Review Article Open Access
Role of the Transcription Factor Nrf2 in Parkinson's Disease: New Insights
Isabel Lastres-Becker*Autonomous University of Madrid, Madrid, Spain
- Corresponding Author:
- Isabel Lastres-Becker
Department of Biochemistry
Faculty of Medicine
Autonomous University of Madrid
Madrid, Spain
Tel: 34915854382
E-mail: ilbecker@iib.uam
Received date: May 11, 2017; Accepted date: June 16, 2017; Published date: June 23, 2017
Citation: Lastres-Becker I (2017) Role of the Transcription Factor Nrf2 in Parkinson’s Disease: New Insights. J Alzheimers Dis Parkinsonism 7:340. doi:10.4172/2161-0460.1000340
Copyright: © 2017 Lastres-Becker I. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Parkinson’s disease (PD) is a slow progressive neurodegenerative disorder associated with motor and nonmotor symptoms, with no neuroprotective therapies. This chronic disease is characterized by loss of dopaminergic neurons from the sustantia nigra pars compacta and the presence of cytoplasmic α-synuclein-positive inclusions called Lewy bodies in the surviving neurons. Although PD has unknown etiology, familiar forms of PD have bring new insights in the causes of the pathology. At the molecular level, PD is characterized by proteinopathy (aggregation of α-synuclein, proteasome dysfunction and autophagy alterations), oxidative stress (increased production of reactive oxidative species, iron accumulation and dopamine oxidation) and neuroinflammation (microgliosis, reactive astrogliosis and increased levels of pro-inflammatory cytokines). A link has been revealed between the transcription factor NRF2 and PD at genetic level, showing that a functional haplotype in the human NFE2L2 gene promoter of NRF2 with slightly increased transcriptional activity, is associated with decreased risk and with delayed age at onset of PD. Moreover, since NRF2 is able to modulate the three main hallmarks of PD, several pharmacological approaches have been used to determine the role of NRF2 targeting in the development of PD. In this review we are going to evaluate the possible role of NRF2 as a pharmacological target to modify the development of PD and how some compounds that have been promising in PD mice models could be transferred to the study in clinical trials.
Keywords
Parkinson’s disease; NRF2; Inflammation; Oxidative stress; Neurodegeneration; Proteinopathy
Abbreviations
ALP: Autophagy-Lysosomal Pathway; DMF: Dimethyl Fumarate; KEAP1: Kelch-like ECH-Associated Protein 1; LPS: Lipopolysaccharide; METC: Mitochondrial Electron Transporter; MPTP: 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine; PD: Parkinson’s Disease; ROS: Reactive Oxygen Species; SFN: Sulforaphane; SNpc: Substantia Nigra pars compacta
Introduction
Parkinson’s disease (PD) is a chronic and progressive disorder of the central nervous system that mainly affects the motor system. PD is the second most common neurodegenerative disease after Alzheimer’s disease. The incidence rate is around 1-4% of the population above the age of 60 and 80 years, although there is a lower incidence in younger people. The major clinical hallmarks are manifested by resting tremor, rigidity, postural instability and akinesia, symptoms that are often accompanied by cognitive impairment. At the molecular level, the main characteristics of PD are the loss of dopaminergic neurons in the substantia nigra pars compacta, the formation of Lewy bodies, oxidative stress and chronic low-grade inflammation. Nowadays, the main standard clinical treatment for PD patients is based on dopamine replacement with levodopa, which manages to ameliorate only motor symptoms and does not delay the neurodegenerative process. Therefore, it is essential to find out new therapies that allow us to improve not only motor symptoms, but non-motor symptoms like cognitive impairment and the dysfunction of the autonomic nervous system, and modulate disease progression. During the last decade, the transcription factor NRF2 has emerged as a suitable target to modulate PD related molecular hallmarks. NRF2 was first described as a master regulator of oxidative stress, but new evidences showed that NRF2 is also implicated in the modulation of inflammatory processes through its crosstalk with the transcription factor NF-κB, the principal regulator of inflammation. Additionally, it has been described that NRF2 is essential in proteostasis, modulating the proteasome and autophagy processes. Thus, pharmacological targeting of NRF2 could be an effective treatment for PD patients.
Parkinson’s Disease Molecular Hallmarks
Parkinson’s disease (PD) is one of the most common neurologic disorders, with evolving layers of complexity, characterised by a large number of motor and non-motor features that can impact on function to a variable degree [1,2]. Nowadays the only pharmacologic treatment of PD is based on symptomatic therapy mainly focused on levodopa treatment, which is not a disease modifying/neuroprotective therapy. Therefore, it is very important to find key targets to focus the treatment for neuroprotection or disease-modifying therapies.
Two of the major neuropathological hallmarks are the loss of dopaminergic neurons from the Substantia Nigra pars compacta (SNpc) and the presence of α-synuclein-containing Lewy bodies (LB) in the surviving neurons [3] (Figures 1 and 2). Most PD cases are sporadic and of unknown etiology, although during the last years the identification of gene mutations responsible for familial forms of PD and the mapping of risk variants for the disease [4] have improved our understanding of the disease. One of the first mutated gene that was found to be associated with familial PD was α-synuclein [5,6] and additional missense mutations and duplications have been found to be rare causes of hereditary PD or PD-like syndromes [7-10]. Moreover, genome-wide association studies have demonstrated an association between non-coding variants in and around the α-synuclein gene and sporadic disease [11]. The relevance of α-synuclein has raised due to its implication in Gaucher disease, a lysosomal storage disease. Decreased turnover of α-synuclein correlates with increased PD susceptibility in people that carry even a single mutation in the glucocerebrosidase gene, responsible for Gaucher disease [12,13]. Furthermore, α-synuclein has been implicated in other important steps of PD like the enteric nervous system dysfunction [14,15] and the cell-to cell transmission [16].
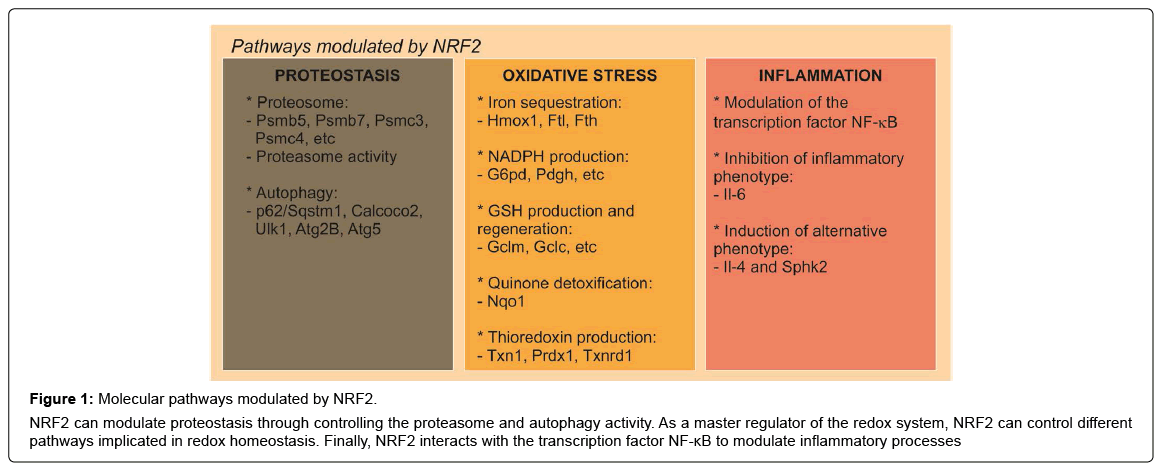
Figure 1: Molecular pathways modulated by NRF2.
NRF2 can modulate proteostasis through controlling the proteasome and autophagy activity. As a master regulator of the redox system, NRF2 can control different pathways implicated in redox homeostasis. Finally, NRF2 interacts with the transcription factor NF-κB to modulate inflammatory processes
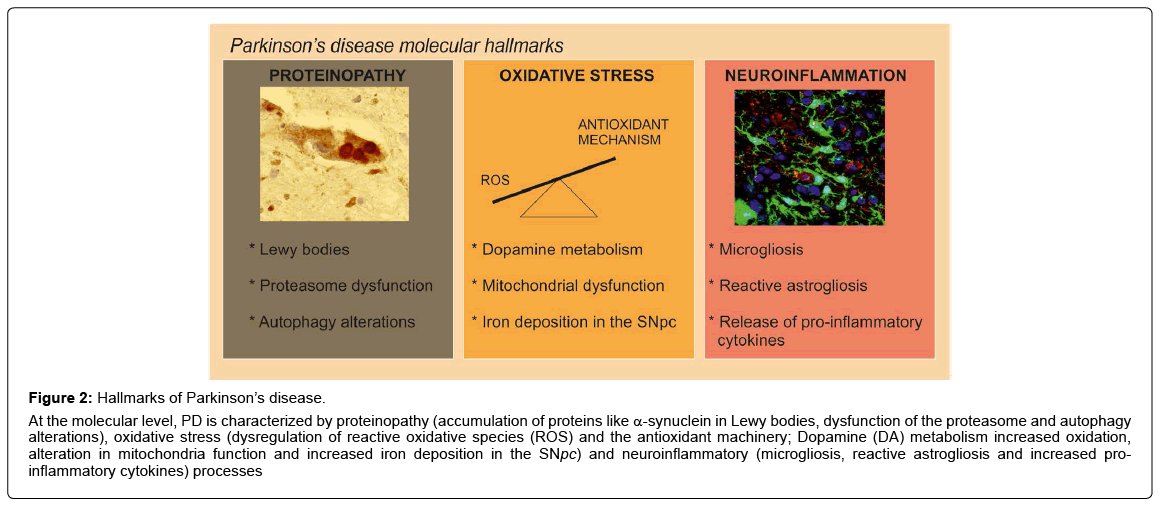
Figure 2: Hallmarks of Parkinson’s disease.
At the molecular level, PD is characterized by proteinopathy (accumulation of proteins like α-synuclein in Lewy bodies, dysfunction of the proteasome and autophagy alterations), oxidative stress (dysregulation of reactive oxidative species (ROS) and the antioxidant machinery; Dopamine (DA) metabolism increased oxidation, alteration in mitochondria function and increased iron deposition in the SNpc) and neuroinflammatory (microgliosis, reactive astrogliosis and increased proinflammatory cytokines) processes
Related to α-synuclein toxicity, in the SNpc of patients with the sporadic disorder it has been suggested that impaired protein clearance is a crucial factor in the pathogenesis of cell death in PD [17]. Disruption of the ubiquitin–proteasome system (UPS), which normally identifies and degrades intracellular proteins, is thought to promote the toxic accumulation of proteins detrimental to neuronal survival, thereby contributing to their demise [18]. These findings were supported by the discovery that mutations in the genes encoding α-synuclein and two enzymes of the ubiquitin-proteasome system, parkin and ubiquitin C-terminal hydrolase L1, are associated with neurodegeneration in some familial forms of PD [19]. Additionally, 20S proteasomal enzymatic activities were impaired in the SNpc in sporadic PD [19]. Importantly, α-synuclein is not degraded only by the proteasome but also by autophagy. A role for autophagy was further supported by the presence of α-synuclein in organelles with the ultrastructural features of autophagic vesicles [20,21]. It has been also described that α-synuclein inclusions are preferred targets for p62-dependent autophagy [22,23] and that α-synuclein overexpression impairs macroautophagy in mammalian cells and in transgenic mice via Rab1a inhibition [24]. In α-synuclein transgenic mice alterations of the UPS have been reported, indicating a role of the UPS in α-synuclein degradation and with increased α-synuclein burden the autophagy-lysosomal pathway (ALP) is recruited. These results provided evidence that the UPS and ALP might be functionally connected such that impairment of one can upregulate the other [25] (Figure 2). These data indicate that the components of the cellular quality control system represent an important focus for the development of targeted and potent therapies for managing PD.
At the molecular level, in both idiopathic and genetic cases of PD, the disease is associated with excess production of reactive oxygen species (ROS), alterations in catecholamine metabolism, modifications in mitochondrial electron transporter chain (METC) function or increase of iron deposition in the SNpc [26] (Figure 2), which leads to oxidative stress implicated in cellular dysfunction and demise. The failure of normal cellular processes that occur in relation to the aging process are also believed to contribute to the increased vulnerability of dopaminergic neurons [27]. There are many evidences indicating that oxidative stress is a key player in PD [28,29]. For example, the SN of PD patients exhibit increased levels of oxidized lipids [30], proteins and DNA [31] and decreased levels of reduced glutathione (GSH) [32]. Related to the vulnerability of the dopaminergic neurons from the SNpc to oxidative stress, one of the causes could be the presence of ROS-generating enzymes such as tyrosine hydroxylase and monoamine oxidase in these neurons. Besides, the nigral dopaminergic neurons contain iron, which catalyzes the Fenton reaction, in which superoxide radicals and hydrogen peroxide can contribute to further oxidative stress [28,33]. Therefore, the major sources of oxidative stress generated for the nigral dopaminergic neurons are produced during dopamine metabolism, mitochondrial dysfunction, and neuroinflammation (Figure 2).
Neuroinflammation is another feature of PD pathology. The presence of an active inflammatory response in the brain mediated primarily by resident astrocytes and microglia has been long recognised. A link between inflammation and PD was first described in a postmortem study by McGeer et al. in 1988, where activated microglia was found in the SN of PD patients [34]. Furthermore, several clinical studies have confirmed this association by reporting increased microglial activation and elevated pro-inflammatory cytokines in post-mortem brains and CSF [35-37] (Figure 2). These data have been also reproduced by experimental studies in animal models of the disease [23,38,39], where neuroinflammation has been shown to be an important contributor to PD progression. Moreover, preclinical PD models suggest that inflammation is a driving force in DA neuron loss [37]. This idea is supported by experiments where chronic intraperitoneal injection of bacterial lipopolysaccharide (LPS) elicits a systemic immune response and leads to DA neuron loss and PD pathology in mice [40,41]. This inflammatory model of PD suggest that inflammatory stress can manifest in DA neuron loss likely through infiltration of peripheral leukocytes. Related to the 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP) PD model, it has been described that this neurotoxin facilitate dopamine neuronal loss at least in part by induction of inflammatory response [42]. Also, in mice, enhanced inflammation has been shown to recapitulate α-synuclein aggregation and oxidation in affected neurons [43]. Altogether, targeting this inflammation with a number of antiÔ?Éinflammatory therapies can be an effective way to halt the progression of chronic neuroinflammationinduced PD.
The Transcription Factor NRF2
Nuclear factor (erythroid-derived 2)-like-2 factor (NRF2) was first described as the master regulator of redox homeostasis that allows cells to adapt to oxidative stress and also promotes cell proliferation, but currently it is known to regulate the expression of about 1% of human genes, which contain in their promoter regulatory regions an enhancer sequence termed Antioxidant Response Element [44]. These genes encode a large variety of cytoprotective proteins implicated in biotransformation, antioxidant reactions, and inflammation [45]. NRF2 belongs to the cap'n'collar (CNC) b-Zip family and is a unstable protein and under homeostatic conditions it is maintained at low basal expression level [46]. The stability of NRF2 is regulated mainly by two different mechanisms. The first mechanism implicates the KEAP1 (Kelch-like ECH-associated protein 1)-dependent ubiquitination and degradation. KEAP1 is an ubiquitin E3 ligase substrate adapter for a Cullin3/Rbx1-dependent E3 ubiquitin ligase complex; henceforth binding of KEAP1 to NRF2 mediates ubiquitination and subsequent proteasomal degradation of NRF2 [47]. Interestingly, KEAP1 contains several cysteine residues capable of undergoing redox modifications and adduct formation with electrophilic compounds. Consequently, NRF2 levels can be modulated pharmacologically to phenocopy this protective NRF2 haplotype.
The second mechanism is related to glycogen synthase kinase-3 (GSK-3), which phosphorylates NRF2 creating a recognition site for β-Transducin Repeat Containing E3 Ubiquitin Protein Ligase (β-TrCP). β-TrCP leads to Cullin-1/Rbx1-mediated NRF2 ubiquitination and its subsequent degradation [48]. It has been described that phosphoinositide 3-kinase (PI3K)-protein kinase B (PKB)/AKT signaling results inhibitory phosphorylation of GSK3, preventing the formation of a DSGIS motif-containing phosphodegron in NRF2 that is recognized by the β-TrCP [48,49].
In response to endogenous and exogenous stresses caused by ROS and electrophiles, NRF2 translocates from the cytoplasm into the nucleus and binds together with small Maf proteins to the Antioxidant Response Element in the regulatory regions of target genes and transactivates expression of genes with antioxidant activity [50]. Small Maf (MafG, MafK and MafF) proteins are b-Zip proteins that lack a transcriptional activation domain. It is known that they form homodimers and heterodimers with other b-Zip proteins including NRF2 [51]. Under basal condition, Maf proteins bind to BACH1 [52], but after induction, BACH1 is replaced by NRF2, resulting in activation and suggesting competition between BACH1 and NRF2 for the same DNA binding site [53].
All together, these different mechanisms of NRF2 regulation indicate that this transcription factor could be a suitable pharmacological target to modulate NRF2-dependent functions.
NRF2 Modulation of Proteostasis, Oxidative Stress and Inflammation
Although NRF2 was first described as the master regulator of redox homeostasis, this transcription factor has been revealed as an essential key in the modulation of proteostasis as well inflammation.
Related to the proteasome, cells that constitutively express NRF2 exhibit elevated levels of proteasome activities [54] while NRF2- deficient cells have impaired proteasome activity and also less expression of proteasome proteins [39] (Figure 1). Related to oxidative stress, it has been observed a NRF2-dependent induction of proteasome required for adaptation to the stress [55]. These data were corroborated by the fact that an NRF2 inducer, sulforaphane (SFN), increases the expression of NRF2-regulated genes as well as the expression of the catalytic subunits of the proteasome and proteasomal peptidase activities [56,57]. The ubiquitin-proteasome system and autophagy are crucial for maintaining the proteostasis and are interdependent pathways. In mice with reduced proteasome activity in their livers, proteasome dysfunction activated autophagy and KEAP1/NRF2 pathway [58]. Recently, NRF2 has been identified as a regulator of autophagy gene expression [59] indicating its potential in regulating cellular proteostasis (Figure 1).
NRF2 controls the basal and induced expression of an array of antioxidant response element-dependent genes to regulate the physiological and pathophysiological outcomes of oxidant exposure [60] (Figure 1). These function can be differentiated in several pathways, depending on the function [61]. First, enzymes regulating iron sequestration, such as heme oxygenase (HMOX1), ferritin heavy chain (FTH) and ferritin light chain (FTL). The second is NADPH production, which is controlled for example, by glucose-6- phosphate dehydrogenase (G6PD), phosphoglycerate dehydrogenase (PHGDH), among others. The third is glutathione (GSH) production and regeneration, which is regulated mainly by glutamate–cysteine ligase complex modifier subunit (GCLM), the GCL catalytic subunit (GCLC). The fourth type are antioxidants that are implicated in quinone detoxification like NAD(P)H quinone oxidoreductase 1 (NQO1). And finally, is thioredoxin (TXN) production, regeneration and utilization, which is regulated by Txn1, thioredoxin reductase 1 (TXNRD1) and peroxiredoxin 1 (PRDX1) [61,62]. These four groups of antioxidant genes have both complementary and overlapping functions. These interactions give the global idea of the huge complexity of the antioxidant system regulated by NRF2 and its implication of the regulation of oxidative stress-related diseases like PD.
The role of NRF2 in inflammation is supported by the fact that NRF2-deficient mice exhibited exacerbated inflammatory process under different stimuli [39,63-67]. In the absence of NRF2, NF-κB lacks a controller to switch-off the inflammatory signal [68] (Figure 1). Consistently, in animals treated with SFN, an NRF2 inducer, the production of inflammatory markers in response to LPS was attenuated [63]. These results are sustained by the presence of a NF-κB binding site in the NRF2 coding gene [69] and by the fact that IκK-β (IκBα kinase) contains an ETGE motif that enables it to bind to KEAP1 [70], the repressor protein of NRF2. In addition, NRF2-Deficiency results in increased ROS levels, which induce IκBα phosphorylation and subsequent degradation, increasing p65-NF-κB levels and NF-κB proinflammatory processes. Moreover, NRF2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. NRF2 binds to the proximity of Il-6 and Il-1 β and inhibits RNA Pol II recruitment, although this inhibition is independent of the NRF2-binding motif [71]. On the other hand, NRF2 activation increased the expression levels of anti-inflammatory markers like Il-4 or sphingosine kinase 2 (Sphk2) in a microglial cell line [23]. Overall, NRF2 is highly involved in the regulation of inflammation and therefore would be very promising to use NRF2 as a target for pharmacological treatment of neuroinflammation associated with PD.
Parkinson’s Disease and its Connection with NRF2
Although there is not a direct link between PD and NRF2, there is circumstantial evidence that connects loss of NRF2 with the disease. So, NRF2 activity declines with ageing, which is the main risk factor for PD. In the dopaminergic neurons from the SNpc, NRF2 is located in the cytosol, whereas in age-matched PD patients, it is found in the nucleus [72] and the NRF2 signature, represented by expression of NQO1 [73] and HO-1 [39,74-76] is up-regulated, suggesting an attempt of brain protection through this pathway [77]. In postmortem samples of PD patients, the cytoprotective proteins associated with NRF2 expression, NQO1 and p62, were partly sequestered in Lewy bodies, suggesting impaired neuroprotective capacity of the NRF2 signature [23]. However, the most compelling evidence comes from the genetic associations showing that a functional haplotype in the human NFE2L2 gene promoter (here termed Nrf2 for the mouse gene), which confers slightly increased transcriptional activity, is associated with decreased risk and with delayed age at onset of the disease [78,79] in an European case-control groups. Further studies have been performed investigating whether genetic variations in and around NFE2L2 modify susceptibility to PD using a large case-control sample recruited via the Queensland Parkinson’s Project. They have identified a number of SNPs associated with a significantly later age at onset as well as common NFE2L2 variants that may reduce PD susceptibility in certain conditions, such as regular exposure to pesticides [80].
Related to familiar PD mutations, leucine-rich repeat kinase 2 (LRRK2) gene mutations are the most common genetic cause of PD, and therefore could be used as a useful tool to find biomarkers. It has been observed a strong positive correlation between NRF2 concentrations with Unified Parkinson’s Disease Rating Scale (UPDRS) in cerebrospinal fluid (CSF) from LRRK2-PD-patients. Partial correlation coefficient calculations indicated that disease duration contributed to the associations of NRF2 levels with UPDRS scores in this group [81]. Another studies with induced pluripotent stem cells (iPSCs) from PARK2 (parkin gene) patients showed increased oxidative stress and enhanced activity of NRF2 pathway, which correlated with abnormal mitochondrial morphology and impaired mitochondrial homeostasis [82].
Other evidence that connect PD with NRF2 is with the disease associated protein DJ-1. Missense, truncation and splice site mutations in DJ-1 lead to an autosomal recessive, early-onset familial form of PD [83]. Interestingly, it has been shown that DJ-1 is involved in the NRF2- dependent oxidative stress response that leads to the upregulation of the 20S proteasome and its regulator, NQO1 [84]. Furthermore, DJ-1 induces thioredoxin 1 expression through NRF2 pathway [85] and that DJ-1 stabilizes NRF2 by preventing association with KEAP1, and NRF2’s subsequent ubiquitination and degradation [86]. DJ-1/- mice did not exhibit widespread neuronal loss in a PD disease model [87,88], but these neurons were more susceptible to death after toxic insults [87], indicating a similar behaviour between DJ-1/- and Nrf2/- mice [89] that could be explained due to the loss of antioxidant gene transcription.
In connection with α-synuclein, it has been demonstrated that expression of human α-SYN in Nrf2/- mice, exhibited exacerbated degeneration of nigral dopaminergic neurons and increased dystrophic dendrites, reminiscent of Lewy neurites, which correlated with impaired proteasome gene expression and activity [39]. Also, dopaminergic neuron loss was associated with an increase in neuroinflammation and gliosis that were intensified in Nrf2/- mice, indicating the relevance of NRF2 expression in the regulation of neurodegenerative and neuroinflammatory processes. α-synuclein was able to induce antioxidant enzyme genes in BV2 microglial cells and these effects was NRF2-dependent [39]. These results were supported by the findings that misfolded α-synuclein directly activates microglia and increased antioxidant enzyme expression [90] and that these enzymes are upregulated in another mouse model of α-synuclein overexpression. In mice that selectively overexpress NRF2 in astrocytes and human mutant α-synuclein (A53T) in neurons, showed delayed onset and extended life span which correlated with increased motor neuron survival, reduced oxidative stress and attenuated gliosis in the spinal cord in comparison to mutant α-synuclein (A53T) mice [91]. In vitro studies in SK-N-SH cells showed that ferrous iron induces α-synuclein aggregation and neurotoxicity by inhibiting NRF2/HO-1. Inhibition of NRF2/HO-1 leads to more α-synuclein aggregation and greater toxicity induced by iron, creating a vicious cycle of iron accumulation, α-synuclein aggregation and HO-1 disruption in PD [92]. All together, these evidence indicate the significant role of NRF2 in PD.
Pharmacologic Targeting of NRF2 as a Disease Modifiying Therapy for Parkinson’s Disease
Due to the fact that NRF2 is controlling the expression of genes related to proteostasis, oxidative stress and inflammation, NRF2 is a promising candidate for pharmacological targeting for the treatment of PD. There is a huge amount of compounds that target NRF2 in different ways, but looking from the clinical perspective, only few of them could be used for treatment of PD patients.
Sulforaphane (SFN), one of the main activators of NRF2, is a compound within the isothiocyanate group of organosulfur compounds. It is obtained from cruciferous vegetables such as broccoli, Brussels sprouts and cabbages. Intraperitoneal administration of the SFN increased NRF2 protein levels in the basal ganglia and led to upregulation of phase II antioxidant enzymes HO-1 and NQO1 [38]. Related to PD, patient-derived cellular model generated from biopsies of the olfactory mucosa (termed olfactory neurosphere-derived (hONS) cells) had a 20% reduction in reduced glutathione levels and MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2- (4-sulfophenyl)-2H-tetrazolium, inner salt] metabolism compared to cultures from healthy control donors. But more importantly, activation of the NRF2 pathway with SFN in PD hONS cultures restored glutathione and MTS metabolism to control levels [93]. In wild-type mice, but not in Nrf2-knockout mice, SFN protected against MPTPinduced death of nigral dopaminergic neurons. The neuroprotective effects were accompanied by a decrease in astrogliosis, microgliosis, and release of pro-inflammatory cytokines [38]. Similar effects have been demonstrated in other animal models of PD [94-96].
Oleanolic acid is a naturally occurring triterpenoid, which has been anti-inflammatory effects. Synthetic triterpenoids such as CDDO have been found to be potent inducers of the transcriptional activity of NRF2, resulting in marked induction of NQO1, HO-1, glutathione transferases, and other cytoprotective enzymes, as well as suppressing induction of iNOS and COX2 [97-100]. CDDO-methyl amide (2-cyano-N-methyl- 3,12-dioxooleana-1,9(11)-dien-28 amide; CDDO-MA) treatment of neuroblastoma SH-SY5Y cells resulted in NRF2 upregulation and translocation from cytosol to nucleus and subsequent activation of ARE pathway genes. Oral administration of CDDO-MA resulted in significant protection against MPTP-induced nigrostriatal dopaminergic neurodegeneration, pathological α-synuclein accumulation and oxidative damage in mice [101]. Two other structural analogues of CDDO (TP-319 and TP-500) had been modified to improve bloodbrain- barrier permeability, and reduced MPTP-induced oxidative stress and inflammation, and ameliorated dopaminergic neurotoxicity in mice. The neuroprotective effect of these TP against MPTP neurotoxicity was dependent on NRF2, since treatment with TP in NRF2 knockout mice failed to block against MPTP neurotoxicity and induce NRF2-dependent cytoprotective genes [102].
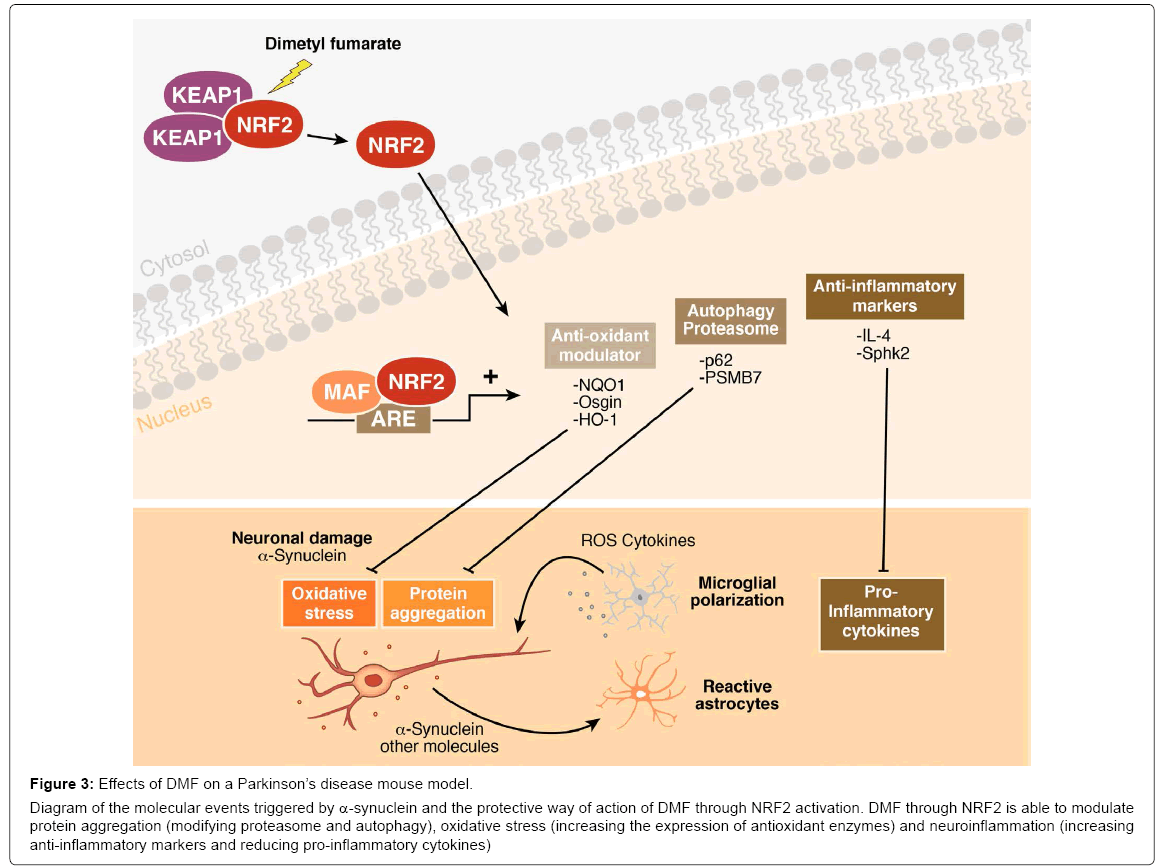
The third class of compound that activates NRF2 and could be used for clinical purposes is dimethyl fumarate (DMF), the methyl ester of fumaric acid and its metabolite, monomethyl fumarate. DMF was initially recognized as a very effective hypoxic cell radiosensitizer. Phase III clinical trials found that DMF (BG-12) successfully reduced relapse rate and increased time to progression of disability in multiple sclerosis (trade name Tecfidera) [103]. The first evidence of the benefits of DMF in PD is as quinone reductase inducer that abolish tetrahydrobiopterin BH4 (an obligatory cofactor for dopamine synthesis, also contributes to the vulnerability of dopamine-producing cells by generating oxidative stress)-induced cell death, suggesting that quinone production plays an important role [104]. More importantly, it has been shown that daily oral gavage of DMF protected nigral dopaminergic neurons against α-synuclein toxicity and decreased astrocytosis and microgliosis after 1, 3 and 8 weeks from stereotaxic delivery to the ventral midbrain of recombinant adeno-associated viral vector expressing human α-synuclein [23]. This protective effect was not observed in Nrf2- knockout mice. In vitro studies indicated that this neuroprotective effect was correlated with altered regulation of autophagy markers SQTSM1/ p62 and LC3 in MN9D, BV2 and IMA 2.1 and with a shift in microglial dynamics toward a less pro-inflammatory and a more wound-healing phenotype (Figure 3). These data demonstrate that NRF2 targeting by DMF could modulate the main hallmarks of PD: proteinopathy, oxidative stress and neuroinflammation. These observations were reinforced by the fact that DMF significantly reduced neuronal cell degeneration of the dopaminergic tract and behavioural impairments induced by injections of the dopaminergic neurotoxin MPTP [105,106]. Interestingly, the pharmacodynamics of DMF are tissue specific and involve NRF2-dependent and -independent mechanisms [107].
Figure 3: Effects of DMF on a Parkinson’s disease mouse model.
Diagram of the molecular events triggered by α-synuclein and the protective way of action of DMF through NRF2 activation. DMF through NRF2 is able to modulate protein aggregation (modifying proteasome and autophagy), oxidative stress (increasing the expression of antioxidant enzymes) and neuroinflammation (increasing anti-inflammatory markers and reducing pro-inflammatory cytokines)
One of the main actions of DMF is modulating inflammation, inhibiting the transcription factor NF-κB, that could be also independent of NRF2 [108-110]. But, NRF2 involvement cannot be ruled out due to Osgin-1, a transcriptional target of NRF2, contributes to monomethyl fumarate-mediated cytoprotection in human astrocytes [111] and is highly regulated in the ventral midbrain after DMF exposure [23] in a NRF2-dependent way.
Conclusion
Parkinson’s disease is the second most common multisystemic neurodegenerative disorder associated with ageing. At the pathologic level, PD is characterized by the selective loss of dopaminergic neurons in the SNpc and accumulation of α-synuclein in Lewy bodies and Lewy neurites [112]. It is a progressive movement disorder, and the main therapeutic treatment is focused on dopamine replacement therapy with levodopa, accompanied with complications related to long-term symptomatic treatment. Moreover, these treatments did not delay or stop disease progression. Although the first description of the disease was made two centuries ago, the treatments have not evolved in a great manner.
Transcription factor NRF2 has emerged as a suitable candidate for pharmacological targeting for the treatment of PD. There are consistent bases relating PD and NRF2 and several basic research based on parkinsonian animal models which reinforce the idea of modulation of the expression of the NRF2 pathway is beneficial for delaying the disease progression. Several compounds that have been promising in PD mouse models could be transferred to the study in clinical trials, for example DMF, that already is used to treat multiple sclerosis patients.
Acknowledgement
This manuscript was supported by a Spanish Ministerio de Ciencia e Innovacion Grant SAF2016-76520-R. We acknowledge Dr. Antonio Cuadrado for thoughtful comments and constructive discussion on the manuscript.
References
- Jankovic J (2008) Parkinson's disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79: 368-376.
- Kalia LV, Lang AE (2015) Parkinson's disease. Lancet 386: 896-912.
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, et al. (1997) Alpha-synuclein in Lewy bodies 388: 839-840.
- Lubbe S, Morris HR (2014) Recent advances in Parkinson's disease genetics. J Neurol 261: 259-266.
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276: 2045-2047.
- Kruger R, Kuhn W, Müller T, Woitalla D, Graeber M, et al. (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet 18: 106-108.
- Nussbaum R.L (2017) The identification of alpha synuclein as the first Parkinson disease gene. J Parkinsons Dis 7: 45-51.
- Zarranz JJ, Alegre J, Gomez Esteban JC, Lezcano E, Ros R, et al. (2004) The new mutation E46K of alpha synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55: 164-173.
- Konno T, Owen A, Ross, Andreas Puschmann, Dennis W, et al. (2016) Autosomal dominant Parkinson's disease caused by SNCA duplications. Parkinsonism Relat Disord 1: 1-6.
- Petrucci S, Ginevrino M ,Valente EM (2016) Phenotypic spectrum of alpha synuclein mutations: New insights from patients and cellular models. Parkinsonism Relat Disord 1: 16-20.
- Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, et al. (2014) Large scale meta-analysis of genome wide association data identifies six new risk loci for Parkinson's disease. Nat Genet 46: 989-993.
- Fishbein I, Yien Ming Kuo, Benoit I, Robert L, Nussbaum, et al. (2014) Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a Gaucher mutation. Brain 137: 3235-3247.
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, et al. (2011) Gaucher disease glucocerebrosidase and synuclein form a bidirectional pathogenic loop in synucleinopathies 146: 37-52.
- Kuo YM, LiZ, Jioa Y, Gaborit N, Pani AK, et al. (2010) Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet 19: 1633-1650.
- Sampson TR, Debelius JW, Thron T, Janseen S, Shastri GG, et al. (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167: 1469-1480.
- Luk, Kelvin C, Victoria M, Kehm, Bin Zhang, et al. (2012) Intracerebral inoculation of pathological alpha synuclein initiates a rapidly progressive neurodegenerative alpha synucleinopathy in mice. J Exp Med 209: 975-986.
- McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P (2001) Failure of the ubiquitin-proteasome system in Parkinson's disease. Nat Rev Neurosci 2: 589-594.
- Lim KL (2007) Ubiquitin proteasome system dysfunction in Parkinson's disease: Current evidence and controversies. Expert Rev Proteomics 4: 769-781.
- McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW (2003) Altered proteasomal function in sporadic Parkinson's disease. Exp Neurol 179: 38-46.
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) Alpha Synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278: 25009-25013.
- Engelender S (2012) Alpha synuclein fate: Proteasome or autophagy? Autophagy 8: 418-420.
- Watanabe Y, Tatebe H, Taguchi K, Endo Y, Tokuda T, et al. (2012) p62/SQSTM1 dependent autophagy of Lewy body-like Alpha synuclein inclusions 7: e52868.
- Lastres Becker, Angel J, Garcia Yague, Robert H, Maria J, et al. (2016) Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson's disease. Antioxid Redox Signal 25: 61-77.
- Winslow AR, Chen CW, Corrochano S, Acevedo Arozena A, Gordon DE, et al. (2010) Alpha synuclein impairs macroautophagy: Implications for Parkinson's disease. J Cell Biol 190: 1023-1037.
- Ebrahimi Fakhari D, Cantuti Cast elvetri L, Fan Z, Rockenstein E, Masliah E, et al. (2011) Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of a-synuclein. J Neurosci 31: 4508-14520.
- Blesa J, Trigo Damas I, Quiroga Varela A, Jackson Lewis VR (2015) Oxidative stress and Parkinson's disease. Front Neuroanat 9: 1-91
- Schapira AH, Jenner P (2011) Etiology and Pathogenesis of Parkinson's disease. Mov Disord 26: 1049-1055.
- Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59: 1609-1623.
- Hwang O (2013) Role of oxidative stress in Parkinson's disease. Neurobiol 22: 11-17.
- Bosco DA, Douglas M Fowler, Quinghai Zhang, Jorge Nieva, Evan T, et al. (2006) Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha synuclein fibrilization. Nat Chem Biol 2: 249-253
- Nakabeppu Y, Tsuchimoto D, Yamaguchi H, Sakumi K (2007) Oxidative damage in nucleic acids and Parkinson's disease. J Neurosci Res 85: 919-934.
- Zeevalk GD, Razmpour R, Bernard LP (2008) Glutathione and Parkinson's disease: Is this the elephant in the room? Biomed Pharmacother 62: 236-249.
- Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson's disease. J Parkinsons Dis 3: 461-491.
- McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38: 1285-1291.
- Taylor JM, Main BS, Crack PJ (2013) Neuroinflammation and oxidative stress: Co-conspirators in the pathology of Parkinson's disease. Int 62: 803-819.
- Duke DC, MoraN LB, Pearce RK, Graeber MB (2007) The medial and lateral substantia nigra in Parkinson's disease: mRNA profiles associated with higher brain tissue vulnerability. Neurogenetics 8: 83-94.
- Stojkovska I, Wagner BM, Morrison BE (2015) Parkinson's disease and enhanced inflammatory response. Exp Biol Med (Maywood) 240: 1387-1395.
- Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernández-Ruiz J, et al. (2011) Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental Parkinsonism. Antioxid Redox Signal 14: 2347-2360.
- Lastres-Becker I, Ulusoy A, Innamorato NG, Sahin G, Rábano A, et al. (2012) alpha-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease. Hum Mol Genet 21: 3173-3192.
- Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, et al. (2008) Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci 28: 10825-10834.
- Qin L, Wu X, Block ML, Liu Y, Breese GR, et al. (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55: 453-462.
- Ramsey CP, Tansey MG (2014) A survey from 2012 of evidence for the role of neuroinflammation in neurotoxin animal models of Parkinson's disease and potential molecular targets. Exp Neurol 256: 126-132.
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, et al. (2008) Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci 28: 7687-7698.
- Rushmore TH, Morton MR, Pickett CB (1991) The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem 266: 11632-11639.
- Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39: 199-218.
- Motohashi H, Yamamoto M (2014) Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10: 549-557.
- Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, et al. (2015) Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 6: 183-197.
- Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, et al. (2011) SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol 31: 1121-1133.
- Hayes JD1, Chowdhry S2, Dinkova-Kostova AT2, Sutherland C3 (2015) Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of β-TrCP and GSK-3. Biochem Soc Trans 43: 611-620.
- Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL (2013) The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol 1: 45-49.
- Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK (2005) Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem 280: 16891-16900.
- Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, et al. (1996) Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol 16: 6083-6095.
- Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, et al. (2002) Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J 21: 5216-5224.
- Kraft DC, Deocaris CC, Wadhwa R, Rattan SI (2006) Preincubation with the proteasome inhibitor MG-132 enhances proteasome activity via the Nrf2 transcription factor in aging human skin fibroblasts. Ann N Y Acad Sci 1067: 420-424.
- Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ (2012) Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J Biol Chem 287: 10021-10031.
- Kwak MK, Cho JM, Huang B, Shin S, Kensler TW (2007) Role of increased expression of the proteasome in the protective effects of sulforaphane against hydrogen peroxide-mediated cytotoxicity in murine neuroblastoma cells. Free Radic Biol Med 43: 809-817.
- Gan N, Wu YC, Brunet M, Garrido C, Chung FL, et al. (2010) Sulforaphane activates heat shock response and enhances proteasome activity through up-regulation of Hsp27. J Biol Chem 285: 35528-35536.
- Kageyama S, Sou YS, Uemura T, Kametaka S, Saito T, et al. (2014) Proteasome dysfunction activates autophagy and the Keap1-Nrf2 pathway. J Biol Chem 289: 24944-24955.
- Pajares M, Jiménez-Moreno N, García-Yagüe A, Escoll M, Ceballos M, et al. (2016) Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy 12: 1902-1916.
- Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53: 401-426.
- Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12: 931-947.
- Niso-Santano M, González-Polo RA, Bravo-San Pedro JM, Gómez-Sánchez R, Lastres-Becker I, et al. (2010) Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death: Modulation by the Nrf2/Trx axis. Free Radic Biol Med 48: 1370-1381.
- Innamorato NG, Rojo AI, García-Yagüe AJ, Yamamoto M, de Ceballos ML, et al. (2008) The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol 181: 680-689.
- Rojo AI, Innamorato NG, Martín-Moreno AM, De Ceballos ML, Yamamoto M, et al. (2010) Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia 58: 588-598.
- Lastres-Becker I, Innamorato NG, Jaworski T, Rábano A, Kügler S, et al. (2014) Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain 137: 78-91.
- Granado N, Lastres-Becker I, Ares-Santos S, Oliva I, Martin E, et al. (2011) Nrf2 deficiency potentiates methamphetamine-induced dopaminergic axonal damage and gliosis in the striatum. Glia 59: 1850-1863.
- Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, et al. (2017) Correction: disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol 198: 3755.
- Cuadrado A, Martín-Moldes Z, Ye J, Lastres-Becker I (2014) Transcription factors NRF2 and NF-κB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J Biol Chem 289: 15244-15258.
- Rushworth SA, Zaitseva L, Murray MY, Shah NM, Bowles KM, et al. (2012) The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood 120: 5188-5198.
- Lee DF, Kuo HP, Liu M, Chou CK, Xia W, et al. (2009) KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell 36: 131-140.
- Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, et al. (2016) Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7: 11264.
- Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, et al. (2007) Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol 66: 75-85.
- van Muiswinkel FL, de Vos RA, Bol JG, Andringa G, Jansen Steur EN, et al. (2004) Expression of NAD(P)H:quinone oxidoreductase in the normal and Parkinsonian substantia nigra. Neurobiol Aging 25: 1253-1262.
- Castellani R, Smith MA, Richey PL, Perry G (1996) Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res 737: 195-200.
- Schipper HM, Liberman A, Stopa EG (1998) Neural heme oxygenase-1 expression in idiopathic Parkinson's disease. Exp Neurol 150: 60-68.
- Yoo MS, Chun HS, Son JJ, DeGiorgio LA, Kim DJ, et al. (2013) Oxidative stress regulated genes in nigral dopaminergic neuronal cells: Correlation with the known pathology in Parkinson's disease. Brain Res Mol Brain Res 110: 76-84.
- Cuadrado A, Moreno-Murciano P, Pedraza-Chaverri J (2009) The transcription factor Nrf2 as a new therapeutic target in Parkinson's disease. Expert Opin Ther Targets 13: 319-329.
- von Otter M, Landgren S, Nilsson S, Celojevic D, Bergström P, et al. (2013) Association of Nrf2-encoding NFE2L2 haplotypes with Parkinson's disease. BMC Med Genet 11: 36.
- von Otter M, Bergström P, Quattrone A, De Marco EV, Annesi G, et al. (2014) Genetic associations of Nrf2-encoding NFE2L2 variants with Parkinson's disease - a multicenter study. BMC Med Genet 15: 131.
- Todorovic M, Newman JR, Shan J, Bentley S, Wood SA, et al. (2015) Comprehensive Assessment of Genetic Sequence Variants in the Antioxidant ‘Master Regulator’ Nrf2 in Idiopathic Parkinson’s Disease. PLoS ONE 10: e0128030.
- Loeffler DA, Smith LM, Coffey MP, Aasly JO, LeWitt PA, et al. (2016) CSF Nrf2 and HSPA8 in Parkinson's disease patients with and without LRRK2 gene mutations. J Neural Transm (Vienna) 123: 179-187.
- Imaizumi Y, Okada Y, Akamatsu W, Koike M, Kuzumaki N, et al. (2012) Mitochondrial dysfunction associated with increased oxidative stress and alpha-synuclein accumulation in PARK2 iPSC-derived neurons and post-mortem brain tissue. Mol Brain 5: 35.
- Cookson MR (2012) Parkinsonism due to mutations in PINK1, parkin, and DJ-1 and oxidative stress and mitochondrial pathways. Cold Spring Harb Perspect Med 2: a009415.
- Moscovitz O, Ben-Nissan G, Fainer I, Pollack D, Mizrachi L, et al. (2015) The Parkinson's-associated protein DJ-1 regulates the 20S proteasome. Nat Commun 6: 6609.
- Im JY, Lee KW, Woo JM, Junn E, Mouradian MM (2012) DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum Mol Genet 21: 3013-3024.
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP (2006) DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A 103: 15091-1506.
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, et al. (2005) Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A 102: 5215-5220.
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, et al. (2005) Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron 45: 489-496.
- Innamorato NG, Jazwa A, Rojo AI, García C, Fernández-Ruiz J, et al. (2010) Different susceptibility to the Parkinson's toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS ONE 5: e11838.
- Béraud D, Hathaway HA, Trecki J, Chasovskikh S, Johnson DA, et al. (2013) Microglial activation and antioxidant responses induced by the Parkinson's disease protein alpha-synuclein. J Neuroimmune Pharmacol 8: 94-117.
- Gan L, Vargas MR, Johnson DA, Johnson JA, et al. (2012) Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J Neurosci 32: 17775-17787.
- He Q, Song N, Jia F, Xu H, Yu X, et al. (2013) Role of α-synuclein aggregation and the nuclear factor E2-related factor 2/heme oxygenase-1 pathway in iron-induced neurotoxicity. Int J Biochem Cell Biol 45: 1019-1030.
- Cook AL, Vitale AM, Ravishankar S, Matigian N, Sutherland GT, et al. (2011) NRF2 activation restores disease related metabolic deficiencies in olfactory neurosphere-derived cells from patients with sporadic Parkinson's disease. PLoS ONE 6: e21907.
- Morroni F, Tarozzi A, Sita G, Bolondi C, Zolezzi Moraga JM, et al. (2013) Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson's disease. Neurotoxicology 36: 63-71.
- Trinh K, Moore K, Wes PD, Muchowski PJ, Dey J, et al. (2008) Induction of the phase II detoxification pathway suppresses neuron loss in Drosophila models of Parkinson's disease. J Neurosci 28: 465-472.
- Advedissian T, Deshayes F, Poirier F, Viguier M, Richarme G (2016) The Parkinsonism-associated protein DJ-1/Park7 prevents glycation damage in human keratinocyte. Biochem Biophys Res Commun 473: 87-91.
- Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, et al. (2005) Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A 102: 4584-4589.
- Liby K, Hock T, Yore MM, Suh N, Place AE, et al. (2005) The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res 65: 4789-4798.
- Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, et al. (2006) Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun 351: 883-889.
- Honda T, Honda Y, Favaloro FG Jr, Gribble GW, Suh N, et al. (2002) A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg Med Chem Lett 12: 1027-1030.
- Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, et al. (2009) Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS ONE 4: e5757.
- Kaidery NA, Banerjee R, Yang L, Smirnova NA, Hushpulian DM, et al. (2013) Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson's disease. Antioxid Redox Signal 18: 139-157.
- Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, et al. (2012) Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 367: 1098-1107.
- Choi HJ, Lee SY, Cho Y, Hwang O (2005) Inhibition of vesicular monoamine transporter enhances vulnerability of dopaminergic cells: Relevance to Parkinson's disease. Neurochem Int 46: 329-335.
- Michela C, Giovanna C, Flavia B, Rosalia C, Marika C, et al. (2017) The neuroprotective effect of dimethyl fumarate in an MPTP-mouse model of Parkinson's disease: Involvement of reactive oxygen species/nuclear factor-kappaB/nuclear transcription factor related to NF-E2. Antioxid Redox Signal.
- Ahuja M, Ammal Kaidery N, Yang L, Calingasan N, Smirnova N, et al. (2016) Distinct Nrf2 signaling mechanisms of fumaric acid esters and their role in neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced experimental Parkinson's-like disease. J Neurosci 36: 6332-6351.
- Brennan MS (2016) Pharmacodynamics of dimethyl fumarate are tissue specific and involve NRF2-dependent and independent mechanisms. Antioxid Redox Signal 24: 1058-1071.
- Gillard GO, Collette B, Anderson J, Chao J, Scannevin RH, et al. (2015) DMF, but not other fumarates, inhibits NF-κB activity in vitro in an Nrf2-independent manner. J Neuroimmunol 283: 74-85.
- Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, et al. (2016) Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci U S A 113: 4777-4782.
- Peng H, Li H, Sheehy A, Cullen P, Allaire N, et al. (2016) Dimethyl fumarate alters microglia phenotype and protects neurons against proinflammatory toxic microenvironments. J Neuroimmunol 299: 35-44.
- Brennan MS, Matos MF, Richter KE, Li B, Scannevin RH, et al. (2017) The NRF2 transcriptional target, OSGIN1, contributes to monomethyl fumarate-mediated cytoprotection in human astrocytes. Sci Rep 7: 42054.
- Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, et al. (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338: 949-953.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 5808
- [From(publication date):
August-2017 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 4836
- PDF downloads : 972