Safety Evaluation of Rayner's Flavor Banana® in Swiss Albino Mice
Received: 27-Jun-2018 / Accepted Date: 03-Aug-2018 / Published Date: 06-Aug-2018 DOI: 10.4172/2476-2067.1000136
Abstract
Safety evaluation of Rayner’s Flavor was carried out in Swiss albino mice and 52 mice were used for the evaluation. Forty out of 52 mice were used for determination of median lethal dose (LD50) using modified arithmetical method of Reed and Muench. The LD50 was determined at dose rate of 0.35 ml. Another 12 mice were divided into two groups of six per group. The first and second groups were administered Rayner’s flavor® (0.05 ml) and water (0.05 ml) respectively. Blood samples were collected for hematology and biochemistry. Haematology revealed significant (P<0.05) increased PCV and neutrophils of the group administered Flavor. But biochemistry showed increased levels (P<0.05) of SG0T, sodium ion and decreased levels of bicarbonate ion (HCO-3), chloride ion (Cl-) and urea, respectively. More so, Rayner’s flavor® caused dullness, dizziness, gasping for air, jerking movement, lying in ventrally recumbency, shallow respiration and death. Hence, caution should be exercised when using Rayner’s flavor®.
Keywords: Safety; Toxicity; Rayner’s flavor®; Albino mice
Introduction
Flavor agents are synthetic chemicals that mimic natural flavors, which are characterized by combination of smell, taste and mouth-feel. Cooking and processing food involves the use of chemical technology to create a harmonious product using color, smell, taste, texture and mouth-feel [1]. However, Rayner’s flavor® contains propylene glycol, water, E102 and E110 manufactured in England by Rayner Company Limited. Flavor agents are typically named after initial extract prepared from the source material and have been classified into three classes: those with simple chemical structures, efficient modes of metabolism and low order of toxicity (class 1) and class II agents have structural features that are less innocuous than those in class I and class III agents have structural features that permit no strong initial presumption of safety, or may even suggest significant toxicity [2]. Nevertheless, acute toxicity tests are now used to establish an approximate lethal dose of a compound in different species using different routes. The intravenous route should usually be used, since it gives best estimate of intrinsic acute toxicity uncomplicated by such factor like absorption, as the plasma concentration of a compound is a more relevant parameter than the administered dose [3].
Propylene glycol, a component of Rayner’s flavor is a clear, viscous liquid, miscible with water used in cosmetics and as occlusive dressing for ichthyosis [4]. The physical properties of propylene glycol are similar to those of ethyl alcohol but it is much less toxic. Hence, it is used as solvent, cosmetic, lotion, ointment, and in food material. Its elimination is slower and its actions prolonged [5]. Percutaneously absorbed propylene glycol is oxidized in liver to lactic acid and pyruvic acid with subsequent utilization in general body metabolism [6].
Since many people are consuming Rayner’s flavor, either according to the dosage guides given by Rayner’s company or otherwise there is need for determination of safety level of the flavor.
Methodology
Rayner’s flavor banana® was purchased from a provision shop in Makurdi, Benue State, Nigeria. The contents of the flavor were propylene glycol, water, E102 (tartrazine) and E110 (synthetic coal tar). Fifty-two Swiss albino mice of either sex weighing 25 ± 2.5 g were used for the study. The mice of about 8-weeks-old were purchased from Veterinary Research Institute (NVRI) Vom, Plateau state, Nigeria. They were acclimatized for a period of 2 weeks and fed Grower’s marsh® formulated by Grand Cereals and Oil-mills Limited in Jos, Nigeria. Forty out of 52 mice were used for determination of median lethal dose (LD50) using modified arithmetical method of Reed and Muench [7]. Forty mice were divided into four groups of ten each. The 1st, 2nd, 3rd and 4th group was administered the flavor at 0.00, 0.05, 010 and 0.75 ml/25 g of mice, intravenously. All the animals were observed for a period of 48hrs and thereafter for 12 days for observation of toxicity signs including death. The remaining 12 mice were divided into two groups of six each. First group was administered 0.05 ml of Rayner’s flavor® intravenously while the 2nd group was administered distilled water (0.05 ml) intravenously. One day after, blood samples (0.5 ml) were collected into ethylene diammine tetraacetate (EDTA) bottles for hematology and serum biochemistry. All the experimental animals were observed for toxicity signs. Total blood cells count was done using the method of Baker [8]; whereas biochemical parameters were quantitatively determined using hemanalyzer [9].
Statistical analysis
Hematological and biochemical parameters were expressed as mean ± S.D. Tests for significance between mean parameters in respect of control and experimental values were performed using student t-test unpaired [10].
Results
The results of median lethal dose (LD50) determination showed 6, 2 and 5 deaths after 48 hours when 0.1, 0.05 and 0.75 ml was administered. LD50 of Rayner’s flavor was determined at dose rate of 0.35 ml (Table 1). Other toxicity signs observed are weakness, dullness, dizziness, gasping for air, jerking movement, lying ventrally, shallow respiration and death.
| Group | Dose (ml/25 g mouse) | No. of Mice | No. of death | No. of survivals |
|---|---|---|---|---|
| 1 | 0.00 | 10 | 0 | 10 |
| 2 | 0.05 | 10 | 2 | 8 |
| 3 | 0.10 | 10 | 6 | 4 |
| 4 | 0.75 | 10 | 5 | 5 |
Table 1: Median lethal dose (LD50) determination of Rayner’s flavor in Swiss albino mice.
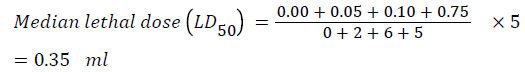
LD50 of the Rayner’s flavor was 0.35 ml per 25 g of mouse. This translates to 14 ml per 1 kg signifying high safety profile of the flavor.
Hematology revealed significantly (P<0.05) increased level of packed cell volume and neutrophils in the group administered Rayner’s Flavor. Other hematological parameters were not affected significantly (p>0.005) (Table 2). Serum biochemistry revealed significantly (P<0.05) increased SGOT, sodium ion and decreased alkaline phosphatase, SGPT, chloride ion, bicarbonate ion and urea, respectively (Table 3).
| Parameters (%) | Control | Experimental |
|---|---|---|
| Packed cell volume | 32.00 ± 3.46 | 38.66 ± 5.16a |
| White blood cells | 40.5 ± 14.4 | 44.16 ± 12.10 |
| Neutrophils | 43.00 ± 7.48 | 48.16 ± 12.04a |
| Lymphocytes | 54.66 ± 4.54 | 48.00 ± 12.24 |
| Eosinophils | 2.00 ± 0.89 | 1.83 ± 0.75 |
| Monocytes | 2.00 ± 0.89 | 2.00 ± 0.89 |
| Basophils | 0.00 ± 0.00 | 0.00 ± 0.00 |
t-test level of significance=5%
Table 2: Effects of single intravenous bolus of Rayner’s flavor on hematological parameters of Swiss albino mice.
| Parameters | Control | Experimental |
|---|---|---|
| Total protein (g/L) | 74.80 ± 8.81 | 71.50 ± 3.11b |
| Albumin (g/L) | 3.34 ± 0.48 | 3.60 ± 0.42 |
| Alkaline phosphatase (µg/L) | 112.2 ± 8.31 | 104.25 ± 19.82b |
| Total bilirubin (µmol/L) | 14.02 ± 1.40 | 14.12 ± 0.85 |
| Conjugated bilirubin (µmol/L) | 2.75 ± 0.49 | 3.10 ± 0.28 |
| Serum glutamic oxaloacetic transaminase (ug/L) | 7.17 ± 1.89 | 9.27 ± 3.45a |
| Serum glutamic pyruvic transaminase (ug/L) | 9.25 ± 1.89 | 6.05 ± 2.92b |
| Sodium ion (mmol/L) | 117.00 ± 7.21 | 135.20 ± 4.55a |
| Potassium ion (mmol/L) | 3.72 ± 0.94 | 3.94 ± 0.6 |
| Chloride ion (mmol/L) | 111.6 ± 12.13 | 102.20 ± 2.05b |
| Bicarbonate ion (mml/L) | 25.6 ± 1.34 | 21.40 ± 2.07b |
| Urea (mmol/L) | 99.2 ± 16.39 | 81.10 ± 18.87b |
t-test level of significance=5%; aSignificantly higher (P<0.05); bSignificantly lower (P<0.05)
Table 3: Effects of Rayner’s flavor®on biochemical parameters of Swiss albino mice.
Discussion
The median lethal dose (0.35 ml) of Rayner’s flavor® determined in Swiss albino mice agrees with the report of Dreisbach et al. indicating that LD50 is the amount of chemical that will kill approximately 50% of a group of test animals. Humburger reported that the ability of a compound to cause death in half of the animals when a certain dose is administered defines the toxicity of the compound. However, the dangerous dose for humans may be 1%-10% more or less of the indicated dose [11]. In the present study the LD50 (0.35 ml) translates to 14 ml/kg, suggesting relative safety of the flavor. Despite the fact that the concentration of Rayner’s flavor in gram was not known, our finding agrees with the report of Newbold, indicating that chemical does not become a medicine until the potential utility observed in the laboratory has been proven in clinical trials and regulatory agencies have accepted the results of exhaustive tests for efficacy, safety and quality [12]. Our observation of other toxicity signs such as weakness, dullness, dizziness, gasping for air, jerking movement, lying in ventral recumbency, shallow respiration and death disagrees with the report of WHO indicating that class 1 flavor agents have simple chemical structure, efficient modes of metabolism and low order of toxicity. But the long observed central nervous system (CNS) effects in the experimental animals may be due to the presence of propylene glycol. This agrees with the report of Hardman et al. indicating that elimination of propylene glycol is slower and its actions are thus prolonged. This in-turn is confirmed by the report of Dreisbach et al. indicating that propylene glycol has a fatal dose of 100 mg/day with CNS effects including convulsions. Hence, the concentration of propylene glycol in Rayner’s Flavor may be relatively high. Moreover, the coloring agents (E102 and E110) may also be relatively toxic. E110 approved by European Union (EU) as synthetic colorant, is a yellow synthetic coal tar, highly soluble in water. Saganuwan reported that coal tar may be converted to central nervous system depressant. E102 (tartrazine), a synthetic azo dye may cause CNS depression and allergic reaction [13]. Therefore, toxicity study of chemicals is a scientific process necessary for identification of a potential toxicant [14,15]. Hence, there is need for Rayner Company Limited to give elaborate explanation of the contents.
The increased packed cell volume observed in the group administered flavor (38.66% ± 5.16%) as compared to the group administered water (32.00% ± 3.46%) is suggestive of hemopoietic effect of the flavor. But the increased neutrophils observed may be suggestive of immune-stimulatory activity of the flavor. The significant increased level of SGOT and sodium ion in the flavor group as compared to the water group, may suggest that the flavor have effect on liver and heart. But the significant decreased level of bicarbonate ion may suggest the ability of Rayner’s flavor® to cause metabolic acidosis. But the decreased urea, chloride ion and bicarbonate ion may suggest safety of the kidney. Hence, the use of Rayner’s flavor® by public should be considered as an issue that is worthy of caution.
Conclusion
The median lethal dose of Rayner’s flavor was estimated to be 0.35 ml in Swiss albino mice. Other toxicity signs include weakness, dullness, dizziness, gasping for air, jerking movement, lying in ventral recumbency, shallow respiration and death. The Flavor caused increase packed cell volume, sodium ion (Na+) and decreased bicarbonate ion (HCO3-), chloride (Cl-) ion and urea. Hence the use of Rayner’s flavor® by public should be reviewed. I recommend that the Rayner Company Limited should conduct a study on safety evaluation and also give explanation in relation to the contents of Rayners’ flavor®.
References
- Foskett D, Ceserani V, Kinton R (2003) The Theory of Catering, (10th edn). Hodder & Stoughton, London, UK. pp: 187.
- WHO (2005) Evaluation of certain food additives. 63rd Report of the Joint FAO/WHO Expert Committee on Food Additives, Geneva.
- Brimblecombe RW, Dayan (1993) Preclinical toxicity testing In: (Burley, D. M., Clarke, J.M. and Lusanga, L.) Pharmaceutical Medicine. Great Britain pp: 13-32.
- Tripathi K D (2003) Drugs acting on skin and mucous membrane. Essentials of Medical Pharmacology, (5th edn. ) pp: 793.
- Hardman J G, Limbird L E, Molinoff P B, Ruddon R W, Gilman A G (1996) Non-metallic environmental toxicants. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, (9th edn.) London McGraw – Hill, UK pp: 1683.
- Katzung B G (2004) Dermatologic pharmacology. In: Basic and Clinical Pharmacology, London (9th edn.) McGraw – Hill, UK pp: 1028-1029.
- Saganuwan SA (2011) A modified arithmetical method of Reed and Muench for determination of a relatively ideal median lethal dose (LD50). Afr J Pharm Pharmacol 5 : 1543-1546.
- Baker F J (1985) The Full blood counts. In: Baker F J Silverton, R E Kilshaw, D Shannon, R Egglestone, et al. (eds.) Introduction to Medical Laboratory Technology (6th edn.). London, Butterworth & Co. Ltd, UK pp: 320-330.
- Willard M. D, Tvedten H, Turnwald G H (1989) Small Animal Clinical Diagnosis by Laboratory Methods(5th edn.)WB Saunders Company, Philadelphia pp: 1-380.
- Petrie A, Watson P (2002)  Hypothesis test 1 – The test comparing one or two means. Statistics for Veterinary Science (3rd edn.) London, Blackwell science Ltd., U. K. pp: 78-88.
- Dreisbach R H, Robertson W O (1987) Alcohols and glycols. Handbook of poisoning: Prevention, Diagnosis & Treatment (13th edn.) London, Appleton & large, UK Â pp: 176.
- Newbold B. B. (1993) How are medicines discovered In: (Burley, D. M., Clarke, J. M and Lusanga, L.) Pharmaceutical Medicine, Great Britain pp: 2-3.
- Saganuwan SA (2017a) Functional chemical groups that may likely become source for synthesis of novel central nervous system (CNS) acting drugs. Central Nervous System Agents in Medicinal Chemistry 17: 1-9.
- Saganuwan SA (2017b) Therapeutic causes of Stevens Johnson syndrome: A mini review. Open Acc J of Toxicol 1: 1-4.
- Saganuwan SA (2017c) Toxicity studies of drugs and chemicals in animals: An overview. Bulg J Vet Med 18: 1-18.
Citation: Saganuwan SA (2018) Safety Evaluation of Rayner’s Flavor Banana® in Swiss Albino Mice. Toxicol Open Access 4: 136. DOI: 10.4172/2476-2067.1000136
Copyright: © 2018 Saganuwan AS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
