Role of Synthetic Hormones on Reproductive Performance in Etroplussuratensis
Received: 01-Aug-2017 / Accepted Date: 30-Aug-2017 / Published Date: 04-Sep-2017 DOI: 10.4172/2332-2608.1000248
Abstract
The present study was conducted to experiment the role of synthetic hormones on reproductive performance in Etroplus suratensis. The performance of these hormones were assessed by studying the fecundity, egg size, percentage of egg fertilization, percentage of deformed larvae development, percentage of hatching, milt collection, sperm motility and time of sperm survival. The observation from this showed that the above said reproductive parameters were found to be maximum in the HCG+LHRH administration followed by ovaprim administration. The findings of this study state that the combination of HCG+LHRH is advisable for the induced maturation for E. suratensis than individual hormone treatment.
Keywords: Etroplus suratensis; Synthetic hormone; HCG; LHRH; Fecundity; Striping response; Percentage of fertilization; Sperm motility
Introduction
The Etroplus suratensis is an economically very important estuarine fish and at the same time most sensitive in confined culture condition and more over the induced spawning of this fish is troublesome one and hence, a few study have been conducted in this estuarine fish [1]. Reproduction in fishes is regulated by external environmental factors that trigger internal mechanisms into actions. The final event of the reproductive cycle, the release of eggs and sperm resulting in spawning can be controlled by either placing the fish in an appropriate environment or by changing the fish’s internal regulating factors with injected hormones or other substances. The internal mechanisms that regulate spawning are similar for most fishes; the external environmental factors that control reproductions however, vary considerable among species. For this reason, more is known about the internal regulatory mechanism of fish reproduction by applying hormones than the specific environmental requirements for spawning [2].
It is well established that in vertebrates, gonadotropins are the primary hormone to regulate gametogenesis [3]. However it appears that gonadotropins do not act directly. But work through the gonadal biosynthesis of steroid hormones which in turn mediate the stages in gametogenesis. The results on the hormonal regulation of oocyte growth, oocyte maturation, spermatogenesis, and sperm maturation in fish was also reviewed [3].
A few works have been carried out in this direction of larval propagation in E. suratensis [4]. The ongoing research in the breeding biology of E. suratensis mainly depends on the natural spawning in captive conditions [5]. Induced breeding by applying hormones, were not encouraging in earlier research works carried out by earlier researchers [6-8]. Keeping in mind about the practical difficulties in the induced breeding of these estuarine fish, the present work was designed.
Materials and Method
Experimental setup
Three set of experimental animals were maintained. The weights of the female and male animals were 250 ± 50 and 200 ± 50 g, respectively. They all were collected from an estuary in Rajakkamangalam, Kanyakumari district, India and from the same group. Three spawners were randomly sacrificed from a catch group for assessing the stages of the ovary. The experimental spawners were selected from the catch group which possessed the 2nd stage of gonad development (Contain spherical and opaque ova owing to the commencement of yolk deposition. Length of the ovary varies from 35 to 52 mm. The diameter of the ova ranges from 0.5 mm to 1.75 mm). Each set comprised of ten matured male and female animals. The state of maturation was assessed by the body size and weight. Each set of animals were separately treated with HCG+LHRH, Ovaprime and Physiological saline (control). All the studied parameters were repeated three times. For each set, triplicates were also maintained. The data were subjected for statistical analysis. The culture and maturation study were conducted following the method described by Dhas A et al. [1].
a) Gonadosomatic Index %: Gonadosomatic index (GSI) is the calculation of the gonad mass (g) as a proportion of total body mass (g). It is represented by the formula [9]. This was estimated only in female.
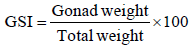
b) Determination of egg size: The length and width of different stages of eggs include Oocyte, Pre-vitrllogenic and matured were measurement microscopically using a calibrated micrometer eye piece. The average length and width of 20 eggs from each stage were individually measured for egg size.
c) Fecundity: Fecundity of a spawner was calculated by counting the total eggs released and deposited at a clutch that happens for 2-3 days by an individual spawner [1]. Counting was made by lifting the egg attached substratum just below the water column and counted by visual method. In the same way, the eggs attached in five different spawning tanks were counted separately and average was taken for the final result.
d) Percentage of egg fertilization: Percentage of egg fertilization was calculated by taking 100 eggs from the spawning tank and examining under of the compound microscope. Fertilized eggs were identified by the presence of fertilization membrane and embryo development.

e) Percentage of deformed embryo development: Hundred eggs were collected from the hatching tank and observed using Stereo Zoom microscope (Coslab, India). The embryo having abnormal cell division, deformed fertilization membrane, abnormal egg sac development and sluggish movement during hatching were considered as deformed embryo. In the same way, the embryo was sampled from five spawning tanks. From the average of five sampling, the percentage of deformed larvae was calculated.
f) Hatching: Hatching was carried out by aerating the spawned eggs in the hatching tank. The process of hatching started 3-4 days after the egg release and got completed within 24 hours, depending upon the egg condition, temperature and aeration. When eggs were removed from the parents along with the substratum from the spawning tank and separately incubated in hatching tanks with the same parents in the assumption that they will take care of the transferred brood, curiously to protect from fungal and bacterial attachment on eggs. The incubation period of the egg was 3-4 days based on temperature and oxygen content of the tank water. After 3rd day, the eggs were noticed for hatching. After complete hatching, the hatched out larvae were scooped out in to separate larval rearing tank having 50 litre water.
g) Percentage of hatching: Percentage of hatching was calculated by dividing the number of hatched larvae with total number of eggs spawned and found attached on substratum. After the hatching process, the larvae were separated with the help of 1000 μm net. Thus, all the larvae were carefully collected and sacrificed with lugol solution for easy counting [1].
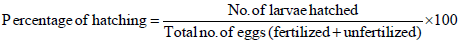
h) Milt collection: Male fish from aquaculture stocks were starved one day before collection to minimize faces contamination. The common anaesthetic agent, MS222 was used to anaesthetize the fish, E. suratensis. Dry towel was used to wipe abdomen and urogenital papillae until dry. A tube was inserted in to genital pore of E. suratensis and its abdomen was gently pressed to expel milt. The milt was collected in a syringe to avoid contamination [10].
i) Sperm quality assessment: Micropipette was used to drop about 2 μL of milt and about 50 μL of activating solution (4.5% NaCl, fertilization solution or freshwater for freshwater fish) nearby on slide. A tiny glass rod was used to tip the two drops for activate sperm to motile, and then looked for motile sperm under microscope at 100 X magnification of a compound microscope [11,12]. Three parameters were taken in to account for measuring sperm quality.
j) Percentage of motile sperm: The sperm those move straight and actively under microscope are consider as good sperm and the sperm that showed circular motion or moving towards water current are treated as low quality sperm [13].The percentage of motility was calculated using the following formula:
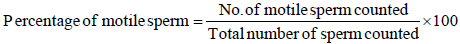
k) Moving time
It is the time in seconds required for 90% of sperms to stop movement. The moving time was recorded. As the moving time is species specific, it was necessary to record the data for E. Suratensis [1].
l) Counting of Active Sperm Cells, Sperm Motility and Time of Sperm Survival in Water
Before the semen collection, fish were anaesthetized in 2-phenoxyethanol solution (0.3 mL/L). Milt samples were collected by stripping, avoiding the water, urine, and faeces contamination and transported on ice (2°C) to the laboratory. Seminal plasma was obtained by centrifugation (8 000 g, 10 min) within 12 h after sampling and stored at –70°C until used. Sperm motility (in 0.5% NaCl activating solution) was estimated using a light microscope (400X) and expressed as a percentage of motile spermatozoa. The dilution of sperm/solution ratio was approximately 1:6 (5 μL of milt and 30 μL of NaCl). Only the progressive straight-line movement of spermatozoa was noted. Sperm concentration was estimated using a spectrophotometric method described by Ciereszko and Dabrowski [14]. Sperm concentration and motility were statistically analyzed using a one-way analysis of variance (ANOVA) [15].
Results
The hormone had influence in the egg size. The egg in oocyte, previtellogenic and matured stage had variation in size based on the type of hormone administration. The HCG+LHRH combination evoked the maximum egg size and followed by Ovaprim and control (Table 1). The GSI was maximum (11.84) in HCG+LHRH administered spawner. The other treatments had 9.84 and 7.82% respectively in Ovaprim and Physiological saline administered groups. The fecundity was the maximum in HCG+LHRH (1010 in number), followed by ovaprim (792 in number) and it was the minimum (612 in number) in control. As like the fecundity, the striping response was also high in HCG+LHRH treatment (1.23 mL), followed by ovaprim (0.84 mL) and in controls it was very minimum level (0.42 mL). The percentage of fertilization, percentage of hatching and percentage of deformed embryo were also measured. The percentage of fertilization was the highest in HCG+LHRH (82.54%) and followed by ovaprim (76.42%) and in control it was the lowest (65.34) level. The percentage of hatching was also very high in HCG+LHRH (80.83%) followed by ovaprim (73.69%), and it was very low (60.76%) in control. The percentage of deformed embryo was the lowest in HCG+LHRH (6.52%), followed by Ovaprim (8.75%), and control (11.27%). The data are presented in Table 2. Statistical analysis (T test) showed that the effectiveness of various hormones treatment on fecundity, striping response, fertilization, hatching and larval production are statistically significant (P<0.05) when compare with control.
| Egg Stage | Length (µm) | Width (µm) | ||||
|---|---|---|---|---|---|---|
| HCG+LHRH(1000 IU+1 mL/kg fish) | Ovaprim (1 mL/kg fish) | Control (Physiological saline/1mL/kg fish) | HCG+LHRH(1000 IU+1 mL/kg fish) | Ovaprim (1 mL/kg fish) | Control (Physiological saline/1 mL/kg fish) | |
| Oocyte | 188.66 ± 10.27 | 174.23 ± 9.35 | 167.25 ± 10.58 | 149.58 ± 13.18 | 127.20 ± 12.38 | 115.68 ± 18.44 |
| Pre-vitrllogenic egg | 602.72 ± 32.19 | 565.00 ± 27.26 | 516.52 ± 21.6 | 342.31 ± 30.27 | 325.14 ± 29.88 | 311.40 ± 76.53 |
| Mature eggs | 1956.65 ± 76.79 | 1892.64 ± 68.35 | 1860.32 ± 55.00 | 985.65 ± 65.55 | 946.25 ± 72.32 | 895.36 ± 82.5 |
Table 1: Length and width of E. suratensis eggs.
| Sl. No. | Parameters | Control | Hormonated | |
|---|---|---|---|---|
| (Physiological saline/1 mL/kg fish) | Ovaprim (1mL/kg fish) | HCG+LHRH (1000 IU+1 mL/kg fish) | ||
| 1 | Gonadosomatic index (GSI %) | 7.82 ± 0.35a | 9.37 ± 0.25b | 11.84 ± 0.42c |
| 2 | Fecundity (No of eggs/clutch) | 612 ± 26.65a | 792 ± 31.68b | 1010 ± 43.42c |
| 3 | Stripping response (mL/male) | 0.42 ± 0.48a | 0.84 ± 0.26b | 1.23 ± 0.52c |
| 4 | Percentage of Fertilization | 65.34 ± 3.92a | 76.42 ± 5.24b | 82.54 ± 6.12c |
| 5 | Percentage of Hatching | 60.76 ± 3.82a | 73.69 ± C3.75b | 80.83 ± 4.22c |
| 6 | Percentage of deformed embryo | 11.27 ± 0.29a | 8.75 ± 0.21b | 6.52 ± 0.18c |
The same superscript given in the control and experiments are statistically not significant and the different superscripts are statistically significant (P < 0.05); The ‘t’ test was conducted for the was data given in the table.
Table 2: Effect of various hormones on fecundity, striping response, fertilization, hatching and larval production in E. suratensis.
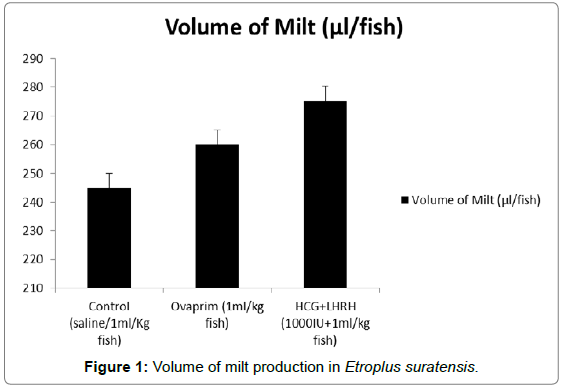
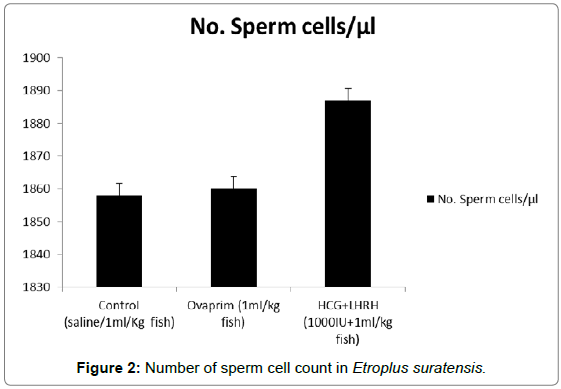
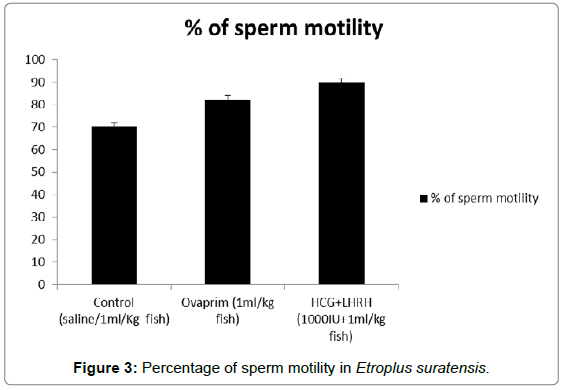
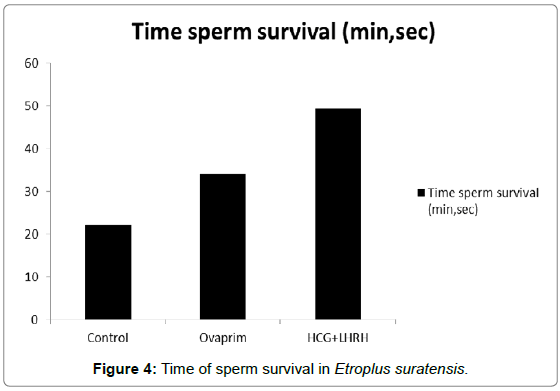
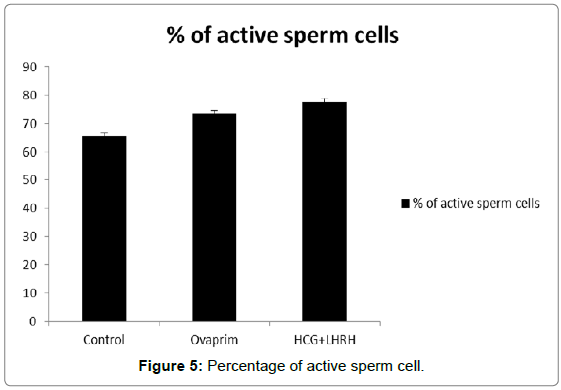
The influence of hormones such as ovaprim and HCG+LHRH on sperm cells count, volume of milt, percentage of sperm motility, time of sperm survival and percentage of active sperm cells were carried out to understand the influence of these hormones on these parameters. The data showed that the volume of milt was maximum in HCG+LHRH treatment (275 μL/fish), followed by ovaprim (260 μL/fish), and it was the minimum in the control (245 μL/fish). The number of sperm cells were also increased by HCG+LHRH treatment (1887 no/μL), followed by ovaprim (1860 no/μL), and it was minimum in the control (1858 no/μL). The percentage of sperm motility was also increased by HCG+LHRH treatment (89.78%), followed by ovaprim (82.34%), and it was the lowest in control (70.23%). As like the percentage of sperm motility, the time of sperm survival also increased in HCG+LHRH treatment (49’35”), followed by ovaprim (34’15”), and it showed a shortest time in the control (22’25”). The percentage of active sperm cells were also found to be increased to the highest level in HCG+LHRH treatment (77.78%), followed by ovaprim (73.51%) and in control the percentage of motility was the lowest (65.66%). The results are given in Figures 1-5.
Discussion
The other spawning parameters such as gonadosomatic index, fecundity, stripping response, percentage of fertilization, hatching, and formed larvae showed an increase in hormone administration such as HCG+LHRH and ovaprim and that showed an influence of these hormones in increase of these parameters. The increased gonadosomatic index, fecundity and the other parameters in the hormone administered treatment was already reported [16,17]. Earlier, Eyo and Mgbenka [17] had established a linear relationship between fecundity, GSI and somatic weight of C. gariepinus. This showed a strong influence of synthetic hormones in increase of all these spawning parameters and the hormones of the present study HCG+LHRH and ovaprim treatments also stated the same from the data investigated.
Nwokoye et al. [16] stated that ovaprim treatment had a significantly higher number of fertilized eggs than the homoplastic hormones in Heteropneustes bidorsalis. In a similar study using ovaprim to induce breed the catfish H. fossilis, Haniffa and Sridhar, 2002 had only a low rate of fertilized egg output H. fossilis given 1000 IU ovaprim. The difference in egg output of Haniffa and Sridhar [18] when compared to the study of Nwokoye et al. [16] even when the same quantity of ovaprim was used may be due to differences in species and weight of spawners.
The findings of the earlier study of Nwokoye et al. [16] co-inside with the present work in recorded lower number of deformed larvae in the hormone treatments such as HCG+LHRH, followed by ovaprim and that showed support to the result of the present work. Nwokoye et al. [16] stated that ovaprim recorded low deformities when compared with homoplastic hormone. In a similar study, Nwadukwe [19] using frog pituitary extract to induce breed H. longifilis reported a similar result to that of Nwokoye et al. [16]. Studies of this present report and the earlier reports revealed that hormones have correlating effect on spawning parameters, as the most hormones are promoters or inhibitors, depending on dosage.
The volume of milt, number of sperm cells, percentage of sperm motility, time of sperm survival and percentage of active sperm cells estimated in the present study showed all these parameters were increased in to the highest level by HCG+LHRH, followed by the ovaprim administration and that showed a positive influence of the hormones on these parameters. It also revealed that the synthetic hormones induce the volume and number of sperm cell production and causing an increase in the percentage of sperm motility, time of sperm survival and percentage of active sperm cells.
Earlier studies stated that the spontaneous sperm released during the breeding season in to very low in the channel catfish and in Silurus glanis [20] and males have to be injected with hormones to stimulate spermiation. This was due to their low GSI and low spermatogenetic production. Various hormones have been used successfully to stimulate spermiation especially HCG, LHRH, ovaprim in S. glanis [21], Pangasius sutchi and C. macrocephalus [22], Eurotropius depressirostris [23] and Ictalurus furcatus [24]. Carp pituitary homogenates were used in S. glanis [21,25]. In C. gariepinus, Van der Waal [26] stated that contrary to untreated fish, it was possible to collect semen by stripping from males injected with Clarius pituitary homogenates. GnRH was injected alone or in combination with Domperidone (Ovaprim) in S. gluni with limited success [21]. GnRH implants had also a limited effect [25]. Some performances of these various treatments are reported in S. glanis. Tambasen-Cheong et al. [27] found a significant increase in semen volume and decrease in sperm density after 24 h treatment with Ovaprim in C. rnacrocephulus. As stated in the observations made on hormone administration in different fishes by various authors on the different parameters such as volume and number of sperm cells, percentage of sperm motility, time of sperm survival and percentage of active sperm cells, in the present experimental fish E. suratensis also the hormones such as HCG+LHRH and ovaprim caused influence on the tested parameters of sperm quality.
Acknowledgments
The authors express the sincere gratitude to their beloved Guide, Late Professor Dr. Peter Marian, for having enlightened the research interest in them. One of the authors M. Micheal Babu also acknowledges IFS (International Foundation for Science) for the grant support through the fund (F/3291-2) to carry out this work.
References
- Dhas AS, Thangaswamy S, Citarasu T, Babu MM (2015) Effect of supplemented diet with maturation plant extract on reproductive performance of Etroplus suratansis. Aquaculture Reports 2: 58-62.
- Aquarium Science Association of the Philippines (1996) Aquarium species in the Philippines. ASAP Aquarist Database. Quezon City, Philippines. Asian Fish Soc Spec Publ 3: 154.
- Nagahama Y (1994) Endocrine regulation of gametogenesis in fish. Int J Bio 38: 217-229.
- Eschmeyer WN (1990) The genera of recent fishes. California Academy of Sciences, San Francisco, USA: 697.
- Rassa TS (1983) Fish. In: Sokolov VE editor. Life of animals, Prosveschenie, Moscow 4: 575.
- Garibaldi L (1996) List of animal species used in aquaculture. FAO Fish: 38.
- Klinkhardt M, Michael T, Hartmut G (1995) Database of fish chromosomes. Westarp Wissenschaften, Magdeburg.
- Kornfield I (1984) Descriptive genetics of cichlid fishes. In: Turner BJ editor. Evolutionary genetics of fishes, New York: 591-616.
- Barber BJ, Blake NJ (2006) Reproductive Physiology Scallops: Biology, Ecology and Aquaculture. In: Shumway SE, Parsons GJ editors. Elsevier Science Publishers 2nd (edn.), pp: 357-416.
- Zilli L, Schiavone R, Storelli C, Vilella S (2008) Molecular Mechanisms Determining Sperm Motility Initiation in Two Sparids (Sparus aurata and Lithognathus mormyrus). Biology of Reproduction 79: 356-366.
- Viveiros ATM, Godinho HP (2009) Sperm quality and cryopreservation of Brazilian freshwater fish species: a review. Fish Physiol Biochem 35:137-150.
- Sarosiek, Cejko BI, Glogowski J, Kucharczyk D, Targo?ska K, et al.(2012) Spermatozoa motility and short-term sperm storage of colourful orfe (Leuciscus idus aberr orfus). Ital J Anim Sci 11: 270-274.
- Canyurt MA, Akhan S (2008) Effect of Ascorbic Acid Supplementation on Sperm Quality of Rainbow trout (Onchorynchus mykiss). Turkish Journal of Fisheries and AquaticSciences 8: 171-175.
- Ciereszko A, Dabrowski K (1993) Estimation of sperm concentration of rainbow trout, whitefish and yellow perch using a spectrophotometric technique. Aquaculture 109: 367-373.
- Krol J, Glogowski J, Hliwa P, Zakes DK (2006) Quality of semen and histological analysis of testes in Eurasian perch Perca fluviatilis L. during a spawning period. Czech J Anim Sci 51: 220-226.
- Nwokoye CO, Nwuba LA, Eyo JE (2007) Induced propagation of African clariid catfish, Heterobranchus bidorsalis (Geoffrey Saint Hillarie, 1809) using synthetic and homoplastic hormones. African Journal of Biotechnology 6: 2687-2693.
- Eyo JE, Mgbenka BO (1992) Aspects of the biology of Clarias gariepinus in Anambra River basin I: oocyte diameter, fecundity and sex ratio. Journal of Agriculture Science and Technology 2: 47-51.
- Haniffa MAK, Sridhar S (2002) Induced spawning of spotted murrel Channa punctatus) and catfish Heteropneustes fossilis using human chorionic gonadotropin and synthetic hormone (Ovaprim). Vet Arhiv 72: 51-56.
- Nwadukwe FO (1993) Inducing oocyte maturation, ovulation and spawning in the African catfish Heterobranchus longifilis (Valences Pisces: Clariidae) using frog pituitary extract. Aquacul. Fish Man 24: 625-630.
- Hilge V (1983) Investigation of induced breeding of the European catfish Silurus glanis L. and the African catfish, Clarias lazera (C & V) (in German). Inf Fischwirtsch 30: 149-151.
- Redondo-Müller Ch (1990) Induction ala espermiation del siluro europeo, Silurus glanis L., mediante la application de extractos hipofisarios de Carpa (EHC). Repr. Anim. Ins. Artif. Zaragoza. Spain: 211-214.
- Na-Nakorn U, Wikrom R, Sunkul W, Saijai (1993) Suitable conditions for induclion of gynogenesis in the catfish, Clariasmacrocephalus, using sperm of Pangasius sutchi. Aquaculture 118: 53-62.
- Kruger EJ, Polling L (1984) First attempts at artificial breeding and larval rearing of the butter catfish, Eutropius depressirostris (Schilbeidae: Pisces). Water SA: 97-104.
- Dunham RA (1993) Observations on controlled artificial spawning of channel catfish. Progress Fish Cult 55: 60-61.
- Linhart O, Billard R (1994) Spermiation and sperm quality of European catfish (Silurus glanis) after implantation of GNRH analogues and injection of carp pituitary extract. J Appl Ichthyol 10: 182-188.
- Van der Waal BCW (1985) Stripping male C1urias gariepinus standard. Aquaculture 42: 137-142.
- Tambasen CMVP, Fermin JDT, Garcia LMB, Baldevarona RB (1995) Milt-egg ratio in artificial fertilization of the Asian freshwater catfish, Clurias macrocephalus, injected salmon gonadotropin-releasing hormone analogue and domperidone. Aquat Living Resour 8:303-307.
Citation: Dhas SA, Selvaraj T, Citarasu T, Punitha SMJ, Babu MM (2017) Role of Synthetic Hormones on Reproductive Performance in Etroplus suratensis. J Fisheries Livest Prod 5: 248 DOI: 10.4172/2332-2608.1000248
Copyright: © 2017 Dhas SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4837
- [From(publication date): 0-2017 - Mar 05, 2025]
- Breakdown by view type
- HTML page views: 4078
- PDF downloads: 759