commonly in oral CII opioid-treated patients (IRR 0.28, p=0.029). The rates of serious pneumonia, cellulitis, and urinary discomfort appeared to be significantly higher among CII opioid cohort (IRR 0.34, 0.29, and 0.16, respectively, p<0.05).
| CIII Buprenorphine (N=545) |
CII Opioids (N=951) |
Incidence Rate Difference (95% CI) |
Incidence Rate Ratio (95% CI) | P-value* | |
|---|---|---|---|---|---|
| Cardiac Adverse Events | |||||
| QT prolongation | 6.95 | 0.00 | 6.95 (-13.26 - 27.16) | - | 0.501 |
| Hypotension | 0.00 | 30.62 | -30.62 (-5.92 - (-2.03)) | 0.00 (0.00 - 2.42) | 0.036 |
| Atrial Fibrillation | 34.73 | 183.70 | -148.97 (-232.30 - (-6.56)) | 0.19 (0.05 - 0.58) | 0.001 |
| Coronary Artery Disease | 111.15 | 45.92 | 65.23 (-22.87 - 153.33) | 2.42 (0.69 - 12.96) | 0.147 |
| Hypertension | 13.89 | 61.23 | -47.34 (-96.84 - 2.17) | 0.23 (0.02 - 1.58) | 0.094 |
| Central Nervous System-related Adverse Events | |||||
| Dizziness | 69.47 | 122.46 | -52.99 (-138.73 - 32.76) | 0.57 (0.20 - 1.65) | 0.243 |
| Somnolence | 0.00 | 0.00 | - | - | - |
| Confusion | 27.79 | 398.01 | -370.22 (-480.90 - (-259.50)) | 0.07 (0.02 - 0.20) | |
| Seizures | 6.95 | 0.00 | 6.95 (-13.26 - 27.16) | - | 0.501 |
| Syncope | 20.84 | 275.54 | -254.70 (-347.30 - (-162.10)) | 0.08 (0.01 - 0.26) | |
| Cerebrovascular Accident | 27.79 | 153.08 | -125.29 (-200.90 - (-49.70)) | 0.18 (0.04 - 0.63) | 0.003 |
| Nervousness | 0.00 | 0.00 | - | - | - |
| Visual Discomfort | 6.95 | 0.00 | 6.95 (-13.26 - 27.16) | - | 0.501 |
| Suicidal Ideation | 0.00 | 15.31 | -15.31 (-35.52 - 4.90) | 0.00 (0.00 - 17.70) | 0.138 |
| Sleep Disturbances | 0.00 | 91.85 | -91.85 (-141.35 - (-42.33)) | 0.00 (0.00 - 0.39) | |
| Opioid Use Disorder-related Adverse Events | |||||
| OAD | 13.89 | 0.00 | 13.89 (-14.69 - 42.48) | - | 0.341 |
| Opioid Poisoning | 41.68 | 15.31 | 26.37 (-27.10 - 79.85) | 2.72 (0.33 - 125.25) | 0.377 |
| General Adverse Events | |||||
| Headache | 13.89 | 122.46 | -108.57 (-172.50 - (-44.60)) | 0.11 (0.01 - 0.57) | 0.002 |
| Fatigue | 145.89 | 168.39 | -22.49 (-136.82 - 91.84) | 0.87 (0.40 - 1.99) | 0.691 |
| Allergic Reactions | 6.95 | 15.31 | -8.36 (-36.94 - 20.22) | 0.45 (0.01 - 35.63) | 0.624 |
| Dehydration | 145.89 | 199.00 | -53.11 (-170.95 - 64.74) | 0.73 (0.35 - 1.59) | 0.382 |
| Dry Mouth | 0.00 | 0.00 | - | - | - |
| Xerostomia | 0.00 | 0.00 | - | - | - |
| Sweating | 0.00 | 0.00 | - | - | - |
| Hot Flushes | 0.00 | 0.00 | - | - | - |
| Sinusitis | 0.00 | 0.00 | - | - | - |
| Gastrointestinal Adverse Events | |||||
| Nausea and Vomiting | 138.94 | 168.39 | -29.45 (-141.97 - 83.09) | 0.83 (0.38 - 1.91) | 0.603 |
| Constipation | 41.68 | 244.93 | -203.25 (-298.00 - (-108.40)) | 0.17 (0.05 - 0.46) | |
| Hepatotoxicity | 0.00 | 0.00 | - | - | - |
| Cholecystitis | 55.58 | 30.62 | 24.96 (-38.95 - 88.87) | 1.82 (0.36 - 17.55) | 0.482 |
| Abdominal Pain | 111.15 | 244.93 | -133.78 (-248.10 - (19.40)) | 0.45 (0.21 - 0.97) | 0.029 |
| Diarrhea | 6.95 | 15.31 | -8.36 (-36.94 - 20.22) | 0.45 (0.01 - 35.63) | 0.624 |
| Anorexia/Loss of Appetite | 13.89 | 0.00 | 13.89 (-14.69 - 42.48) | - | 0.341 |
| Hormonal Adverse Events | |||||
| Adrenal Insufficiency | 0.00 | 0.00 | - | - | - |
| Musculoskeletal Adverse Events | |||||
| Bone Fractures | 90.31 | 15.31 | 75.00 (-0.62 - 150.62) | 5.90 (0.89 - 250.73) | 0.044 |
| Osteoarthritis | 34.73 | 122.46 | -87.73 (-160.59 - (14.85)) | 0.28 (0.07 - 0.98) | 0.029 |
| Respiratory Adverse Events | |||||
| Respiratory Depression | 284.83 | 398.01 | -113.18 (-278.60 - 52.30) | 0.72 (0.43 - 1.22) | 0.187 |
| Pneumonia | 125.05 | 367.39 | -242.34 (-373.30 - (-111.30)) | 0.34 (0.17 - 0.65) | 0.001 |
| Skin-related Adverse Events | |||||
| Cellulitis | 62.52 | 214.31 | -151.79 (-248.70 - (-54.80)) | 0.29 (0.11 - 0.72) | 0.004 |
| Pruritus | 0.00 | 0.00 | - | - | - |
| Erythema | 6.95 | 0.00 | 6.95 (-13.26 - 27.16) | - | 0.501 |
| Rash | 0.00 | 15.31 | -15.31 (-35.52 - 4.90) | 0.00 (0.00 - 17.70) | 0.138 |
| Skin Irritation | 0.00 | 0.00 | - | - | - |
| Urinary Adverse Events | |||||
| Urinary Discomfort | 34.73 | 214.31 | -179.58 (-267.70 - (91.50)) | 0.16 (0.05 - 0.48) | |
| *Incidence rate ratio test was performed to assess statistical differences between study cohorts. If the rate of adverse event was zero in one of the cohorts, p-value reflects the difference between study cohorts in the absolute incidence rate difference Abbreviations: OAD: Opioid abuse/dependence; CI: Confidence interval |
|||||
Table 5: Serious TEAE rates (per 1,000 person-years) during the CIII buprenorphine and CII opioid treatment in the primary analysis.
Sub-analysis #1: belbuca® vs. CII opioids
Non-matched population: Demographic and clinical characteristics of 26,178 patients treated with Belbuca® (203 patients) and CII Opioids (25,976 patients) before the PSM are provided in the Supplement (Table A7,A8).
Matched population: A total number of 421 patients were identified in the final sample of matched patients (124 patients in Belbuca® cohort and 297 patients in CII opioid cohort). As the demographic and clinical characteristics were used as a basis for PSM analysis, there were no statistical differences in these measures implying the cohorts were well-balanced. The list of demographic and clinical characteristics is shown in the Supplement (Table A9,A10).
There was no observed all-grade TEAEs with significantly higher rates in Belbuca® compared to CII opioid-treated Medicare patients diagnosed with cLBP. Otherwise, it was demonstrated that all-grade atrial fibrillation (IRD -194.33, p=0.010), confusion (IRD -194.33, p=0.010), headache (IRR 0.12, p=0.035), constipation (IRR 0.15, p=0.001), cellulitis (IRD -242.92, p=0.004), and urinary discomfort (IRR 0.11, p<0.001) occurred more frequently in CII opioid cohort. The list of all-grade TEAE rates in sub-analysis #1 matched sample is provided in Table 6.
| Belbuca® (N=124) |
CII Opioids (N=297) |
Incidence Rate Difference (95% CI) |
Incidence Rate Ratio (95% CI) | P-value* | |
|---|---|---|---|---|---|
| Cardiac Adverse Events | |||||
| QT prolongation | 0.00 | 0.00 | - | - | - |
| Hypotension | 29.52 | 0.00 | 29.52 (-44.71 - 103.74) | - | 0.436 |
| Atrial Fibrillation | 0.00 | 194.33 | -194.33 (-342.80 - (-45.90)) | 0.00 (0.00 - 0.92) | 0.010 |
| Coronary Artery Disease | 0.00 | 97.17 | -97.17 (-202.15 - 7.79) | 0.00 (0.00 - 3.23) | 0.070 |
| Hypertension | 0.00 | 48.58 | -48.58 (-122.82 - 25.63) | 0.00 (0.00 - 23.69) | 0.200 |
| Central Nervous System-related Adverse Events | |||||
| Dizziness | 88.56 | 0.00 | 88.56 (-40.01 - 217.11) | - | 0.177 |
| Somnolence | 59.04 | 0.00 | 59.04 (-45.94 - 164.00) | - | 0.270 |
| Confusion | 0.00 | 194.33 | -194.33 (-342.80 - (-45.90)) | 0.00 (0.00 - 0.92) | 0.010 |
| Seizures | 59.04 | 0.00 | 59.04 (-45.94 - 164.00) | - | 0.270 |
| Syncope | 29.52 | 194.33 | -164.81 (-330.80 - 1.10) | 0.15 (0.00 - 1.53) | 0.079 |
| Cerebrovascular Accident | 236.16 | 97.17 | 138.89 (11.77 - 351.05) | 2.43 (0.48 - 23.49) | 0.267 |
| Nervousness | 0.00 | 0.00 | - | - | - |
| Visual Discomfort | 29.52 | 0.00 | 29.52 (-44.71 - 103.74) | - | 0.436 |
| Suicidal Ideation | 0.00 | 48.58 | -48.58 (-122.82 - 25.63) | 0.00 (0.00 - 23.69) | 0.200 |
| Sleep Disturbances | 236.16 | 388.67 | -152.51 (-449.50 - 144.30) | 0.61 (0.20 - 1.86) | 0.329 |
| Opioid Use Disorder-related Adverse Events | |||||
| OAD | 177.12 | 97.17 | 79.95 (-130.03 - 289.86) | 1.82 (0.33 - 18.46) | 0.495 |
| Opioid Poisoning | 0.00 | 0.00 | - | - | - |
| General Adverse Events | |||||
| Headache | 29.52 | 242.92 | -213.40 (-395.30 - (-31.60)) | 0.12 (0.00 - 1.09) | 0.035 |
| Fatigue | 944.64 | 825.92 | 118.72 (-401.10 - 638.00) | 1.14 (0.62 - 2.20) | 0.666 |
| Allergic Reactions | 0.00 | 0.00 | - | - | - |
| Dehydration | 118.08 | 145.75 | -27.67 (-224.09 - 168.67) | 0.81 (0.14 - 5.53) | 0.779 |
| Dry Mouth | 0.00 | 0.00 | - | - | - |
| Xerostomia | 29.52 | 0.00 | 29.52 (-44.71 - 103.74) | - | 0.436 |
| Sweating | 0.00 | 0.00 | - | - | - |
| Hot Flushes | 0.00 | 0.00 | - | - | - |
| Sinusitis | 29.52 | 0.00 | 29.52 (-44.71 - 103.74) | - | 0.436 |
| Gastrointestinal Adverse Events | |||||
| Nausea and Vomiting | 147.60 | 388.67 | -241.07 (-508.80 - 26.50) | 0.38 (0.10 - 1.32) | 0.093 |
| Constipation | 88.56 | 583.00 | -495.44 (-782.00 - (-207.10)) | 0.15 (0.03 - 0.56) | 0.001 |
| Hepatotoxicity | 0.00 | 0.00 | - | - | - |
| Cholecystitis | 29.52 | 0.00 | 29.52 (-44.71 - 103.74) | - | 0.436 |
| Abdominal Pain | 442.80 | 437.25 | 5.55 (-358.21 - 369.05) | 1.01 (0.42 - 2.62) | 0.990 |
| Diarrhea | 88.56 | 145.75 | -57.19 (-239.04 - 124.59) | 0.61 (0.08 - 4.54) | 0.560 |
| Anorexia/Loss of Appetite | 383.76 | 534.42 | -150.66 (-514.40 - 212.80) | 0.72 (0.30 - 1.77) | 0.423 |
| Hormonal Adverse Events | |||||
| Adrenal Insufficiency | 0.00 | 0.00 | - | - | - |
| Musculoskeletal Adverse Events | |||||
| Bone Fractures | 0.00 | 48.58 | -48.58 (-122.82 - 25.63) | 0.00 (0.00 - 23.69) | 0.200 |
| Osteoarthritis | 442.80 | 534.42 | -91.62 (-470.24 - 286.72) | 0.83 (0.36 - 1.99) | 0.633 |
| Respiratory Adverse Events | |||||
| Respiratory Depression | 0.00 | 97.17 | -97.17 (-202.15 - 7.79) | 0.00 (0.00 - 3.23) | 0.070 |
| Pneumonia | 295.20 | 194.33 | 100.87 (-176.90 - 378.50) | 1.52 (0.44 - 6.63) | 0.502 |
| Skin-related Adverse Events | |||||
| Cellulitis | 0.00 | 242.92 | -242.92 (-408.90 - (-77.00)) | 0.00 (0.00 - 0.66) | 0.004 |
| Pruritus | 0.00 | 97.17 | -97.17 (-202.15 - 7.79) | 0.00 (0.00 - 3.23) | 0.070 |
| Erythema | 0.00 | 48.58 | -48.58 (-122.82 - 25.63) | 0.00 (0.00 - 23.69) | 0.200 |
| Rash | 88.56 | 0.00 | 88.56 (-40.01 - 217.11) | - | 0.177 |
| Skin Irritation | 29.52 | 0.00 | 29.52 (-44.71 - 103.74) | - | 0.436 |
| Urinary Adverse Events | |||||
| Urinary Discomfort | 88.56 | 825.92 | -737.36 (-1,069.40 - (-405.50)) | 0.11 (0.02 - 0.37) | |
| *Incidence rate ratio test was performed to assess statistical differences between study cohorts. If the rate of adverse event was zero in one of the cohorts, p-value reflects the difference between study cohorts in the absolute incidence rate difference Abbreviations: OAD: Opioid abuse/dependence; CI: Confidence interval |
|||||
Table 6: All-grade TEAE rates (per 1,000 person-years) during the Belbuca® and CII opioid treatment among matched sample in sub-analysis #1.
There were no serious TEAEs that occurred significantly more in Belbuca®- vs. CII opioid-treated Medicare patients diagnosed with cLBP. Significantly lower rates of fatigue (IRR 0.20, p=0.43), constipation (IRR 0.12, p=0.035), osteoarthritis (IRD -340.08, p<0.001), and urinary discomfort (IRD -194.33, p=0.010). All serious TEAE rates among sub-analysis #1 matched sample are listed in Table 7.
| Belbuca® (N=124) |
CII Opioids (N=297) |
Incidence Rate Difference (95% CI) |
Incidence Rate Ratio (95% CI) | P-value* | |
|---|---|---|---|---|---|
| Cardiac Adverse Events | |||||
| QT prolongation | 0.00 | 0.00 | - | - | - |
| Hypotension | 0.00 | 0.00 | - | - | - |
| Atrial Fibrillation | 0.00 | 0.00 | - | - | - |
| Coronary Artery Disease | 0.00 | 0.00 | - | - | - |
| Hypertension | 0.00 | 48.58 | -48.58 (-122.82 - 25.63) | 0.00 (0.00 - 23.69) | 0.200 |
| Central Nervous System-related Adverse Events | |||||
| Dizziness | 0.00 | 0.00 | - | - | - |
| Somnolence | 0.00 | 0.00 | - | - | - |
| Confusion | 0.00 | 48.58 | -48.58 (-122.82 - 25.63) | 0.00 (0.00 - 23.69) | 0.200 |
| Seizures | 29.52 | 0.00 | 29.52 (-44.71 - 103.74) | - | 0.436 |
| Syncope | 0.00 | 0.00 | - | - | - |
| Cerebrovascular Accident | 236.16 | 48.58 | 187.42 (-35.10 - 410.20) | 4.86 (0.65 - 216.63) | 0.104 |
| Nervousness | 0.00 | 0.00 | - | - | - |
| Visual Discomfort | 0.00 | 0.00 | - | - | - |
| Suicidal Ideation | 0.00 | 48.58 | -48.58 (-122.82 - 25.63) | 0.00 (0.00 - 23.69) | 0.200 |
| Sleep Disturbances | 0.00 | 0.00 | - | - | - |
| Opioid Use Disorder-related Adverse Events | |||||
| OAD | 0.00 | 0.00 | - | - | - |
| Opioid Poisoning | 0.00 | 0.00 | - | - | - |
| General Adverse Events | |||||
| Headache | 29.52 | 48.58 | -19.06 (-124.05 - 85.90) | 0.61 (0.01 - 47.68) | 0.756 |
| Fatigue | 59.04 | 291.50 | -232.46 (-442.50 - (-22.60)) | 0.20 (0.02 - 1.13) | 0.043 |
| Allergic Reactions | 0.00 | 0.00 | - | - | - |
| Dehydration | 88.56 | 145.75 | -57.19 (-239.04 - 124.59) | 0.61 (0.08 - 4.54) | 0.560 |
| Dry Mouth | 0.00 | 0.00 | - | - | - |
| Xerostomia | 0.00 | 0.00 | - | - | - |
| Sweating | 0.00 | 0.00 | - | - | - |
| Hot Flushes | 0.00 | 0.00 | - | - | - |
| Sinusitis | 0.00 | 0.00 | - | - | - |
| Gastrointestinal Adverse Events | |||||
| Nausea and Vomiting | 29.52 | 48.58 | -19.07 (-124.05 - 85.90) | 0.61 (0.01 - 47.68) | 0.756 |
| Constipation | 29.52 | 242.92 | -213.40 (-395.30 - (-31.60)) | 0.12 (0.00 - 1.09) | 0.035 |
| Hepatotoxicity | 0.00 | 0.00 | - | - | - |
| Cholecystitis | 0.00 | 0.00 | - | - | - |
| Abdominal Pain | 118.08 | 291.50 | -173.42 (-408.20 - 61.20) | 0.41 (0.08 - 1.71) | 0.172 |
| Diarrhea | 29.52 | 0.00 | 29.52 (-44.71 - 103.74) | - | 0.436 |
| Anorexia/Loss of Appetite | 0.00 | 97.17 | -97.17 (-202.15 - 7.79) | 0.00 (0.00 - 3.23) | 0.070 |
| Hormonal Adverse Events | |||||
| Adrenal Insufficiency | 0.00 | 0.00 | 0.00 (0.00 - 0.00) | - | - |
| Musculoskeletal Adverse Events | |||||
| Bone Fractures | 0.00 | 48.58 | -48.58 (-122.82 - 25.63) | 0.00 (0.00 - 23.69) | 0.200 |
| Osteoarthritis | 0.00 | 340.08 | -340.08 (-536.50 - (-143.80)) | 0.00 (0.00 - 0.42) | 0.001 |
| Respiratory Adverse Events | |||||
| Respiratory Depression | 0.00 | 97.17 | -97.17 (-202.15 - 7.79) | 0.00 (0.00 - 3.23) | 0.070 |
| Pneumonia | 206.64 | 145.75 | 60.91 (-173.88 - 295.56) | 1.42 (0.32 - 8.49) | 0.645 |
| Skin-related Adverse Events | |||||
| Cellulitis | 0.00 | 0.00 | - | - | - |
| Pruritus | 0.00 | 0.00 | - | - | - |
| Erythema | 0.00 | 0.00 | - | - | - |
| Rash | 29.52 | 0.00 | 29.52 (-44.71 - 103.74) | - | 0.436 |
| Skin Irritation | 0.00 | 0.00 | - | - | - |
| Urinary Adverse Events | |||||
| Urinary Discomfort | 0.00 | 194.33 | -194.33 (-342.80 - (-45.90)) | 0.00 (0.00 - 0.92) | 0.010 |
| *Incidence rate ratio test was performed to assess statistical differences between study cohorts. If the rate of adverse event was zero in one of the cohorts, p-value reflects the difference between study cohorts in the absolute incidence rate difference Abbreviations: OAD: Opioid abuse/dependence; CI: Confidence interval |
|||||
Table 7: Serious TEAE rates (per 1,000 person-years) during the Belbuca® and CII opioid treatment among matched sample in sub-analysis #1.
Sub-analysis #2: Belbuca® vs. Buprenorphine patches
Non-matched population: Demographic and clinical characteristics of 1,020 patients before the PSM, including 203 patients initially prescribed Belbuca® and 817 patients initially prescribed buprenorphine patches, are provided in the Supplement (Table A11,A12).
Matched population: The sample of matched patients included 62 patients in each of the compared cohorts. Patient demographic and clinical characteristics were well-balanced with no remaining statistical differences between the cohorts (Supplement Table A13,A14).
There were differences observed between study cohorts in 7/44 all-grade TEAEs. Belbuca® treatment was associated with lower rates of all-grade cerebrovascular accident (IRD -248.26 per 1,000 person-years, p=0.038), and opioid abuse and dependence (IRR 0.07, p<0.001), dehydration (IRR 0.08, p=0.002), and nausea and vomiting (IRR 0.25, p=0.025). Contrary, all-grade confusion (IRD 520.38, p=0.004), fatigue (IRR 2.68, p=0.012), and osteoarthritis (IRR 10.71, p<0.001) more commonly occurred in Belbuca® cohort. The list of all-grade TEAE rates in sub-analysis #2 matched sample is reported in Table 8.
| Belbuca® (N=62) |
Bup. patch (N=62) |
Incidence Rate Difference (95% CI) |
Incidence Rate Ratio (95% CI) | P-value* | |
|---|---|---|---|---|---|
| Cardiac Adverse Events | |||||
| QT prolongation | 0.00 | 0.00 | - | - | - |
| Hypotension | 57.82 | 0.00 | 57.82 (-59.60 - 175.21) | - | 0.335 |
| Atrial Fibrillation | 0.00 | 0.00 | - | - | - |
| Coronary Artery Disease | 0.00 | 0.00 | - | - | - |
| Hypertension | 0.00 | 124.13 | -124.13 (-290.20 - 41.90) | 0.00 (0.00 - 4.96) | 0.143 |
| Central Nervous System-related Adverse Events | |||||
| Dizziness | 404.74 | 124.13 | 280.61 (-71.70 - 632.70) | 3.26 (0.62 - 32.16) | 0.134 |
| Somnolence | 57.82 | 0.00 | 57.82 (-59.60 - 175.21) | - | 0.335 |
| Confusion | 520.38 | 0.00 | 520.38 (168.00 - 872.40) | - | 0.004 |
| Seizures | 57.82 | 0.00 | 57.82 (-59.60 - 175.21) | - | 0.335 |
| Syncope | 0.00 | 62.06 | -62.06 (-179.48 - 55.33) | 0.00 (0.00 - 36.32) | 0.300 |
| Cerebrovascular Accident | 0.00 | 248.26 | -248.26 (-483.10 - (-13.50)) | 0.00 (0.00 - 1.41) | 0.038 |
| Nervousness | 0.00 | 0.00 | - | - | - |
| Visual Discomfort | 57.82 | 0.00 | 57.82 (-59.60 - 175.21) | - | 0.335 |
| Suicidal Ideation | 0.00 | 0.00 | - | - | - |
| Sleep Disturbances | 173.46 | 372.39 | -199.07 (-551.20 - 153.20) | 0.47 (0.08 - 2.18) | 0.294 |
| Opioid Use Disorder-related Adverse Events | |||||
| OAD | 115.64 | 1,551.61 | -1,436.17 (-2,046.30 - (-826.20)) | 0.07 (0.01 - 0.30) | |
| Opioid Poisoning | 0.00 | 0.00 | - | - | - |
| General Adverse Events | |||||
| Headache | 57.82 | 62.06 | -4.24 (-170.30 - 161.76) | 0.93 (0.01 - 73.10) | 0.964 |
| Fatigue | 1,329.86 | 496.52 | 832.86 (179.20 - 1,486.60) | 2.68 (1.16 - 6.92) | 0.012 |
| Allergic Reactions | 0.00 | 0.00 | - | - | - |
| Dehydration | 57.82 | 682.71 | -625.11 (-1,031.70 - (218.30)) | 0.08 (0.00 - 0.58) | 0.002 |
| Dry Mouth | 0.00 | 0.00 | - | - | - |
| Xerostomia | 0.00 | 0.00 | - | - | - |
| Sweating | 0.00 | 0.00 | - | - | - |
| Hot Flushes | 0.00 | 0.00 | - | - | - |
| Sinusitis | 57.82 | 124.13 | -66.31 (-269.69 - 137.00) | 0.47 (0.01 - 8.94) | 0.585 |
| Gastrointestinal Adverse Events | |||||
| Nausea and Vomiting | 173.46 | 682.71 | -509.25 (-948.70 - (-70.10)) | 0.25 (0.05 - 0.96) | 0.025 |
| Constipation | 289.10 | 310.32 | -21.22 (-392.61 - 349.91) | 0.93 (0.21 - 4.05) | 0.913 |
| Hepatotoxicity | 0.00 | 0.00 | - | - | - |
| Cholecystitis | 0.00 | 0.00 | - | - | - |
| Abdominal Pain | 404.74 | 806.84 | -402.30 (-927.40 - 122.70) | 0.50 (0.17 - 1.35) | 0.142 |
| Diarrhea | 115.64 | 0.00 | 115.64 (-50.40 - 281.60) | - | 0.172 |
| Anorexia/Loss of Appetite | 693.84 | 434.45 | 259.39 (-252.60 - 770.90) | 1.60 (0.58 - 4.79) | 0.334 |
| Hormonal Adverse Events | |||||
| Adrenal Insufficiency | 0.00 | 0.00 | - | - | - |
| Musculoskeletal Adverse Events | |||||
| Bone Fractures | 0.00 | 0.00 | - | - | - |
| Osteoarthritis | 1,329.86 | 124.13 | 1,205.73 (618.30 - 1,792.30) | 10.71 (2.65 - 93.71) | <0.001 |
Respiratory Adverse Events
References
Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Citation: Grbic D, Stanicic F, Vukicevic D, Zah V (2024) Safety Profile of Schedule III Buprenorphine and Schedule II Oral Opioids in Elderly with Chronic Low Back Pain: A Retrospective US Medicare Claims Analysis. J Pain Relief 13: 656. DOI: 10.4172/2167-0846.1000656 Copyright: © 2024 Grbic D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Select your language of interest to view the total content in your interested language Share This ArticleRecommended JournalsOpen Access JournalsArticle ToolsArticle Usage
Peer Reviewed JournalsMake the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals Journals by SubjectClinical & Medical JournalsInternational Conferences 2025-26
Conferences by CountryMedical & Clinical Conferences | |||||