Review Article Open Access
Roles of Sodium-Calcium Exchanger Isoform-3 toward Calcium Ion Regulation in Alzheimers Disease
Henok KA1,3*, Tongmei Zhang2,3, Hao Li2,3 and Youming Lu2,31Department of Pathology and Pathophysiololgy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
2Department of Physiology, School of Basic Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
3Institute for Brain Research, Collaborative Innovation Center for Brain Science, Huazhong University of Science and Technology, Wuhan, China
- *Corresponding Author:
- Henok Kessete Afewerky
Department of Pathology and Pathophysiology
Tongji Medical College, Huazhong University of Science and Technology
Wuhan, Hubei, 430030, China
Tel: 0086-13260654649
E-mail: henokessete@hust.edu.cn
Received date: December 01, 2016; Accepted date: December 12, 2016; Published date: December 19, 2016
Citation: Henok KA, Zhang T, Li H, Lu Y(2016) Roles of Sodium-Calcium Exchanger Isoform-3 toward Calcium Ion Regulation in Alzheimers Disease. J Alzheimers Dis Parkinsonism 6:291. doi:10.4172/2161-0460.1000291
Copyright: © 2016 Henok KA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer’s disease (AD) is a late-onset progressive neurodegenerative disorder that leads to cognitive, memory and behavioural impairments. Substantial evidence indicates that disrupted neuronal calcium homeostasis is an early event in AD that could mediate synaptic dysfunction and neuronal toxicity. Sodium calcium exchangers (NCXs) play important roles in regulating intracellular calcium, and accumulated data suggests that reduced NCX function, following aberrant proteolytic cleavage of these exchangers, may contribute to neurodegeneration. This review, characterizes the expression and activity of NCX as a prominent feature of AD brain, identifies the molecular mechanisms underlying the effects of NCX isoforms, and pinpoints the molecular determinants responsible for the effects of NCX. Our findings suggest that calpain mediates cleavage of NCX3 in AD brain and therefore that reduced NCX3 activity contributes to the sustained increases in intraneuronal calcium concentrations that are associated with caspase-12 activation and neuronal death in AD.
Keywords
Alzheimers disease; Sodium; Calcium; Calpain; Caspase-12; Sodium calcium exchanger.
Background of the Study
Alzheimers disease
AD is a late-onset progressive neurodegenerative disorder that results in the irreversible loss of cholinergic cortical neurons, particularly in the associative neo-cortex and hippocampus. The principal risk factor for AD is age, with the main onset time observed in people aged over 60, in particular between the age of 70 and 80 [1,2]. With the increasing longevity of our population, AD is already approaching epidemic proportions with no cure or preventative therapy available [3,4].
Currently, it is estimated that one in 10 persons over 65 age and more than a third of all people over 80 have AD. According to United Nations population projections, it is estimated that 370 million people will be older than 80 years by 2050 [5]. The aging of the world’s population, therefore, will potentially pose an immense social and economic burden on future societies as this susceptible cohort continues to rapidly expand. Thus, a better understanding of the clinical and molecular events underlying AD will no doubt prove invaluable for combating this affliction.
Clinically, AD is characterized by the progressive impairment of higher cognitive function, loss of memory and altered behaviour that follows a gradual progression [6]. Pathological hallmarks of AD characterised at autopsy include: the presence of senile plaques composed of extracellular amyloid-beta (Aβ) protein aggregates, intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated forms of the microtubule associated tau (τ) protein deposits, the shrinkage of the cerebral cortex due to extensive neuronal loss and neuronal death [7,8]. These histopathological lesions do not occur diffusely throughout the brain, but are restricted to selective regions, particularly the hippocampus and neo-cortex. The pathogenetic mechanism that causes AD is unknown, but the discovery that mutations in presenilins (which cleave APP) and in APP itself (which is a substrate of the presenilin containing γ-secretase complex) in FAD patients gave rise to the most dominant, and commonly accepted, hypothesis to explain pathogenicity in both FAD and SAD, namely the “amyloid cascade hypothesis” [9].
Protein abnormalities in Alzheimer’s disease: Aβ and Tau protein: The pathological AD hallmarks are the presence of cerebral senile plaques composed of extracellular amyloid-beta (Aβ) protein aggregates, intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau (τ) protein deposits and the shrinkage of the cerebral cortex due to extensive neuronal loss. Although the precise cause of AD remains elusive, it has been suggested that neuronal loss in AD is attributed to the accumulation of toxic protein β–amyloid (Aβ) [10]. The β-site amyloid precursor protein–cleaving enzyme 1 (BACE1 or β-secretase), the principal actor in amyloid precursor protein (APP) processing in AD [11], is a stress-response protein involved in several neurologic diseases including stroke [12], amyloid angiopathy, inflammation, and oxidative damage.
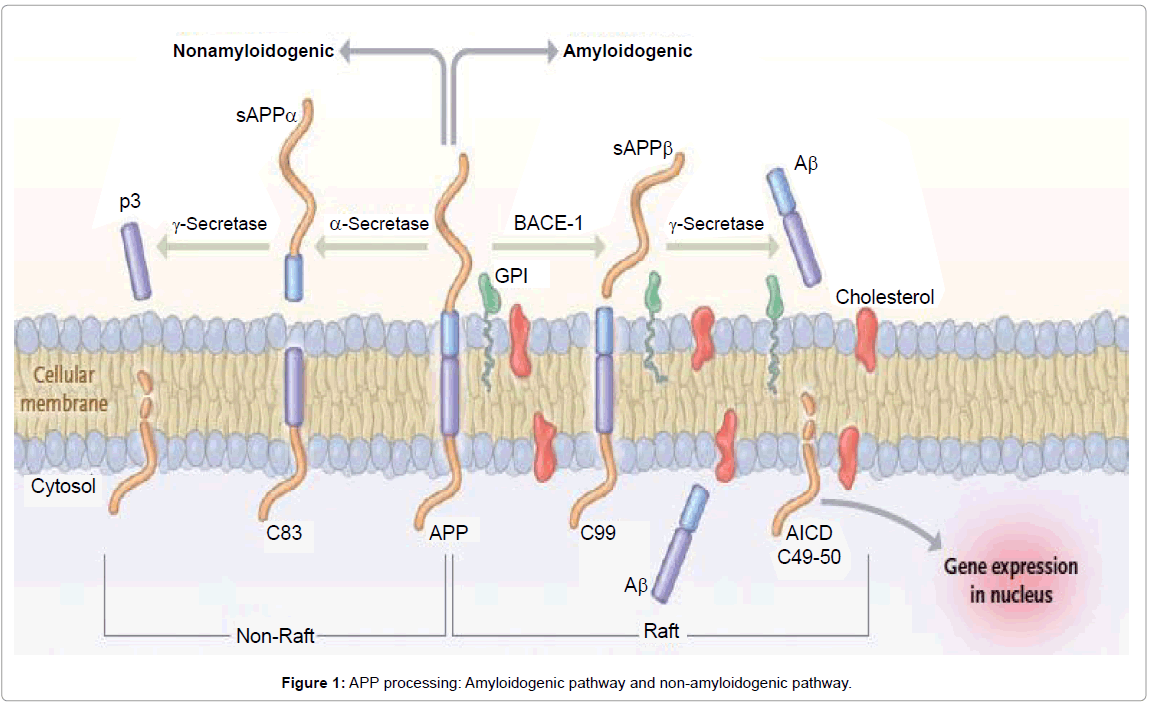
Aβ peptides are natural products of metabolism consisting of 39 to 43 amino acids, and it mainly exists in two isoforms: soluble Aβ1- 40 (∼80-90%) and insoluble Aβ1-42 (∼5-10%) [13,14]. Monomers of Aβ40 are much more prevalent than the aggregation-prone and damaging Aβ42 species. Aβ peptides originate from proteolysis of the APP by the sequential enzymatic actions of BACE-1 and γ-secretase, a protein complex with presenilin-1 at its catalytic core [3,15] (Figure 1). Its primacy has been manifested in the ‘amyloid cascade hypothesis’, which posits that the aggregation and accumulation of Aβ (resulting from overproduction, altered processing or imbalance between production and clearance) is the initiating molecular event that triggers neurodegeneration in sporadic and familial AD [10].
This process, called “amyloid cascade hypothesis”, remains the main pathogenetic model, as suggested by familial AD, mainly associated with mutation in APP and presenilin genes [9]. Aβ peptides spontaneously self-aggregate into multiple coexisting forms. One form consists of oligomers (2 to 6 peptides), which link into intermediate assemblies [16] (Figure 1). Aβ peptides can also grow into fibrils, which arrange themselves into β-pleated sheets to form the insoluble fibers of advanced amyloid plaques. Soluble oligomers and intermediate amyloids are the most neurotoxic forms of Aβ [17]. The severity of the cognitive defect in AD correlates with levels of oligomers in the brain, not the total Aβ [18].
As for NFTs, it has been found that their major constituent is the protein tau. Experimental evidence indicates that Aβ accumulation precedes and drives tau aggregation [19]. Tau is normally an abundant soluble protein in axons that promotes assembly and stability of microtubules and vesicle transport. Hyperphosphorylated tau is instead insoluble, lacks affinity for microtubules and self-associates into paired helical filament structures. Several factors might be involved in tau hyperphosphorylation, including Aβ-mediated caspases activation, Aβ- mediated oxidative stress, chronic oxidative stress, and reduced insulinlike growth factor 1-mediated oxidative stress [20]. Like Aβ oligomers, the aggregates of abnormal tau molecules (neurofibrillary tangles) are cytotoxic and impair cognition. These filamentous inclusions are sited in pyramidal neurons and their number is a pathologic marker of the severity of AD.
Increased oxidative stress, the impaired protein-folding function of the endoplasmic reticulum (ER) and deficient proteasome-mediated and autophagicmediated clearance of damaged proteins - all of which are also associated with aging - accelerate the accumulation of amyloid and tau proteins in AD [21].
APP processing and Aβ generation: Aβ is generated by the sequential cleavage of APP, a type I integral membrane protein anchored to the plasma membrane and internal membranes of the ER, Golgi and trans-Golgi apparatus [22,23]. Aβ is generated in very small quantities in normal healthy individuals and does not typically build up to very high levels [24]. However, in individuals afflicted with AD, differential processing of APP or the failure to degrade Aβ leads to its excessive accumulation.
Endoproteolysis of parental APP is achieved by the sequential cleavage of APP by groups of enzymes or enzyme complexes termed α-, β- and γ-secretases (Figure 1). For α-secretase there are currently three members of the ADAM family (a disintegrin- and metalloproteinasefamily enzyme): ADAM-9, ADAM-10 and ADAM-17 [25]. ADAM- 17 is also referred to as tumour necrosis factor α converting enzyme (or TACE) [26]. Several groups have identified the β-secretase (β-site APP cleaving enzyme or BACE) as a type I integral membrane protein belonging to the pepsin family of aspartyl proteases [27-29]. The identity of the γ-secretase has been identified as a complex of enzymes composed of presenilin-1 or -2, (PS1 and PS2), nicastrin, anterior pharynx defective (aph-1) and presenilin enhancer 2 (pen-2) [30].
The cleavage and processing of APP can be divided into a “nonamyloidogenic pathway” and an “amyloidogenic pathway” (Figure 1).
In the prevalent non-amyloidogenic pathway, a large portion of APP is cleaved by the α-secretase at a position 83 amino acids from the carboxyl (C) terminus, producing a large amino (N)-terminal ectodomain (sAPPα) which is secreted into the extracellular medium [3,15]. The resulting 83-amino-acid C-terminal fragment (C83) in the membrane is retained within cells and subsequently cleaved by the γ-secretase, producing a short fragment termed p3. Importantly, cleavage by the α-secretase occurs within the Aβ region, thereby precluding formation of Aβ [31].
The amyloidogenic pathway is an alternative cleavage pathway for APP which leads to Aβ generation, under physiological conditions. The initial proteolysis is mediated by the β-secretase at a position located 99 amino acids from the C-terminus. This cut results in the release of a slightly smaller form of APP (sAPPβ) into the extracellular space, and leaves the slightly larger 99-amino-acid C-terminal fragment (known as C99) within the membrane, with the newly generated N-terminus corresponding to the first amino acid of Aβ [5]. Subsequent cleavage of this fragment (between residues 38 and 43) by the γ-secretase liberates an intact Aβ peptide. Most of the full-length Aβ peptide produced is 40 residues in length (Aβ1-40), whereas a small proportion (approximately 10%) is the 42 residue variant (Aβ1-42). The Aβ1-42 variant is more hydrophobic and more prone to fibril formation than Aβ1-40; it is also the predominant isoform found in cerebral plaques [32]. Aβ can exist in a variety of forms, including monomers, oligomers, and fibrils [33].
Genetic mutations and Aβ production: Most cases of AD are not caused by a specific genetic defect but are sporadic in nature and are typically characterized by a later age of onset. However, there are a significant number of cases that are inherited in an autosomal dominant manner and generally these forms manifest at an earlier age of onset [34].
Mutations in three genes -APP, PS-1 and PS-2 - are known to cause autosomal dominant AD, which generally manifests with an earlyonset pathogenesis [34]. All these mutations affect the metabolism or stability of Aβ. Most of the mutations occur within the APP gene and cluster around the various secretase sites [5]. Surprisingly, APP mutations account for a small percentage of FAD cases. Mutations in the genes encoding PS-1 and PS-2, found on chromosomes 14 and 1, respectively, serve as the major loci for FAD [35,36]. Whilst over 135 individual mutations have been linked with PS-1, only 10 have thus far been linked with PS-2 [37]. PS-1 mutations cause an aggressive form of FAD with a particularly early age of onset, whilst PS-2 mutations result in a form of FAD that is more akin to sporadic AD, bearing a later age of onset [37].
A key feature of these identified mutations is that, all causes elevated production of Aβ1-42. Hence, these genetic mutations have been used to generate transgenic mouse models of the AD. One common mutation in APP is known as the Swedish mutation (APPSwe), in which a double amino acid change leads to increased cleavage of APP by the β-secretase [38]. Other mutations, such as the Arctic mutation (APPArc), increase the aggregation of Aβ, leading to early onset, aggressive forms of the disease [39]. Mutations in the presenilins, such as the PS1M146V mutation, increase levels of Aβ1-42 [40], which aggregates more readily than Aβ1-40. Increased dosage of the APP gene also results in AD [41].
Calcium
Calcium can be considered a ubiquitous intracellular messenger within cells acting as a regulator in multiple physiological functions. As a divalent cation, calcium can bind to several proteins, receptors and ion channels. All of these properties are of great importance within neurons, where continuous firing of action potentials leads to calcium cycling and it implies an influx through the calcium channels at the plasma membrane level, intracellular buffering and an efflux through the calcium plasma membrane transporters.
Loss of calcium (Ca2+) regulation is common to several neurodegenerative disorders and studies indicate that Ca2+ dyshomeostasis is one of the earliest molecular defects in the pathogenesis of AD [42]. In AD, elevated concentrations of cytosolic calcium ([Ca2+]i) stimulate Aβ aggregation and amyloidogenesis [43]. The presenilins modulate Ca2+ balance. Presenilin mutations might disrupt Ca2+ homeostasis in ER [44]. However, the main effect of the mutations is to increase Aβ42 levels, which in turn increases Ca2+ stores in the ER and the release of Ca2+ into the cytoplasm [6]. The relevance of these mechanisms to sporadic AD is delineated. A chronic state of excitatory amino acid (glutaminergic) receptor activation is thought to aggravate neuronal damage in late-stage AD [45]. Glutamate increases [Ca2+]i, which in turn stimulates calcium-release channels in the ER. Aβ forms voltage-independent, cation channels in lipid membranes [46], resulting in Ca2+ uptake and degeneration of neuritis [47]. Indirectly, glutamate activates voltage-gated calcium channels. The L-type voltage-gated calcium-channel blocker, MEM 1003, is in a phase 3 trial and an ionotropic glutamate receptor blocker, N-methyl- D-aspartate (NMDA) receptor blocker, is approved by the Food and Drug Administration.
Calcium regulation of Aβ production and linkage to AD: By screening genes located in known AD linkage regions, Marambaud et al. [48] discovered a novel calcium-conducting channel, with polymorphisms associated with increased risk for the development of Sporadic AD (SAD) [49].
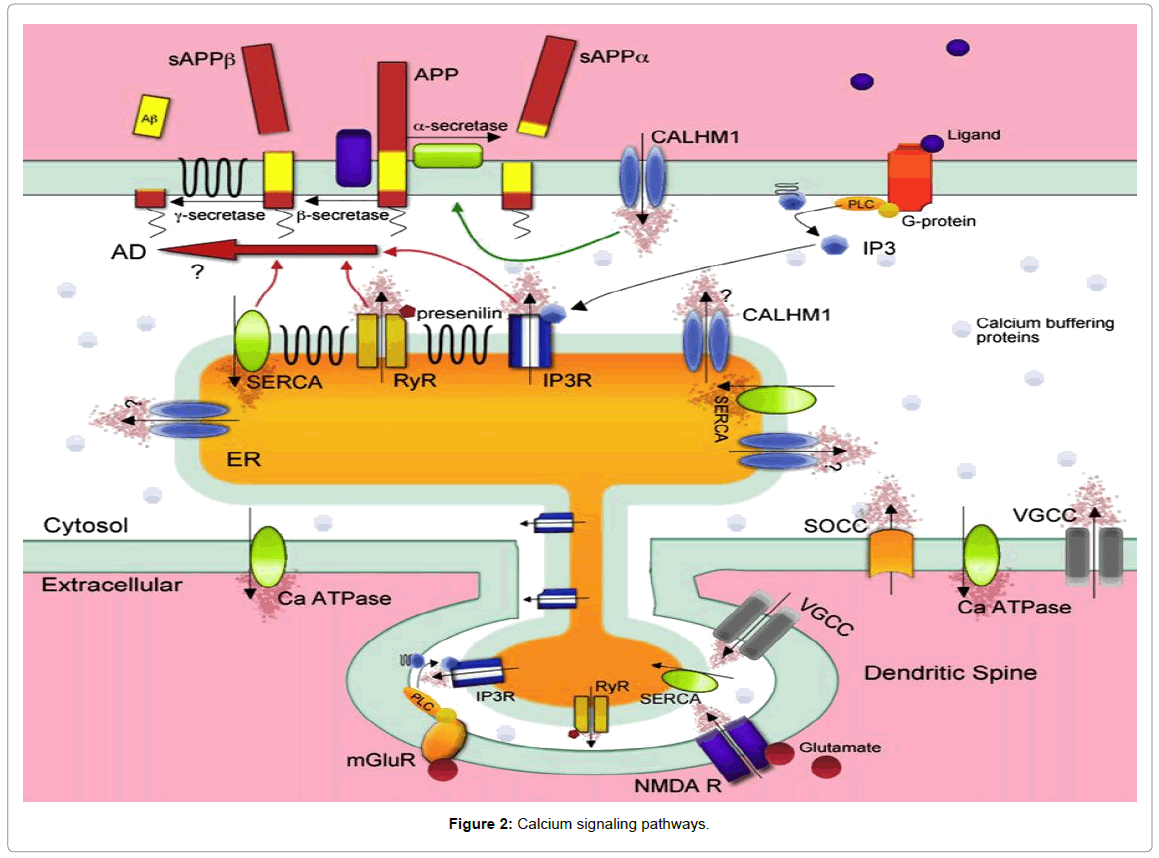
They called this novel calcium channel Calcium Homeostasis Modulator 1 (CALHM1). It is a three-transmembrane domain containing glycoprotein. Expression of CALHM1 was found in all brain regions and cells of neuronal lineage. CALHM1 localized predominantly to the ER but also exists at the plasma membrane where it mediates a novel Ca2+ influx to the cytosol, which is unaffected by specific blockers of store-operated Ca2+ influx or voltage-gated calcium channels but inhibited by nonspecific cation channel blockers such as cobalt (Figure 2). CALHM1 appears to exist as multimeric complexes, forming a functional ion channel, and has structural similarities with the NMDA receptor within the ion selectivity region.
Critically, Ca2+ influx through CALHM1 decreases Aβ production and is accompanied by increases in soluble amyloid precursor protein alpha (sAPPα) [50]. The mechanism underlying this effect has not been elucidated but presumably involves a calcium-dependent effect on α-secretase, which are enzymes that are known to cleave APP 83 amino acids from the carboxyl terminus and can thereby prevent Aβ formation. Conversely, increases in Aβ occur after siRNA knockdown of endogenous CALHM1 in cells when combined with calcium influx.
Curiously, this observation is contradictory to the vast majority of studies published on cytosolicCa2+ entry and Aβ production, which indicate that increasingCa2+ influx into the cytosol, either from the extracellular media or from ER stores, increases Aβ production [51]. An unexplored possibility could be that CALHM1 and the polymorphism P86L variant (that decrease Ca2+ permeability and also increases Aβ) exert their effects on Aβ processing via their location in the ER rather than the smaller pool found on the plasma membrane, given that the vast majority of CALHM1 was localized to the ER. It is unknown whether CALHM1 forms a functional cation-conducting pore within the ER, which could facilitate Ca2+ influx or efflux from the stores [52].
As the channel appears to be constitutively open (as membrane depolarization was not required for Ca2+ influx), it may exist as a potential leak channel at the ER, which would increase [Ca2+]i and would be diminished by the P86L variant.
This finding would then be in agreement with previous studies showing how ER Ca2+ regulation modulates Aβ production. Basal Ca2+ levels are tightly regulated by a number of calcium pumps and binding proteins, which sequester free cytosolic Ca2+ so that it cannot affect local enzymes and signaling cascades.
Calcium enters into the cytosol from the extracellular space through ionotropic receptor-operated (ligand-gated) channels (ROCs), voltage-operatedCa2+channels (VOCCs), and also through store-operated calcium-entry channels. ROCs permeable to Ca2+ include the N-methylo-D-aspartate receptors (NMDARs), some a-amino-3-hydroxy-5-methylisoxazole- 4-propionate acid receptors (AMPARs). Calcium can also enter into the cytosol from intracellular stores such as the ER via the inositol 1, 4, 5-trisphosphate receptor (IP3R) [53] and the ryanodine receptors (RyR), as well as from the mitochondria [54,55]. When [Ca2+]i increases are large, mitochondria become rapidly-sequestering Ca2+ buffers, ensuring protection against excess of Ca2+ [56,57]. Indeed, slower Ca2+ clearance is mediated by Ca2+ pumps and exchangers located at plasma membrane level. Ca2+ ions are pumped out against a concentration gradient of four orders of magnitude by a plasma membrane Ca2+ ATPase (PMCA).Ca2+ is also removed from the cytoplasm by Na+/Ca2+ exchanger (NCX) located in the cell membrane; NCX has low affinity but high capacity for Ca2+ compared with PMCA [58] for this it is perfectly suited to extruding large amounts of this ion.
Conversely, [Ca2+]i is reduced via the presence of calcium-binding proteins, such as calbindin, acting as buffers, and also through the extrusion of Ca2+ either into intracellular stores, such as the ER via the sarco-endoplasmic reticulum calcium ATPase (SERCA) [59] or out across the plasma membrane via plasmalemmal calcium pumps and exchangers (Figure 2).
Presenilins and calcium homeostasis: In addition to direct effects on Aβ formation, presenilin mutations have profound effects on cellular calcium homeostasis [60]. Familial Alzheimer’s Disease (FAD)-associated mutations in the presenilins were found to enhance IP3-mediated Ca2+ release from the ER stores [61]. The presenilins were identified in 1995 as multi-transmembrane proteins, which predominantly localized to the ER, and were postulated to form a novel ion channel. Their involvement in the AD pathogenesis was cemented with the discovery that the presenilins formed the catalytic core of the γ-secretase complex, which liberates Aβ from the membrane fragment C99 (Figure 1). These FAD mutations lead to the formation of the more predominantly 42 amino acid long version Aβ, which aggregates more readily.
The effects of FAD presenilin mutations on Ca2+ are very significant given that FAD presenilin mutations enhance Ca2+ release from the ER via the IP3 receptor [53], the ryanodine receptor via caffeine [54,55] and through endogenous calcium leak channels [62], it was thought that these results could all be explained by an increase in ER Ca2+ load. However, the same FAD-linked mutations have also shown a reduction in ER Ca2+ load and ER release with SERCA inhibition [59]. Thus, it is unclear whether all mutations increase ER Ca2+ or not.
Foskett et al. [63] performed direct IP3 channel recordings via single channel patch-clamp electrophysiology of the ER membrane, expressing presenilin or FAD-linked mutants. Overexpression of mutant presenilin 1 or 2 directly increased IP3 channel activity by prolonging the channel open time [64]. Presenilin mutants appear to modulate the IP3 receptors directly, as they were found to physically interact and are known to co-localize to the ER membrane [65]. Presenilin have been shown to interact with the ryanodine receptor, via its N terminus and to increase the open channel probability and mean current [66], similar to that described with the IP3 receptor.
Furthermore to these ‘‘gain-of-function’’ interactions with native ER calcium receptors, FAD-linked presenilin mutations have also been shown to have a ‘‘loss-of-function’’ effect on ER Ca2+ dynamics by reducing endogenous Ca2+ leak from the ER [65]. Overexpression of wild-type presenilins also accelerates the sequestration of cytosolic Ca2+, an effect that can be blocked by pharmacological inhibition of SERCA, suggesting that presenilins modulate SERCA function and that SERCA pumping is impaired in the absence of both presenilins. Taken together, presenilins appear to interact and modulate Ca2+ influx into the ER via SERCA and Ca2+ extrusion from the ER via interactions with the ryanodine and IP3 receptors. ER Ca2+ regulation results to be a critical determinant for the production of Aβ, in addition to plasma membrane influx pathways such as with CALHM1.
The sodium/calcium exchanger
The Na+/Ca2+ exchanger (NCX) is a plasma membrane transporter that plays a major role in the maintenance of Ca2+ and Na+ homeostasis in various excitable and non-excitable cells [67]. NCX belongs to the Ca2+ cation antiporter superfamily (CaCA) and catalyzes the exchange of Na+ and Ca2+ across the plasma membrane with a 3:1 stoichiometry [67]. Depending on the electrochemical gradient across the plasma membrane NCX can either extrude intracellular Ca2+ in its forward mode or take up extracellular Ca2+ in its reverse mode.
The regulation of Ca2+ and Na+ homeostasis is a crucial physiological phenomenon in excitable cells. In fact, Ca2+ ions play a key role as a second messenger in the cytosol and in the nucleus [68], while the Na+ ion regulates the cellular osmolarity, induces action potentials [69] and it is involved in the signal translation [30]. The control of this regulation is delegated to ionic channels selective for Ca2+ and Na+, to Na+ pumps, Ca2+ ATP-dependent and to NCX [70].
Molecular biology of NCX: NCX belongs to the superfamily of membrane proteins comprising the following members:
1) The NCX family, which exchanges three Na+ ions for one Ca2+ ion or four Na+ ions for one Ca2+ ion depending on [Na+]i and [Ca2+]i [67];
2) The Na+/Ca2+ exchanger K+-dependent family, which exchanges four Na+ ions for one Ca2+ plus one K+ ion [69];
3) The bacterial family which probably promotes Ca2+/H+ exchange [71];
4) The nonbacterial Ca2+/H+ exchange family, which is also the Ca2+ exchanger of yeast vacuoles;
5) The Mg2+/H+ exchanger, an electrogenic exchanger of protons with Mg2+ and Zn2+ ions [72].
These membrane proteins are all peculiarly characterized by the presence of α-repeats, the regions involved in ion translocation. In mammals, the NCX family consists of three separate isoforms: NCX1, NCX2 and NCX3 [73]. NCX1 is predominantly expressed in heart, kidney and brain [74], NCX2 is most abundantly expressed in brain [75] and NCX3 is expressed in excitable tissues such as brain and skeletal muscle [76]. These three genes appear to be dispersed, since NCX1, NCX2 and NCX3 have been mapped in mouse chromosomes 17, 7 and 12, respectively [76]. At the post-transcriptional level, at least 12 NCX1 and 3 NCX3 proteins are generated through alternative splicing of the primary nuclear transcripts. These variants arise from a region of the large intracellular f-loop, are encoded by six small exons defined A to F and are used in different combinations in a tissue-specific manner. To maintain an open reading frame, all splice variants must include either exon A or B, which are mutually exclusive [75].
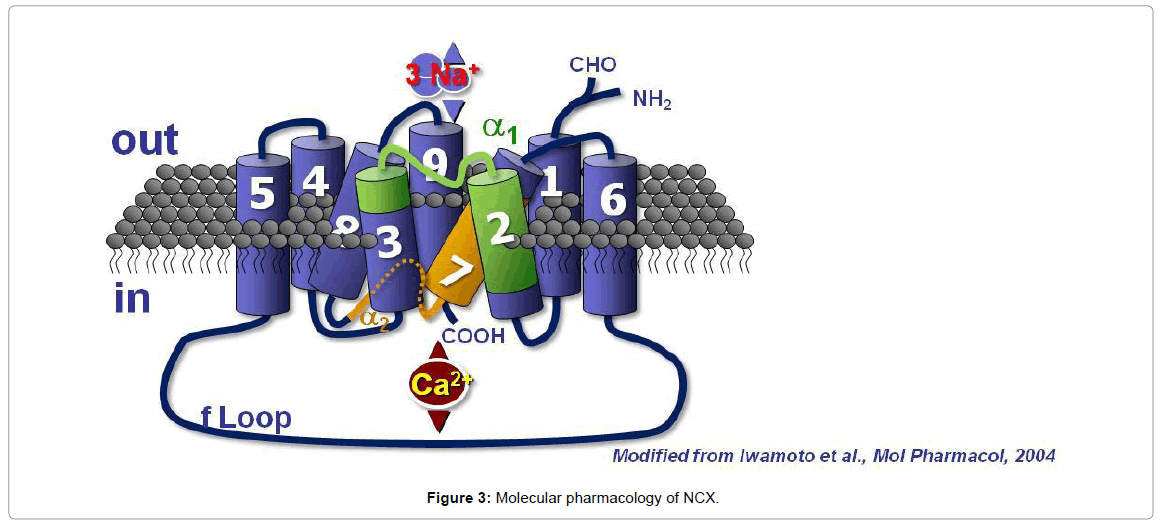
NCX1 is composed of 938 amino acids in the canine heart and has a molecular mass of 120 kDa and contains nine transmembrane segments (TMS). NCX1 amino terminus (N-terminal) is located in the extracellular space, whereas the carboxyl terminus (C-terminal) is located intracellularly (Figure 3). The nine transmembrane segments can be divided into an N-terminal hydrophobic domain, composed of the first five TMS (1–5) and into a C-terminal hydrophobic domain, composed of the last four TMS (6–9). These two hydrophobic domains are important for the binding and the transport of ions. The first (1– 5) TMS are separated from the last four (6–9) TMS through a large hydrophilic intracellular loop of 550 amino acids, named the f-loop [77]. Although the f-loop is not implicated in Na+ and Ca2+ translocation, it is responsible for the regulation of NCX activity elicited by several cytoplasmic messengers and transductional mechanisms, such as Ca2+ and Na+ ions, NO, phosphatidylinositol 4,5 bisphosphate (PIP2), protein kinase C (PKC), protein kinase A (PKA), phosphoarginine (PA) and ATP. In the center of the f-loop, a region of approximately 130 amino acids in length (371–508 amino acids) has been reported to exert a Ca2+ regulatory function. This region is characterized by a pair of three aspartyl residues and by a group of four cysteines [77]. At the N terminal end of the f-loop near the membrane lipid interface, an autoinhibitory domain, rich in both basic and hydrophobic residues and consisting of a 20-aminoacid sequence (219-238), named exchange inhibitory peptide (XIP) [78], has been identified. The f-loop is also characterized by alternative splicing sites named α1-repeat and α2- repeat. The NCX protein amino acid sequence found between TMS2 and TMS3 is called α-1 repeat, whereas the one found between TMS7 and TMS8 is named α-2 repeat [79]. With electrophoretic gels and under non reducing conditions, NCX1 migrates as a 120 and a 70 kDa band. The 120-kDa band represents the native protein, and the 70-kDa protein is a proteolytic fragment, which includes a large part of the f-loop and retains an NCX activity.
Interestingly, NCX2 and NCX3 have been found only in the brain and in the skeletal muscle. These two gene products consist of 921 and 927 amino acids and are characterized by molecular masses of 102 and 105 KDa, respectively. In addition, NCX2 displays a 65% sequence identity with NCX1, whereas NCX3 possesses a 73% sequence identity with NCX1 and 75% sequence identity with NCX2 [76]. All three NCX gene products share the same membrane topology.
NCX can facilitate both Ca2+ and Na+ flow in a bidirectional way through the plasma membrane [67] with a stoichiometry of 3 Na+ ions versus 1 Ca2+ ion. Depending on the intracellular levels of Na+ and Ca2+, NCX can operate in the forward mode by extruding one Ca2+ against three entering Na+, using the Na+ gradient across the plasma membrane as a source of energy [67]. Alternatively, in the reverse mode, NCX can function as Na+ efflux–Ca2+ influx. Because of its high exchange capacity, NCX is well-suited for rapid recovery from high intracellular Ca2+ concentrations ([Ca2+]i) and may play an important role in maintaining Ca2+ homeostasis and protecting cells from Ca2+ overload and eventual death [67].
NCX regulation: Several factors are involved in the regulation of NCX activity: the two transported ions, Na+ and Ca2+, the intracellular pH, metabolic related compounds, ATP, PA, PIP2, PKA and PKC, redox agents, hydroxyl radicals, H2O2, dithiothreitol (DTT), O2-, Fe3+, Fe2+, Cu2+, OH°, glutathione reduced (GSH) and glutathione oxidized (GSSG) and finally the gaseous mediator, NO.
Intracellular Ca2+ concentrations: The site level at which [Ca2+] i regulates NCX activity is different from the one required for Ca2+ transport. In fact, submicromolar concentrations (0.1–0.3 μM) of intracellular Ca2+ are needed to activate the antiporter [80]. Indeed, the removal of intracellular Ca2+ ions completely blocks NCX activity [81]. This regulatory function of low micromolar Ca2+ is more evident when the Na+/Ca2+ exchanger is working in the reverse mode. However, it is not completely clear how low μM Ca2+ can also regulate NCX when it operates in the forward mode [78]. The location of such a regulatory site has been identified in the 134 amino acid length region, situated in the center of the intracellular f-loop. This region is characterized by a pair of three aspartyl residues and by a group of four cysteines.
Intracellular Na+ concentrations: In addition to the submicromolar intracellular Ca2+ regulatory site, an increase in [Na+]i can also regulate the Na+/Ca2+ exchanger [82]. In particular, when intracellular Na+ increases, it binds to the transport site of the exchanger molecule, and after an initial fast outward Na+/Ca2+ current, an inactivation process occurs [80]. This inactivation process, very similar to the phenomenon occurring in voltage-dependent ionic channels, is named Na+-dependent inactivation. The region of the intracellular f loop, in which this regulatory site is located, has been identified in a 20-amino acid portion of the N-terminal part of the loop termed XIP [78]. Studies in vitro have characterized a negatively charged region of the intracellular f loop (445-455 amino acids) of the NCX protein that is able to cross link with synthetic XIP, suggesting that this amino acid sequence constitutes the binding site of XIP. On the other hand, since deletion mutagenesis of amino acids 562 to 685 results in an exchange activity that is no longer regulated by XIP, it is likely that XIP interacts with residues 445 to 455 and with another region of the f loop located between residues 562 and 685. Indeed, this region is believed to be a Na+ regulatory site. Regarding the mechanism by which XIP inhibits NCX activity, it has been proposed that when the XIP-binding site is ligand occupied, a conformational change is induced in the C-terminal portion of the f loop, resulting in the inhibition of the ion transport. XIP is provided with relevant pharmacological implications. In fact, those exogenous peptides, having the same amino acid sequence as XIP, act as potent inhibitors of NCX activity [83]. Interestingly, Ca2+ ions, at low micromolar concentrations, binding its regulatory site, decrease the extent of this Na+-dependent inactivation. In fact, mutations in the Ca2+ regulatory binding site alter the activation and inactivation kinetics of exchange currents by modulating Na+-dependent inactivation.
Intracellular H+ concentrations: H+ strongly inhibits NCX activity under steady-state conditions. Changes in intracellular pH values, as little as 0.4 can induce a 90% inhibition of NCX activity. Since this H+ ion modulatory action is α1-chymotrypsin sensitive, the action site of the proton can be attributed to the antiporter’s hydrophilic intracellular loop [84]. Intriguingly, such inhibitory action depends on the presence of intracellular Na+ ions [85]. Hence, the action exerted by H+ ions is pathophysiologically relevant with regards to brain and heart ischemia. In fact, when intracellular H+ and Na+ ion homeostasis are deregulated, the anoxic conditions resulting in these cells may selectively interfere with the activity of the different NCX gene products. In particular, increases of H+ and Na+, as in anoxic conditions, synergically inhibit NCX activity [86].
ATP, PKA, PKC and PIP2: Acting as a phosphoryl donor molecule, ATP may increase the activity of the exchanger in a number of ways. Firstly, ATP directly participates in the NCX molecule phosphorylation process by protein kinase A (PKA) and protein kinase C (PKC). Secondly, it increases phosphatidylinositol-4, 5-phosphate (PIP2) production. Finally, by activating G-protein-coupled receptors, via endogenous and exogenous ligands, ATP can stimulate the activity of the Na+/Ca2+ exchanger through the pathway involving PKC or PKA activation [84]. The mechanism underlying the phosphorylating effect on the exchanger seems to be related to an increase in its affinity for both internal Ca2+ and external Na+ and to a decrease in its inhibition by internal Na+.
Each of the NCX isoforms has distinctive putative phosphorylation sites, although their roles have not yet been elucidated [87]. ATP cellular depletion inhibits NCX1 and NCX2 but does not affect NCX3 activity. The exchange activity of NCX1 and NCX3 is modestly increased by those agents that activate PKA and PKC [87]. More recently, the mechanism by which PKA and PKC activate NCX has been clarified. In fact, it has been demonstrated that the regulation of PKA-induced phosphorylation is due to the existence of an NCX1 macromolecular complex that contains the kinase PKA holoenzyme. This holoenzyme consists of two PKA catalytic subunits and two identical PKA regulatory subunits [88]. Together with PKA, other critical regulatory enzymes are also associated with NCX1, including PKC and serine-threonine protein phosphatases, PP1 and PP2A [88]. Particularly a pathway involving PKC has been shown to stimulate NCX1 [89].
In a more recent paper, it has been demonstrated that PKCdependent regulation of NCX isoforms also involves NCX3 but not NCX2 [90]. In the same paper, three phosphorylation sites in the NCX1 protein, Ser-249, Ser-250 and Ser-357, have been identified. Among these, Ser-250 is the amino acid that is predominantly phosphorylated [90]. The other mechanism by which ATP can activate NCX occurs through PIP2 production. This mechanism of activation is related to the relevant PIP2 influence on Na+-dependent inactivation of NCX. In fact, PIP2 directly interacts with the XIP region of the exchanger, thus eliminating its inactivation and stimulating NCX function. Indeed, exchangers with mutated XIP regions no longer respond to PIP2 or to PIP2 antibodies [80].
Redox agents: In the last 15 years, several groups of investigators using different cellular models, such as cell-expressing cloned splicing variants of the brain, heart isoforms, cardiac sarcolemma vesicles, cells transiently transfected with NCX1 isoform and giant excised patches, have found that the Na+/Ca2+ exchanger is sensitive to different combinations of redox agents [91]. In particular, the stimulation of the exchange activity requires the combination of a reducing agent (DTT, GSH or Fe2+) with an oxidizing agent (H2O2 and GSSG). The effects of both agents are mediated by metal ions (e.g. Fe2+). The antiporter’s sensitivity to changes in the redox status can assume particular relevance during oxidative stress. In fact, in this condition, the modulation of reactive oxygen species (ROS) could affect the transport of Na+ and Ca2+ ions through the plasma membrane.
Nitric oxide: The ubiquitous gaseous mediator Nitric Oxide (NO) seems to be involved in the modulation of NCX activity. In fact, Asano and his colleagues provided evidence that NO, released by NO donors, is able to stimulate NCX in the reverse mode of operation in neuronal preparations and astrocytes through a cGMP-dependent mechanism [92]. In contrast, in C6 glioma cells, the stimulatory action on NCX reverse mode of operation, elicited by the NO donor sodium nitroprusside (SNP) is not elicited by NO release but by the presence of iron in SNP molecule [91]. In addition, a direct relationship between the constitutive form of nitric oxide synthase (NOS), the enzyme involved in NO synthesis, and NCX has recently been demonstrated. Indeed, heat stress by inducing NOS phosphorylation causes NOS complication with NCX, thus decreasing its activity. Secondo and colleagues [92]), demonstrated the selectivity of NO in modulating each isoform at different molecular determinant level.
NCX role in physiological conditions: The NCX protein may play a relevant function in different neurophysiological conditions. In neurons, the level of expression of NCX is particularly high in those sites where a large movement of Ca2+ ions occurs across the plasma membrane, as it happens at the level of synapses [84]. Specifically, during an action potential or after glutamate-activated channel activity, Ca2+ massively enters the plasma membrane. Such phenomenon triggers the fusion of synaptic vesicles with the plasma membrane and promotes neurotransmitter exocytosis. After this event, outward K+ currents repolarize the plasma membrane, thus leading to VOCC closure. According to the diffusion principle, Ca2+ ions are distributed in the cytosolic compartment, reversibly interacting with Ca2+- binding proteins. Residual Ca2+ is then rapidly extruded by the plasma membrane Ca2+ ATPase and by NCX.
The NCX becomes the dominant Ca2+ extrusion mechanism when [Ca2+]i is higher than 500 nM, as it happens when a train of action potentials reaches the nerve terminals. It has been calculated that for these [Ca2+]i values (500 nM), more than 60% of Ca2+ extrusion is mediated by NCX families. In such physiological conditions, NCX activation is consistent with its low-affinity (Kd=500 nM) and highcapacity (5 × 103 Ca2+/s) function. In contrast, in resting conditions or after a single action potential, when [Ca2+]i slightly increases, requiring, therefore, a more subtle control, the high affinity (Kd=100 nM) and low-capacity (102 Ca2+/s) pump, plasma membrane Ca2+-ATPase, assumes a predominant function, thus making the involvement of NCX less relevant [70].
NCX genes knocking-out effect: NCX1-, NCX2- and NCX3- specific knockout mice were generated over the past decade [93-95]. These mouse models are useful tools for elucidating NCX1–3 specific function in physiological and pathophysiological processes in the central nervous system (CNS). NCX1-deficient mice are not viable. NCX1 null-mutation caused embryonic lethality, irregular heartbeats and apoptosis in the heart [95]. Recent studies indicated that cardiacspecific transgenic re-expression of NCX1 was not enough to rescue the lethal phenotype, suggesting an important non-cardiac role for NCX1 during embryogenesis (e.g. vascularization of yolk sac, placental development) [68]. Mice lacking NCX2 exhibit enhanced learning and memory [93]. Targeted disruption of NCX3 leads to defective neuromuscular transmission [94]. Under ischemic conditions, NCX3- deficient mice exhibit increased neuronal damage [96,97]. Studies also showed that NCX plays a major role in restoring baseline Ca2+ levels following glutamate-induced depolarization in cortical and hippocampal neurons [93,98]. These findings highlight NCX function in the regulation of Na+ and Ca2+ following synaptic activity.
NCX role in pathophysiological conditions: The disregulation of [Ca2+]i and [Na+]i homeostasis is involved in neuronal and glial injury occurring in in vitro and in vivo models of hypoxia-anoxia and in several neurodegenerative diseases. In a cellular model of glial cells, C6 glioma, the activation of NCX, in reverse mode, obtained by [Na+]e removal, reduces cell injury induced by chemical hypoxia. Such phenomenon suggests that the antiporter plays a protective role during this pathophysiological condition. Consistent with these results, the pharmacological inhibition of NCX activity worsens cell damage by increasing the intracellular concentration of Na+ ions [91]. Furthermore, the stimulation of NCX activity by redox agents results in a protective effect against hypoxia as well as the overexpression of NCX3 [99]. Published evidence demonstrated that Ca2+ influx due to NCX activity in reverse mode is the main component of the excitotoxicity damage [100].
More papers highlighted the different roles played by the different NCX isoforms in the cell survival modulation in cellular death models. For example, NCX3 is neuroprotective during an ischemia insult in vitro both in neuronal models and in cells transfected with only this isoform [101]. This role is attributable to the NCX3 ability to buffer the cytosolic Ca2+ by the forward mode of operation, like during glutamate increase or chemical ipoxia insult [102]. The molecular mechanisms by which NCX is involved in the pathophysiological conditions involve the cleavage of NCX1 and NCX3 by calpain in brain ischemia and in cultured cerebellar granule neurons exposed to glutamate [103]. Calpains modulate a variety of physiological processes [104] but can also become important mediators of cell death [105]. Ample evidence documents the activation of calpains in brain ischemia and excitotoxic neuronal degeneration [106], leading to speculation for the alteration of the NCX function.
In in vivo models, reproducing human cerebral ischemia through the occlusion of the middle cerebral artery, the inhibition of NCX, induced by selective inhibitors [107] or by the knockout of one of the NCX isoforms (NCX2) [93] or NCX3 [97] aggravates brain infarct, whereas the activation of the antiporter with redox agents reduces the cerebral infarctual area [83].
The role played by NCX in those neurons and glial cells involved in cerebral ischemia should be differentiated according to the anatomical regions involved in the ischemic pathological process. In particular, it is conceivable that, since in the penumbral region ATPase activity is still preserved, NCX may likely operate in a forward mode. As a result, by extruding Ca2+ ions, the exchanger favors the entry of Na+ ions. Therefore, the inhibition of NCX in this area reduces the extrusion of Ca2+ ions, thus enhancing Ca2+-mediated cell injury. In contrast, in the ischemic core region, in which ATP levels are remarkably low and Na+/K+ ATPase activity is reduced, intracellular Na+ ions massively accumulate because of Na+/K+ ATPase failure [108]. Hence, the intracellular Na+ loading promotes NCX to operate in the reverse mode as a Na+ efflux-Ca2+ influx pathway. In conclusion, the NCX pharmacological inhibition in this core region further worsens the necrotic lesion of the surviving glial and neuronal cells as the loading of intracellular Na+ increases [83].
The “Ca2+ hypothesis” provides an attractive mechanism to explain the cell death associated with AD. The theory proposes that cell death results from elevated [Ca2+]i. The acute or chronic in [Ca2+]i rise may exist leading the cell to an irreversible pathway of necrosis and/or apoptosis. If true, derangements in several Ca2+ homeostatic processes could simultaneously contribute to and be responsible for a persistent rise in [Ca2+]i. In particular, multiple evidence points to deregulated endoplasmic reticulum (ER) Ca2+ homeostasis in the aging brain and AD [51]. A large bulk of studies have shown that the neurotoxicity exerted by Aβ protein is intimately related to [Ca2+]i. Indeed, the attenuation of [Ca2+]i increase by Ca2+ channel blockers, growth factors, and cytochalasins results in a reduction of neural damage induced by the Aβ peptide. It has been demonstrated that exposure to the Aβ protein partially reduces Na+-dependent Ca2+ accumulation in plasma membrane vesicles deriving from the human frontal cortex of patients affected by AD. These findings have suggested that Aβ directly interacts with the hydrophobic surface of the NCX molecule, thus interfering with plasma membrane Ca2+ transport [30].
Many evidences in literature are in support of the “Ca2+ hypothesis of AD”. Where does the NCX become involved in this mechanism? NCX would be expected to be neuroprotective in situations where elevations in [Ca2+]i are leading to cell death. This neuroprotective role was proposed to explain increased NCX activity in AD brain [109]. In this model, neurons that survived the neurodegenerative elevations in [Ca2+]i caused by AD were having increased capacity for NCX. This increased capacity for NCX in surviving neurons was manifested as increased Na+-dependent Ca2+ uptake in plasma membrane vesicles derived from AD brain [110].
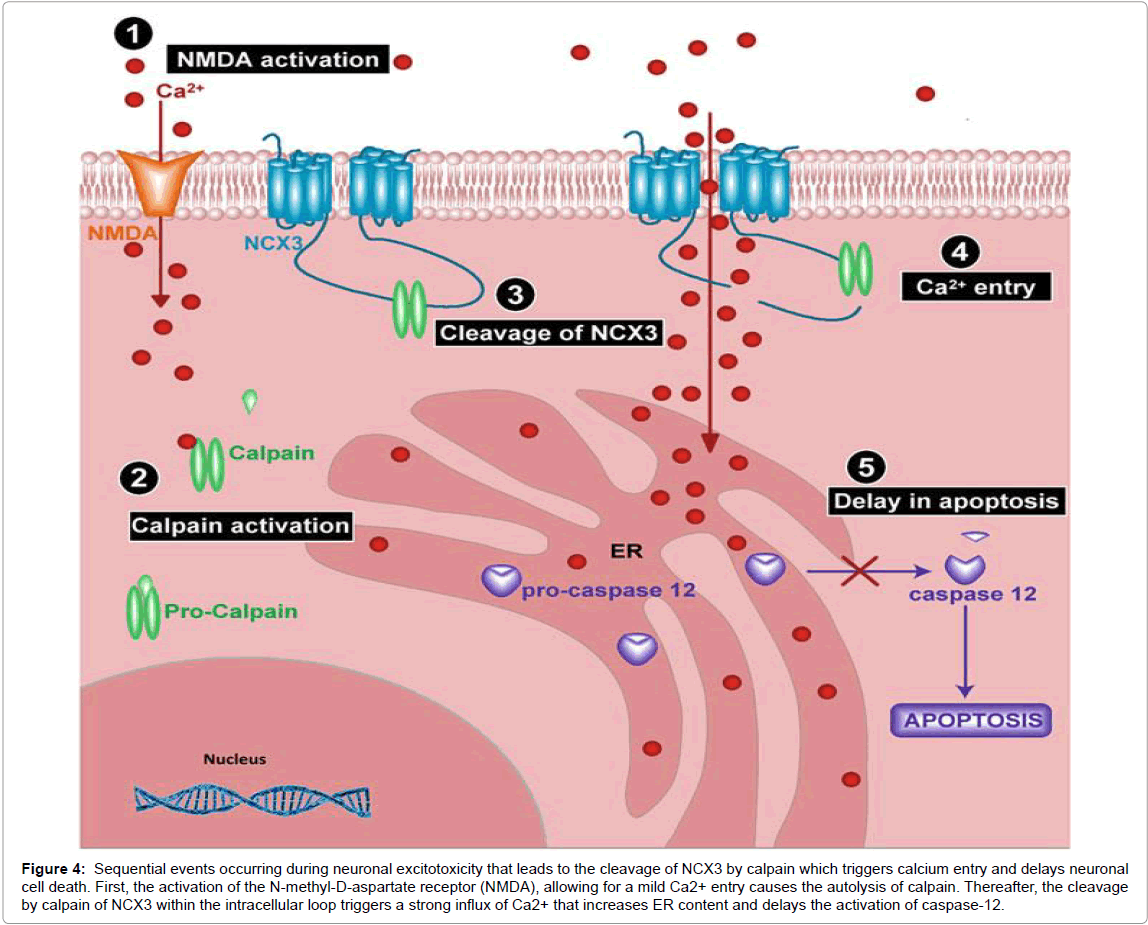
Pathophysiological implications of NCX3: Recently, the Annunziato’s group concluded from their functional studies that the cleaved NCX3 is most likely hyperfunctional in its reverse mode, triggering a significant Ca2+ uptake [100], increasing the Ca2+ content of the ER, and therefore delaying caspase-12 activation and neuronal cell death (Figure 3). Moreover, cleavage of NCX3 is triggered by Aβ1–42 peptide exposure, a peptide well known to accumulate in the brain of AD patients. Furthermore, level of cleaved NCX3 correlates perfectly with the level of Aβ1–42 and calpain activity in AD patients postmortem, a further correlation between the loss of NCX3 with neuronal cell death [111].
Thus, NCX3 is well accepted as having a major role in the Ca2+ dysregulation deriving from excitotoxicity and most likely acting in a neuroprotective manner. Nevertheless, many unresolved issues remain as to its activity after cleavage by calpain, the mechanisms by which NCX3 is implied in both ER and mitochondria refilling and the cleavage pattern of the variants of NCX3. Further studies are, therefore, needed to address these questions and conclude whether this constitutes an aberrant proteolytic cleavage or rather a new degree of posttranslational regulation allowing for a tight regulation of the exchanger that correlates with the stress state of the cell. Finally, NCX3 seems to be implicated not only in the excitotoxic conditions but also in the hyperexcitability state underlying the apparition of seizures, as an inhibition of NCX3 significantly reduces frequency and severity of seizures [112,113] (Figure 4).
Conclusion and Perspectives
The knowledge gained mainly over the past 10 years of research on NCX has implicated NCX3 in pathophysiological conditions. In this respect, some of these implications require further investigation, including the interactions of NCX3 with other proteins and cellular organelles during stress conditions such as excitotoxicity.
Therefore, research on several types of nerve fibers is required to conclude on the exact role of NCX3 in the brain physiology. Such investigation, together with the use of conditional knockout models would allow for a better understanding of their role in a delay of Caspase-12 activation and neuronal death. By pinpointing this example of the Aβ1-42-NCX3 cross talk, researchers have initiated a quest to unravel the different pathologies were NCX3 cleavage is very likely to be involved in neurodegenerative disorders. In this matter, the use of the recently developed technique of CRISPR/Cas might be of great help to generate a mice model with specific mutation of the NCX3 cleavage sites in either a wild-type mouse or a mice already subjected to excitotoxicity as it is the case in the Alzheimer’s disease mice models [114].
Figure 4:Sequential events occurring during neuronal excitotoxicity that leads to the cleavage of NCX3 by calpain which triggers calcium entry and delays neuronal cell death. First, the activation of the N-methyl-D-aspartate receptor (NMDA), allowing for a mild Ca2+ entry causes the autolysis of calpain. Thereafter, the cleavage by calpain of NCX3 within the intracellular loop triggers a strong influx of Ca2+ that increases ER content and delays the activation of caspase-12.
References
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, et al. (2011) Alzheimer's disease. Lancet 377: 1019-1031.
- Reitz C, Mayeux R (2014) Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88: 640-651.
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1: a006189.
- Hu S, Maiti P, Ma Q, Zuo X, Jones MR, et al. (2015) Clinical development of curcumin in neurodegenerative disease. Expert Rev Neurother 15: 629-637.
- Suh YH, Checler F (2002) Amyloid precursor protein, presenilins and alpha-synuclein: Molecular pathogenesis and pharmacological applications in Alzheimer's disease. Pharmacol Rev 54: 469-525.
- LaFerla FM (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci 3: 862-872.
- Schellenberg GD, Montine TJ (2012) The genetics and neuropathology of Alzheimer's disease. Acta Neuropathol 124: 305-323.
- Querfurth HW, LaFerla FM (2010) Alzheimer's disease. N Engl J Med 362: 329-344.
- Tanzi RE, Bertram L (2005) Twenty years of the Alzheimer's disease amyloid hypothesis: A genetic perspective. Cell 120: 545-555.
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297: 353-356.
- Hayley M, Perspicace S, Schulthess T, Seelig J (2009) Calcium enhances the proteolytic activity of BACE1: An in vitro biophysical and biochemical characterization of the BACE1-calcium interaction. Biochim Biophys Acta 1788: 1933-1938.
- Wen PH, Hof PR, Chen X, Gluck K, Austin G, et al. (2004) The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol 188: 224-237.
- Gandy S, Caporaso G, Buxbaum J, Frangione B, Greengard P (1994) APP processing, A beta-amyloidogenesis and the pathogenesis of Alzheimer's disease. Neurobiol Aging 15: 253-256.
- Zhang YW, Xu H (2007) Molecular and cellular mechanisms for Alzheimer's disease: Understanding APP metabolism. Curr Mol Med 7: 687-696.
- Xu X (2009) Gamma-secretase catalyzes sequential cleavages of the AbetaPP transmembrane domain. J Alzheimers Dis 16: 211-224.
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, et al. (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300: 486-489.
- Walsh DM, Selkoe DJ (2007) A beta oligomers - a decade of discovery. J Neurochem 101: 1172-1184.
- Krafft GA, Klein WL (2010) ADDLs and the signaling web that leads to Alzheimer's disease. Neuropharmacology 59: 230-242.
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, et al. (2003) Triple-transgenic model of Alzheimer's disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 39: 409-421.
- Reddy PH (2011) Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria and synaptic deprivation in Alzheimer's disease. Brain Res 1415: 136-148.
- Hoozemans JJ, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, et al. (2005) The unfolded protein response is activated in Alzheimer's disease. Acta Neuropathol 110: 165-172.
- Small SA, Gandy S (2006) Sorting through the cell biology of Alzheimer's disease: Intracellular pathways to pathogenesis. Neuron 52: 15-31.
- Thinakaran G, Koo EH (2008) Amyloid precursor protein trafficking, processing and function. J Biol Chem 283: 29615-29619.
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, et al. (1992) Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258: 126-129.
- Allinson TM, Parkin ET, Turner AJ, Hooper NM (2003) ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res 74: 342-352.
- Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, et al. (1998) Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem 273: 27765-27767.
- Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, et al. (1999) Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci 14: 419-427.
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, et al. (1999) Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402: 537-540.
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, et al. (1999) Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286: 735-741.
- Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, et al. (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature 407: 48-54.
- De Strooper B, Vassar R, Golde T (2010) The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol 6: 99-107.
- Jarrett JT, Berger EP, Lansbury Jr PT (1993)The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer's disease. Biochem 32: 4693-4697.
- Glabe CC (2005) Amyloid accumulation and pathogensis of Alzheimer's disease: Significance of monomeric, oligomeric and fibrillar Abeta. Subcell Biochem 38: 167-177.
- St George-Hyslop PH1, Petit A (2005) Molecular biology and genetics of Alzheimer's disease. C R Biol 328: 119-130.
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, et al. (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375: 754-760.
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, et al. (1995) Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science 269: 973-977.
- Selkoe DJ, Podlisny MB (2002) Deciphering the genetic basis of Alzheimer's disease. Annu Rev Genomics Hum Genet 3: 67-99.
- Haass C, Lemere CA, Capell A, Citron M, Seubert P, et al. (1995) The Swedish mutation causes early-onset Alzheimer's disease by beta-secretase cleavage within the secretory pathway. Nat Med 1: 1291-1296.
- Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, et al. (2001) The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation. Nat Neurosci 4: 887-893.
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, et al. (2004) Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: Evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13: 159-170.
- Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, et al. (2002) Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid 9: 88-102.
- Chakroborty S, Stutzmann GE (2011) Early calcium dysregulation in Alzheimer's disease: Setting the stage for synaptic dysfunction. Sci China Life Sci 54: 752-762.
- Isaacs AM, Senn DB, Yuan M, Shine JP, Yankner BA (2006) Acceleration of amyloid beta-peptide aggregation by physiological concentrations of calcium. J Biol Chem 281: 27916-27923.
- Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, et al. (2007) Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest 117: 1230-1239.
- Ribeiro FM, Paquet M, Cregan SP, Ferguson SS (2010) Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol Disord Drug Targets 9: 574-595.
- Kagan BL, Hirakura Y, Azimov R, Azimova R, Lin MC (2002) The channel hypothesis of Alzheimer's disease: Current status. Peptides 23: 1311-1315.
- Lin H, Bhatia R, Lal R (2001) Amyloid beta protein forms ion channels: Implications for Alzheimer's disease pathophysiology. FASEB J 15: 2433-2444.
- Lambert JC, Campagne F, Marambaud P (2008) CALHM1, a novel gene to blame in Alzheimer disease. Med Sci (Paris) 24: 923-924.
- Bandara S, Malmersjo S, Meyer T (2013) Regulators of calcium homeostasis identified by inference of kinetic model parameters from live single cells perturbed by siRNA. Sci Signal 6: ra56.
- Dreses-Werringloer U, Lambert JC, Vingtdeux V, Zhao H, Vais H, et al. (2008) A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels and Alzheimer's disease risk. Cell 133: 1149-1161.
- Bezprozvanny I, Hiesinger PR (2013) The synaptic maintenance problem: Membrane recycling, Ca2+ homeostasis and late onset degeneration. Mol Neurodegener 8: 23.
- Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, et al. (2008) SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J Gen Physiol 132: i1.
- Demuro A, Parker I (2013) Cytotoxicity of intracellular abeta42 amyloid oligomers involves Ca2+ release from the endoplasmic reticulum by stimulated production of inositol trisphosphate. J Neurosci 33: 3824-3833.
- Ferreiro E, Oliveira CR, Pereira C (2004) Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-beta peptide. J Neurosci Res 76: 872-880.
- Ferreiro E, Resende R, Costa R, Oliveira CR, Pereira CM (2006) An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-beta peptides neurotoxicity. Neurobiol Dis 23: 669-678.
- Collins TJ, Lipp P, Berridge MJ, Bootman MD (2001) Mitochondrial Ca2+ uptake depends on the spatial and temporal profile of cytosolic Ca2+ signals. J Biol Chem 276: 26411-26420.
- Giacomello M, Drago I, Pizzo P, Pozzan T (2007) Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ 14: 1267-1274.
- Scheff NN, Yilmaz E, Gold MS (2014) The properties, distribution and function of Na+-Ca2+ exchanger isoforms in rat cutaneous sensory neurons. J Physiol 592: 4969-4993.
- Zatti G, Burgo A, Giacomello M, Barbiero L, Ghidoni R, et al. (2006) Presenilin mutations linked to familial Alzheimer's disease reduce endoplasmic reticulum and golgi apparatus calcium levels. Cell Calcium 39: 539-550.
- Guo Q, Furukawa K, Sopher BL, Pham DG, Xie J, et al. (1996) Alzheimer's PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid beta-peptide. Neuroreport 8: 379-383.
- Fernandez MA, Klutkowski JA, Freret T, Wolfe MS (2014) Alzheimer presenilin-1 mutations dramatically reduce trimming of long amyloid beta-peptides (Abeta) by gamma-secretase to increase 42-to-40-residue Abeta. J Biol Chem 289: 31043-31052.
- Nelson O, Supnet C, Tolia A, Horré K, De Strooper B, et al. (2011) Mutagenesis mapping of the presenilin 1 calcium leak conductance pore. J Biol Chem 286: 22339-22347.
- Foskett A, Williams C, Boobis L, Tsintzas K (2008) Carbohydrate availability and muscle energy metabolism during intermittent running. Med Sci Sports Exerc 40: 96-103.
- Cheung KH, Shineman D, Müller M, Cárdenas C, Mei L, et al. (2008) Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58: 871-883.
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, et al. (2006) Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell 126: 981-993.
- Rybalchenko V, Hwang SY, Rybalchenko N, Koulen P (2008) The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int J Biochem Cell Biol 40: 84-97.
- Liao J, Li H, Zeng W, Sauer DB, Belmares R, et al. (2012) Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335: 686-690.
- Cho CH, Lee SY, Shin HS, Philipson KD, Lee CO (2003) Partial rescue of the Na+-Ca2+ exchanger (NCX1) knock-out mouse by transgenic expression of NCX1. Exp Mol Med 35: 125-135.
- Lytton J, Li XF, Dong H, Kraev A (2002) K+-dependent Na+/Ca2+ exchangers in the brain. Ann N Y Acad Sci 976: 382-393.
- Blaustein MP, Lederer WJ (1999) Sodium/calcium exchange: Its physiological implications. Physiol Rev 79: 763-854.
- Cunningham KW, Fink GR (1996) Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol 16: 2226-2237.
- Shaul O, Hilgemann DW, de-Almeida-Engler J, Van Montagu M, Inz D, et al. (1999) Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J 18: 3973-3980.
- On C, Marshall CR, Chen N, Moyes CD, Tibbits GF (2008) Gene structure evolution of the Na+-Ca2+ exchanger (NCX) family. BMC Evol Biol 8: 127.
- Kofuji P, Hadley RW, Kieval RS, Lederer WJ, Schulze DH (1992) Expression of the Na-Ca exchanger in diverse tissues: A study using the cloned human cardiac Na-Ca exchanger. Am J Physiol 263: C1241-C1249.
- Quednau BD, Nicoll DA, Philipson KD (1997) Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2 and NCX3 in rat. Am J Physiol 272: C1250-C1261.
- Nicoll DA, Quednau BD, Qui Z, Xia YR, Lusis AJ, et al. (1996) Cloning of a third mammalian Na+-Ca2+ exchanger, NCX3. J Biol Chem 271: 24914-24921.
- Nicoll DA, Ottolia M, Lu L, Lu Y, Philipson KD (1999) A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J Biol Chem 274: 910-917.
- Matsuoka S, Nicoll DA, He Z, Philipson KD (1997) Regulation of cardiac Na+-Ca2+ exchanger by the endogenous XIP region. J Gen Physiol 109: 273-286.
- Nicoll DA, Sawaya MR, Kwon S, Cascio D, Philipson KD, et al. (2006) The crystal structure of the primary Ca2+ sensor of the Na+/Ca2+ exchanger reveals a novel Ca2+ binding motif. J Biol Chem 281: 21577-21581.
- Hilgemann DW, Collins A, Matsuoka (1992) Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol100: 933-961.
- Philipson KD, Nicoll DA (2000) Sodium-calcium exchange: A molecular perspective. Annu Rev Physiol 62: 111-133.
- Michel LY, Verkaart S, Koopman WJ, Willems PH, Hoenderop JG, et al. (2014) Function and regulation of the Na+-Ca2+ exchanger NCX3 splice variants in brain and skeletal muscle. J Biol Chem 289: 11293-11303.
- Pignataro G, Gala R, Cuomo O, Tortiglione A, Giaccio L, et al. (2004) Two sodium/calcium exchanger gene products, NCX1 and NCX3, play a major role in the development of permanent focal cerebral ischemia. Stroke 35: 2566-2570.
- Annunziato L, Pignataro G, Di Renzo GF (2004) Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev 56: 633-654.
- Doering AE, Lederer WJ (1994) The action of Na+ as a cofactor in the inhibition by cytoplasmic protons of the cardiac Na+-Ca2+ exchanger in the guinea-pig. J Physiol 480: 9-20.
- DiPolo R, Beauge L (2002) MgATP counteracts intracellular proton inhibition of the sodium-calcium exchanger in dialysed squid axons. J Physiol 539: 791-803.
- Linck B, Qiu Z, He Z, Tong Q, Hilgemann DW, et al. (1998) Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3). Am J Physiol 274: C415-423.
- Schulze DH, Muqhal M, Lederer WJ, Ruknudin AM (2003) Sodium/calcium exchanger (NCX1) macromolecular complex. J Biol Chem 278: 28849-28855.
- Iwamoto T, Pan Y, Wakabayashi S, Imagawa T, Yamanaka HI, et al. (1996) Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C. J Biol Chem 271: 13609-13615.
- Giladi M, Tal I, Khananshvili D (2016) Structural features of ion transport and allosteric regulation in sodium-calcium exchanger (NCX) proteins. Front Physiol 7: 30.
- Amoroso S, Tortiglione A, Secondo A, Catalano A, Montagnani S, et al. (2000) Sodium nitroprusside prevents chemical hypoxia-induced cell death through iron ions stimulating the activity of the Na+-Ca2+ exchanger in C6 glioma cells. J Neurochem 74: 1505-1513.
- Secondo A, Molinaro P, Pannaccione A, Esposito A, Cantile M, et al. (2011) Nitric oxide stimulates NCX1 and NCX2 but inhibits NCX3 isoform by three distinct molecular determinants. Mol Pharmacol, 79: 558-568.
- Jeon D, Yang YM, Jeong MJ, Philipson KD, Rhim H, et al. (2003) Enhanced learning and memory in mice lacking Na+/Ca2+ exchanger 2. Neuron 38: 965-976.
- Sokolow S, Manto M, Gailly P, Molgó J, Vandebrouck C, et al. (2004) Impaired neuromuscular transmission and skeletal muscle fiber necrosis in mice lacking Na/Ca exchanger 3. J Clin Invest 113: 265-273.
- Wakimoto K, Fujimura H, Iwamoto T, Oka T, Kobayashi K, et al. (2003) Na+/Ca2+ exchanger-deficient mice have disorganized myofibrils and swollen mitochondria in cardiomyocytes. Comp Biochem Physiol B Biochem Mol Biol 135: 9-15.
- Meade AJ, Meloni BP, Cross J, Bakker AJ, Fear MW, et al. (2010) AP-1 inhibitory peptides are neuroprotective following acute glutamate excitotoxicity in primary cortical neuronal cultures. J Neurochem 112: 258-270.
- Molinaro P, Cuomo O, Pignataro G, Boscia F, Sirabella R, et al. (2008) Targeted disruption of Na+/Ca2+ exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J Neurosci 28: 1179-1184.
- Ranciat-McComb NS, Bland KS, Huschenbett J, Ramonda L, Bechtel M, et al. (2002) Antisense oligonucleotide suppression of Na+/Ca2+ exchanger activity in primary neurons from rat brain. Neurosci Lett 294: 13-16.
- Mohammadi E, Bigdeli MR (2013) Effects of preconditioning with normobaric hyperoxia on Na+/Ca2+ exchanger in the rat brain. Neuroscience 237: 277-284.
- Pannaccione A, Secondo A, Molinaro P, D'Avanzo C, Cantile M, et al. (2012) A new concept: Aβ1-42 generates a hyperfunctional proteolytic NCX3 fragment that delays caspase-12 activation and neuronal death. J Neurosci 32: 10609-10617.
- Pignataro G, Boscia F, Esposito E, Sirabella R, Cuomo O, et al. (2012) NCX1 and NCX3: Two new effectors of delayed preconditioning in brain ischemia. Neurobiol Dis 45: 616-623.
- Cross JL, Boulos S, Shepherd KL, Craig AJ, Lee S, et al. (2012) High level over-expression of different NCX isoforms in HEK293 cell lines and primary neuronal cultures is protective following oxygen glucose deprivation. Neurosci Res 73: 191-198.
- Cross JL, Boulos S, Shepherd KL, Craig AJ, Lee S, et al. (2012) High level over-expression of different NCX isoforms in HEK293 cell lines and primary neuronal cultures is protective following oxygen glucose deprivation. Neurosci Res 73: 191-198.
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, et al. (2005) Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120: 275-285.
- Vosler PS, Brennan CS, Chen J (2008) Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol 38: 78-100.
- Neumar RW, Xu YA, Gada H, Guttmann RP, Siman R (2003) Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J Biol Chem 278: 14162-14167.
- Nixon RA (2003) The calpains in aging and aging-related diseases. Ageing Res Rev 2: 407-418.
- Bano D, Munarriz E, Chen HL, Ziviani E, Lippi G, et al. (2007) The plasma membrane Na+/Ca2+ exchanger is cleaved by distinct protease families in neuronal cell death. Ann N Y Acad Sci 1099: 451-455.
- Colvin RA, Bennett JW, Colvin SL, Allen RA, Martinez J, et al. (1991) Na+/Ca2+ exchange activity is increased in Alzheimer's disease brain tissues. Brain Res 543: 139-147.
- Boscia F, Gala R, Pignataro G, de Bartolomeis A, Cicale M, et al. (2006) Permanent focal brain ischemia induces isoform-dependent changes in the pattern of Na+/Ca2+ exchanger gene expression in the ischemic core, periinfarct area and intact brain regions. J Cereb Blood Flow Metab 26: 502-517.
- Colvin RA, Davis N, Wu A, Murphy CA, Levengood J (1994) Studies of the mechanism underlying increased Na+/Ca2+ exchange activity in Alzheimer's disease brain. Brain Res 665: 192-200.
- Atherton J, Kurbatskaya K, Bondulich M, Croft CL, Garwood CJ, et al. (2014) Calpain cleavage and inactivation of the sodium calcium exchanger-3 occur downstream of Abeta in Alzheimer's disease. Aging Cell 13: 49-59.
- Martinez Y, Gouemo PN (2010) Blockade of the sodium calcium exchanger exhibits anticonvulsant activity in a pilocarpine model of acute seizures in rats. Brain Res 1366: 211-216.
- N'Gouemo P (2013) Probing the role of the sodium/calcium exchanger in pentylenetetrazole-induced generalized seizures in rats. Brain Res Bull 90: 52-57.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 22370
- [From(publication date):
December-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 21392
- PDF downloads : 978