Ruptured Distal Mycotic Middle Cerebral Artery Aneurysm with Cortical Haemorrhagic Stroke Treated with NBCA Embolization: Case Report
Received: 10-Jul-2020 / Accepted Date: 24-Jul-2020 / Published Date: 31-Jul-2020 DOI: 10.4172/2167-7964.1000325
Abstract
Mycotic aneurysms are a rare cause of intracranial aneurysms that develop in the presence of infections such as infective endocarditis. They account for a small percentage of all intracranial aneurysms and carry a high mortality rate when ruptured. A 48-year-old male patient was admitted to our hospital with a severe headache and right-sided weakness. Computed tomography scan (CT scan) of the brain and Magnetic Resonance Imaging (MRI) revealed cortical hemorrhagic stroke with perilesional oedema in the left parietal lobe in left middle cerebral artery (MCA) territory and Magnetic Resonance Angiography (MRA) showed an aneurysm located at the distal subcortical mycotic aneurysm in the left MCA. A cerebral angiogram revealed 2 aneurysms; one pseudoaneurysm (approx. 2×2 mm) located at the left M4 distal MCA and the other false aneurysm in right basal ganglia. Findings of echocardiography were mitral valve myxomatous change and prolapse posterior mitral leaflet with oscillating vegetation with moderate mitral regurgitation (MR). Laboratory studies showed Streptococcus viridians positive blood culture of 3 specimens, anaemia, leucocytosis, and elevated inflammatory factors. The patient was treated with appropriate antibiotics therapy for 4 weeks and improved clinically. The follow-up MRA showed an unchanged mycotic aneurysm. We planned for another cerebral angiography and found an increased size of pseudoaneurysm (5×5 mm) and successful embolization with NBCA concentration 1:1 without any complication. Control angiography showed that the mycotic aneurysm was completely resolved. The patient was nearly free of symptoms.
Keywords: Mycotic middle cerebral artery aneurysm; Cortical haemorrhagic stroke; NBCA embolization
Abbreviations
CT scan: Computed Tomography Scan; MRA: Magnetic Resonance Angiography; MCA: Middle Cerebral Artery; Mas: Mycotic aneurysms; IA: Infective Aaneurysms; IA: Infective Aneurysms; SBE: Subacute Bacterial Endocarditis; dMCA: Distal Middle Cerebral Artery; NBCA: N-butyl 2-cyanoacrylate; IE: Infective Endocarditis; DSA: Digital Subtraction Angiography; ESR: Erythrocyte Sedimentation Rate; CRP: C-Reactive Protein; PAO: Parent Artery Occlusion; LEAs: Liquid Embolic Agents; PCA: Posterior Cerebral Artery; ICA: Internal Cerebral Artery.
Introduction
Mycotic aneurysms (MAs), also known as infective aneurysms (IA), are rare inflammatory neurovascular lesions that constitute 4% of all intracranial aneurysms. They are more common at young ages and 80% of the cases are younger than 40 years of age [1,2]. They are also observed in immune suppressed patients and 2-10% of patients with subacute bacterial endocarditis (SBE). MAs develop secondary to the invasion of the muscular layer of the tunica media by an infectious pathogen, most frequently via the blood stream and, occasionally, dissemination through the arterial wall of the inflamed tissues around the arteries, causing necrosis [3]. Infectious aneurysms are generally located in the distal parts of the cerebral arteries and can be multiple in numbers [4]. The resulting aneurysms are usually fusiform and eccentric, without saccular characteristics, and are more common in the anterior circulation [5]. The most frequent location is the distal middle cerebral artery (dMCA). Aneurysm of the dMCA is a rarely seen encountered condition, constituting 1.1-5% of MCA lesions [6,7]. Because their spontaneous rupture results in subarachnoid and intracerebral haemorrhage, they are associated with significant morbidity and mortality, as high as 60%–90% in earlier case studies, and 12–32% in more recent literature reviews [8,9]. We report a patient with an aneurysm in a ruptured right dMCA that originated from the M4 junction of the MCA, presenting with a left intracerebral haemorrhagic stroke and successful endovascular treatment by embolization with N-butyl 2-cyanoacrylate (NBCA).
Case Report
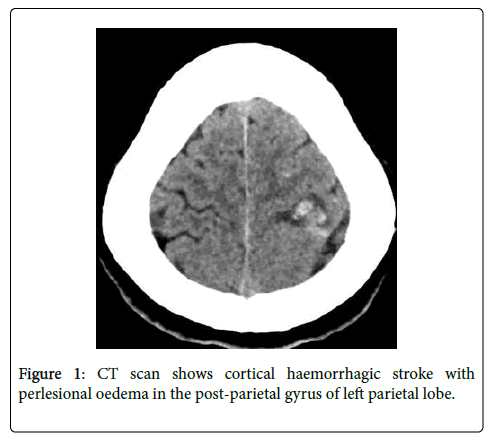
A 48 years old normotensive male presented with recurrent right weakness and headache last 3 months. From the first two episodes he recovered spontaneously with short period of time without attending to the doctor. But the last episode the weakness and headache are more severe and attended to hospital and admitted. He had associated complaints of night sweating and fever weight loss but no history of cough, joint pain, or shortness of breath. Patient was diagnosed as mitral valvular heart disease but was asymptomatic. So, he had no regular follow up or any medication. He had habit of smoking (20 pack/year) and alcohol (>5years) but quitted last 6 months before. On general examination revealed normal except anemia, fever (38.4ᵒC) and painful small erythematous macule in right great toe and his cardiovascular examination revealed a 4/6 pansystolic murmur, the most prominent at the left midclavicular line with radiation to the axilla. Neurological examination showed right sided upper motor lesion (MRC Grade 4) with uneventful fundoscopy. CT scan and MRI showed hemorrhagic stroke with perilesional edema in the postparietal gyrus of left parietal lobe (Figure 1).
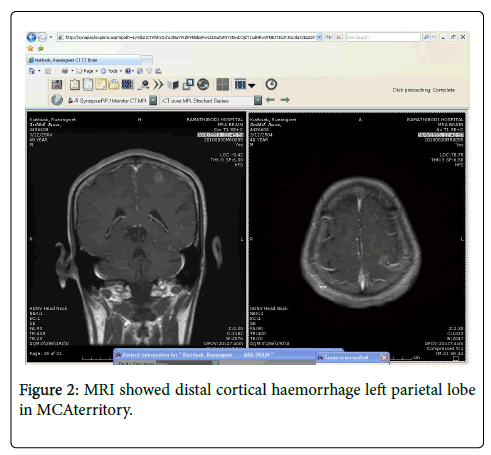
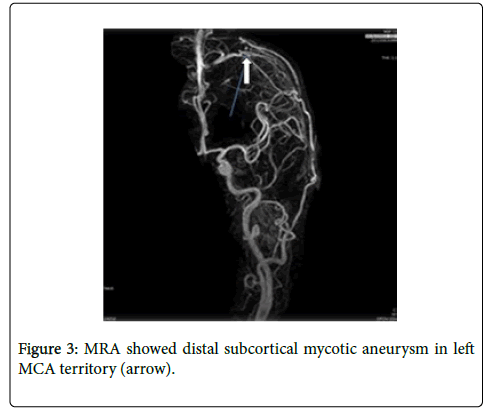
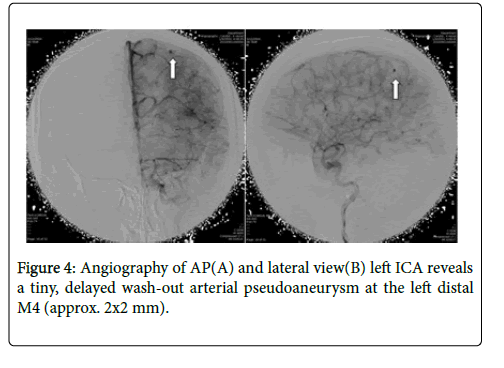
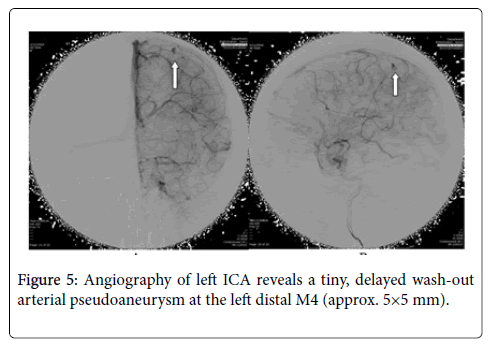
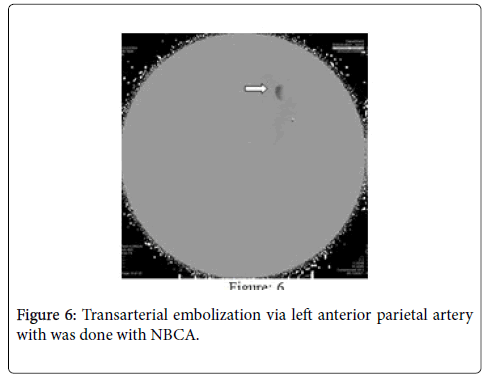
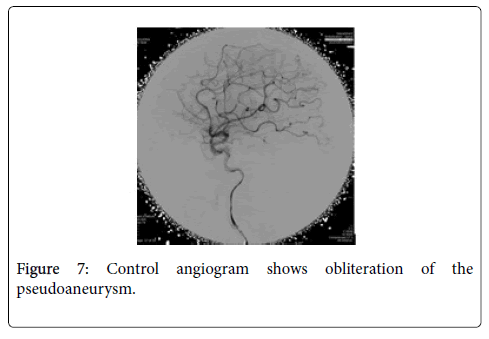
MRA showed distal subcortical mycotic aneurysm in left MCA (Figures 2 and 3) subsequently cerebral angiography was done and revealed2 aneurysms: one was distal M4 MCA pseudoaneurysm (approx. 2 × 2 mm) (Figure 4) and the other false aneurysm in right basal ganglia. The endovascular treatment could not be performed because it is impossible for the smallest micro catheter to reach into aneurysmal site and high-risk complications of embolization on this eloquent area. The echocardiogram visualized mitral valve myxomatous change and prolapse posterior mitral leaflet (PML) with large oscillating vegetation (approx. 1.0 × 0’5 cm) on the mitral valve and moderate MR. The 3 of 3 blood cultures were positive for Streptococcus viridians. The other laboratory investigations were found normal except Leucocytosis, elevated erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), haemoglobin (Hb)% 8.6 gm/dl and haematocrit (Hct) 27.3%. Intravenous Penicillin G and Gentamicin were started immediately, and after 7 days of antibiotics treatment, the patient’s general condition and right facial weakness was improved. We planned for continue antibiotics for total 4weeks and follow up afterwards. After 4 weeks of follow up the patient’s clinical profile was improved and but follow up MRI & MRA showed unchanged mycotic aneurysm. We planned for another cerebral angiography and found increase size of pseudoaneurysm (approx. 5×5 mm) (Figure 5 A & B) and successful embolization was done with NBCA concentration 1:1 (Figure 6). without any complication. The post control angiogram showed complete obliteration of the pseudoaneurysm (Figure 7). The patient's postoperative course was uneventful and was discharged home on the 9th postoperative day. The patient was transferred to a long-term nursing facility and given follow up with the cardiology team for reassessment after a 4-week course of IV antibiotics. At 2 months follow-up examination, he recovered completely.
Discussion
The term “bacterial aneurysm” was introduced to replace “mycotic aneurysm”, which was first used by William Osler in 1885, after high frequency of endocarditis in its etiology. At present, “mycotic aneurysm” is preferred for all infection-related aneurysms. Although a wide variety of bacteria, mycobacteria, viruses, and fungi may cause mycotic aneurysms, Streptococcus viridans and Staphylococcus aureus are the most common etiologic organisms [10].
IntracranialMAs presented with initial symptoms of headache (83%), fever (67%), vomiting (50%), ocular palsy (25%), seizures (21%), behavioural changes (21%), hemiparesis (21%), drowsiness (17%), and loss of consciousness (17%) [11]. Almost half the patients met Duke’s criteria for infective endocarditis (IE) and gave a history of either rheumatic or congenital heart disease.
A scoring system based on the presence of specific clinical and radiographic findings has been proposed for the diagnosis of MA. There are supportive criteria drawn from three domains [12]. Domain A encompasses infection, such as infective endocarditis (IE), meningitis, cavernous sinus thrombophlebitis or orbital cellulitis. Domain B encompasses angiographic features of the aneurysm, such as multiplicity, distal location, and fusiform shapeand change in size or appearance of new aneurysm at follow-up angiogram. Domain C encompasses other features, such as age <45 years, recent history of fever, lumbar puncture or cerebral haemorrhage. Each criterion is given 1 point and the sum under each domain (Asum, Bsum and Csum) and total score are calculated 12. Applying this scheme to our patient prior to Angiography would yield a score of >3. The sensitivity and specificity of this value of our case are 96% and 100% respectively.
The diagnosis of MAs relies on the presence of a predisposing infectious process with an aneurysm documented by vascular imaging. A literature even recommends that screening patient with bacterial endocarditis for intracranial aneurysms were given the strong correlation between the two [13]. Digital subtraction angiography (DSA) continues to be the gold standard for the diagnosis of MA10, although CT angiography and magnetic resonance imaging can be used [13]. Some of the findings on DSA that points toward MA are the fusiform shape, the multiplicity, the distal location, and the change in size on follow-up angiography [13]. Other indicators are positive blood culture (only found in 35.6%), leucocytosis, elevated ESR, and CRP [13]. Depends on location, dMCA aneurysms are classified into four segments M2 (insular segment), M2–M3 junction, M3 (opercular segment) and M4 (cortical segment). Most MCA aneurysms occur at division of the main trunk (M1–M2 junction) [14]. Because, cerebral aneurysms usually arise at the primary bifurcation or trifurcation due to haemodynamic stress and/or congenital factors. dMCA aneurysms are uncommon. In our study showed that ruptured dMCA aneurysm was pseudoaneurysm and originated from the M4 segment in the dMCA with large oscillating vegetation (approx. 1.0 × 0.5 cm) on the mitral valve and moderate MR, positive blood culture,leucocytosis, elevated ESR, and CRP which is consistent dMCA mycotic aneurysm.
For unruptured MAs, the mortality rate is 30%. Following rupture, the prognosis is poor, with an 80% mortality rate 19. Much controversy therefore exists regarding management of these lesions. Over the past decade, the management of infective intracranial aneurysms has been divided into medical, endovascular, and surgical treatment.
The mainstay medical intervention that is uniformly recommended is long-term intravenous antibiotic therapy for at least 6 weeks. The mainstay of treatment is immediate commencement of intravenous antibiotic therapy. It is generally accepted that endocarditis-related aneurysms will follow one of three possible outcomes. There is ample evidence that one-third resolve completely with antibiotic therapy alone and one-third will demonstrate no significant change in size and the remaining third, half enlarge on treatment while the other half reduce in dimensions. Unfortunately, there is no way of predicting if an ICMA will regress or rupture on appropriate antibiotic treatment.
In other study of unruptured MAs showed that they could be undergo spontaneous thrombosis and suggesting that MAs could resolve completely with antibiotic therapy alone [15]. In a review of 20 cases of MAs over a ten-year period seven patients were initially treated conservatively with IV antibiotics alone and followed by serial angiography [16]. In this series, the aneurysms in two patients decreased in size, one did not change, two achieved successful thrombosis, and the remaining two enlarged. Based on this review, the conclusion regarding unruptured MAs is that medical management with 6 weeks of IV antibiotic therapy is reasonable if closely followed by serial angiography. The goal of serial angiography would be to demonstrate improvement in aneurysm size and resolution. Another reported a case in which a MA failed to show radiographic decrease in size after 2 weeks of medical therapy and was successfully treated with endovascular therapy [17].
Ruptured aneurysms on the other hand should be immediately secured by surgical or endovascular means. The success of endovascular or surgical treatment depends mostly on the aneurysm morphology, the comorbidities of the patient, and the presence of an associated intracerebral hemorrhage [18]. The choice between endovascular and open surgery is complex and should be individualized.
Traditionally, the neurosurgical management of MA involves evacuation of the haematoma with ligation of the parent artery [19]. Surgery in the acute setting is technically difficult because of the friable nature of the necrotic tissue. It has been reported that once demonstrated, surgical treatment of a MA is more deleterious than medical treatment alone [20]. Peripherally located aneurysms are a greater challenge and frequently unnecessary brain damage is incurred in attempts to expose the aneurysm. The presence of surrounding clot also makes aneurysm identification particularly difficult.
Endovascular therapy has rapidly evolved in its efficacy and ability to access more distal aneurysms. The safety profile of this intervention is difficult to interpret, as it is based only on anecdotal and case-report data. A meta-analysis was performed on 16 patients in previously published cases who underwent endovascular treatment; 69% had a good outcome, while none had procedural-related complications [21]. The Current strategies in endovascular therapy include an indirect approach by parent artery occlusion (PAO) using coils or liquid embolic agents (LEAs) and direct approach by embolization of the aneurysm using coils, stent-assisted coiling (SAC), flow diverters, and LEAs [22-24]. PAO is attempted when the aneurysm is distally located, dysplastic, involving the whole circumference of the parent vessel, and having a complex morphology, provided that the area of the brain supplied by that artery is non-eloquent. Intracranial balloon test occlusion or amobarbital injection testing can help determining whether the area is eloquent or not when the provider is unsure 22. MAs those are proximal in location such as those arising from cavernous internal cerebral artery (ICA). ICA tends to be more treated by a direct approach, while both approaches are equally used for aneurysms that are distal in location such as those arising from MCA and posterior cerebral artery (PCA). When the aneurysm is difficult to reach, LEAs can be used for distal PAO (NBCA, ethylene-vinyl alcohol copolymer, Onyx). Rapid improvements in technology have resulted in the endovascular approach coming into vogue. Distal intracranial vascular navigation, which is now increasingly possible, has made percutaneous obliteration of peripheral MA a feasible and effective therapeutic option [25,26].
In another retrospective review performed on 14 patients with intracranial MAs who underwent endovascular intervention at between 1991 and 1999 at a major French hospital 29, no deaths or complications were reported, 11 of the 14 patients showed stable lesions on follow up angiography 6-24 months after endovascular embolization, and 9 had complete resolution of their presenting neurologic deficit 27. The use of endovascular therapy for mycotic aneurysms has been reported in series of cases by using NBCA in one case, autologous clot in another and platinum coils in the third. Treatment was successful in all these previously reported cases.
So, by using NBCA to embolize the distal parent vessel prevents retrograde collateral filling of the aneurysm. The major advantage of the endovascular techniques is that they avoid craniotomy and surgical handling of the swollen brain. Post-intervention recovery is less complicated and usually quicker. We performed successful embolization with NBCA with complete obliteration of our dMCAmycotic aneurysm without any complication. So, our opinion, endovascular techniques should be the primary treatment option in MAs requiring intervention after trial of medical therapy.
References
- Bohmfalk GL, Story JL, Wissinger JP, Brown WE (1978) Bacterial intracranial aneurysms. J Neurosurg 48:369-382.
- Horten BC, Abbott GF, Porro RS (1976) Fungal aneurysms of intracranial vessels. Arch Neurol 33:577-579.
- Suzuki J, Ohara H (1978) Clinicopathological study of cerebral aneurysms. Origin, rupture, repair and growth.J Neurosurg 48:505-514.
- McCormick WF (1971) Problems and pathogenesis of intracranial aneurysms in cerebral vascular diseases; L Moossy, R Janeway (eds): Grune& Stratton, New York 219-231.
- Nakahara M, Taha M, Higashi T (2006) “Different modalities of treatment of intracranial mycotic aneurysms: report of 4 cases,†Surgical Neurology 66:405-409.
- Dashti R, Hernesniemi J, Niemelä M, Rinne J, Lehecka M, Shen H (2007) Microneurosurgical management of distal middle cerebral artery aneurysms. Surgical Neurology 67:553-563.
- Yasargil MG, Verlag GT (1984) Stuttgart: clinical considerations, surgery of the intracranial aneursyms and results; Microneurosurgery 124-164.
- Watanabe A, Hirano K, Ishii R (1998) “Cerebral mycotic aneurysm treated with endovascular occlusion-case report,†Neurologia Medico-Chirurgica 38:657-660.
- Kannoth S, Thomas SV (2009) “Intracranial microbial aneurysm (infectious aneurysm): current options for diagnosis and management,†Neurocritical Care 11:120-129.
- Kannoth S, Iyer R, Thomas SV (2007) “Intracranial infectious aneurysm: presentation, management and outcome,†Journal of the Neurological Sciences 256:3-9.
- Kannoth S, Thomas SV, Nair S, Sarma PS (2008) “Proposed diagnostic criteria for intracranial infectious aneurysms,†Journal of Neurology, Neurosurgery and Psychiatry 79: 943-946.
- Ducruet F, Hickman ZL, Zacharia BE (2010) “Intracranial infectious aneurysms: a comprehensive review,†Neurosurgical Review 33:37-45.
- Gibo H, Carver CC, Rhoton AL, Lenkey C, Mitchell RJ (1981) Microsurgical anatomy of the middle cerebral artery. Journal of Neurosurgery 54:151-169.
- Morawetz RB, Karp RB (1984) “Evolution and resolution of intracranial bacterial (mycotic) aneurysms,†Neurosurgery 15:43-49.
- Chun Y, Smith W, Van Halbach V, Higashida RT, Wilson CB, et al. (2001) “Current multimodality management of infectious intracranial aneurysms,†Neurosurgery 48:1203-1214.
- Zhao PC, Li J, He M, You C (2010) “Infectious intracranial aneurysm: endovascular treatment with onyx case report and review of the literature,†Neurology India 58:131-134.
- Sugg RM, Weir R, Vollmer DG, Cacayorin ED (2006) “Cerebral mycotic aneurysms treated with a neuroform stent: technical case report,†Neurosurgery 58:E381.
- Pruitt AP, Rubin RH, Karchmer AW, Duncan GW (1978) Neurological complications of bacterial endocarditis. Medicine 57:329-343.
- van der Meulen JHP, Weststrate W, van Gijn J (1992) Is cerebral angiography indicated in infective endocarditis? Stroke 23:1662-1667.
- Eddleman CS, Surdell D, Di Patri A, Tomita T, Shaibani A (2008) “Infectious intracranial aneurysms in the pediatric population: endovascular treatment with Onyx,†Child's Nervous System 24:909-915.
- Gross BA, Puri AS (2013) “Endovascular treatment of infectious intracranial aneurysms,†Neurosurgical Review 36:11-19.
- Cloft HJ, Kallmes DF, Jensen ME, Lanzino G, Dion JE (1998) Endovascular treatment of ruptured cerebral aneurysm; parent artery occlusion with short Guglielmi detachable coils. Am J Neuroradiol 20:308-310.
- Scotti G, Li MH, Righi C, Simionato E, Rocca A (1996) Endovascular treatment of bacterial intracranial aneurysms. Neuroradiology 38:186-189.
- Chapot R, Houdart E, Saint-Maurice JP (2001) “Endovascular treatment of cerebral mycotic aneurysms.†Radiology 389-396.
- Khayata MH, Aymard A, Casasco A, Herbreteau D, Woimant F, et al. (1993) Selective endovascular techniques in the treatment of cerebral mycotic aneurysms. J Neurosurg 1993; 78:661-665.
- Frizzell RT, Vitek JJ, Hill DL, Fisher WS (1993) Treatment of bacterial (mycotic) intracranial aneurysm using an endovascular approach. Neurosurgery 32:852-854.
Citation: Rahman A, Pongpech S, Jiarakongmun P, Chanthanaphak E, Takong W, et al. (2020) Ruptured Distal Mycotic Middle Cerebral Artery Aneurysm with Cortical Haemorrhagic Stroke Treated with NBCA Embolization: Case Report. OMICS J Radiol 9: 1000325. DOI: 10.4172/2167-7964.1000325
Copyright: © 2020 Rahman A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3644
- [From(publication date): 0-2020 - Nov 04, 2025]
- Breakdown by view type
- HTML page views: 2756
- PDF downloads: 888