Role of Procalcitonin in the Timely Detection of Ischemia and Necrosis in Children with Intestinal Obstruction
Received: 18-Nov-2022 / Manuscript No. JGDS-22-80269 / Editor assigned: 21-Nov-2022 / PreQC No. JGDS-22-80269 (PQ) / Reviewed: 05-Dec-2022 / QC No. JGDS-22-80269 / Revised: 02-Feb-2023 / Manuscript No. JGDS-22-80269 (R) / Published Date: 09-Feb-2023
Abstract
Introduction: Procalcitonin (PCT) has been studied for early identification of Ischemia and/or Necrosis (I/N) in children with Intestinal Obstruction (IO) secondary to Adhesive Small Bowel Obstruction (ASBO). However, the causes of IO in children are numerous.
Purpose: To correlate the level of PCT with the presence of I/N.
Results: Fifty-seven patients were analyzed. The incidence of I/N was 36%. PCT median was statistically higher in patients with I/N compared to those patients with normal intestine: 4.13 (13.9) vs. 0.11 (0.28) ng/ml, p=<0.001. A PCT threshold >1.17 ng/ml for predicting I/N yielded a sensitivity of 90%, a specificity of 97%, a Positive Predictive Value (PPV) of 95%, a Negative Predictive Value (NPV) of 94%, p=<0.001, Relative Risk (RR) 17.57 (95% CI, 4.54-67.90). Similarly, a PCT threshold >1.41 ng/ml for predicting intestinal necrosis yielded a sensitivity of 92%, a specificity of 88%, a PPV of 72%, a NPV of 97%, p=<0.001, RR 28.16 (95% CI, 3.98-119.12).
Conclusion: This study corroborates the association of PCT with IN in children with IO and expands the evidence of its use in this field. Similarly, suggests a PCT threshold >1.17 ng/ml and >1.41 for predicting IN and intestinal necrosis respectively.
Keywords
Procalcitonin; Adhesive small bowel obstruction; Intestinal ischemia; Intestinal necrosis; Pediatric small bowel obstruction
Introduction
Intestinal Obstruction (IO) is a serious condition that has to be identified and treated promptly to avoid severe complications [1]. In children a variety of causes have been reported such as, small bowel obstruction, incarcerated inguinal hernia, intussusception, intestinal malrotation, intestinal atresia, tumors, inflammatory bowel disease and anorectal malformations [2-5]. Clinical, laboratory and radiological parameters have been studied to identify the presence of Ischemia and/or Necrosis (I/N) in patients with IO [6-11]. However, none of them have shown sufficient sensitivity and specificity to be considered as the gold standard.
Procalcitonin (PCT) is a protein of 116 amino acids, the precursor of the calcitonin hormone discovered by Moya and collaborators in 1975 [12]. It is produced by the C cells of the thyroid gland and converted entirely to calcitonin within these cells. In normal conditions, the serum levels of PCT in the blood are very low <0.1-0.5 ng/ml [13-15]. In 1993, Assicot et al. reported the association between increased PCT levels and bacterial infection [16]. Subsequently, multiple studies have suggested the PCT levels as a guide in antibiotic treatment [17-19].
In 2005, Ayten et al. reported for the first time the association between PCT increase and I/N in rabbits [20]. Subsequently, multiple studies investigated the role of PCT in the management and prognosis of adult patients with Adhesive Small Bowel Obstruction (ASBO) showing encouraging results [21-26]. In 2017, we published the first study in pediatric patients that demonstrated a correlation between the increase in PCT and the presence of I/N in patients with ASBO and suggested that a PCT greater than 1.0 ng/ml should be indicative of surgical management due to this association [27]. Nonetheless, this study was exclusive for patients with ASBO including those managed with medical treatment and surgery; we got some false negatives, which we believe were from taking PCT every 24 hours or maybe because we granted that ABSO that didn’t require surgery had no I/N. In the current study, we only included patients with IO who required surgical intervention, meaning we were certain of the presence/ absence of I/N and we attempted to reduce false negatives by taking the last sample of PCT immediately before surgery knowing the report that recommended taking PCT more frequently.
The objective of the present study was to include only subjects who required surgery with any cause of IO, to justify the association of PCT and I/N and to determine if PCT threshold greater than 1.0 ng/ml is still the most accurate to predict I/N.
Materials and Methods
A prospective, longitudinal, monocentric, comparative clinical study was carried out.
Population
Consecutive patients between 0 and 18 years of age with a diagnosis of IO (bilious vomiting, abdominal distension and absence of stools) were included, corroborated by abdominal X-ray (distended loops, air levels and absence of gas below obstruction), treated with surgery in a tertiary level pediatric hospital between July 2018 and November 2019. Exclusions were patients who did not undergo surgery because the intestine conditions could not be evaluated, as well as those who were on antibiotic treatment since they do not elevate PCT as expected and those who had sepsis because PCT is regularly elevated [28]. We eliminated patients who did not authorize PCT sample recollection and those in which PCT sample processing was poor.
Study design
In our institution, patients with ASBO undergo surgery when conservative treatment (fasting, gastric tube and IV solutions) fails after 72 hours, in presence of acute abdomen (defined by the evaluating surgeons based on severe abdominal pain, often with associated nausea or vomiting and ill appearance. At the physical exam, abdominal pain and signs of peritoneal irritation (rebound tenderness and guarding). Surgical treatment is indicated immediately in a newborn presenting with IO and no prior abdominal surgery. In the case of intussusception, reduction by enema is performed except in cases of acute abdomen or if intussusception is present for more than 24 hours, in which case, they are managed surgically, according to the protocol from our hospital. Similarly, a serum PCT is taken at admission in all patients with IO and then every 24 hours until resolution of the condition or if surgery is indicated. In the present study, if the patient had a PCT measurement longer than 12 hours before surgery, a new PCT sample was obtained in the operating room before incision (ORPCT), with the prior consent of the parents. If the last PCT sample was within 12 hours before the surgery, that measurement was considered ORPCT.
PCT assay
2 ml of blood were taken in a heparin free tube for each sample, which was centrifuged and the serum was used to measure PCT levels by a quantitative CLIA assay (LIAISON® BRAHMS PCT® II GEN). Taking our previous report as a reference, the normal reference value for PCT was considered <1.0 ng/ml and a PCT level ≥ 1.0 ng/ml was considered elevated.
Main result
Final condition of the intestine (presence or absence of I/N). The presence of I/N was divided into 2 groups: Intestinal ischemia and intestinal necrosis.
Assessment of the main outcome
The presence of I/N was evaluated during surgery: If the occluded bowel had normal color and adequate perfusion it was defined as a normal intestine. Intestinal ischemia was diagnosed when the bowel proximal to the obstruction was edematous, violaceous, and with pulse present. Intestinal necrosis was considered when the intestine was white or blackish, with no pulse present in the mesentery. In cases of necrosis, the intestine was resected and necrosis was confirmed by histopathology. In ischemia cases, the cause was corrected, warm towels were placed over the intestinal loops and bowel condition was reevaluated 10 minutes later. If the intestine recovered, the procedure was completed. If there was no recovery, it was classified as necrosis and the affected intestine was resected, proceeding to perform an anastomosis or intestinal diversion (same as in necrosis cases).
Statistical analysis
For descriptive quantitative variables we used median and Interquartile Range (IQR), for the comparative analysis Mann-Whitney U test was used and for the categorical variables χ2 chi-squared test or Fisher's exact test was applied. For the comparison of the last PCT measure vs. the ORPCT, we used the Wilcoxon test. We considered statistical significance at p<0.05 and when a variable had a statistically significant association with I/N, the effect size was calculated using Relative Risk (RR) and the 95% Confidence Interval (CI). A multivariate analysis was performed with logistic regression including the variables associated with I/N in the univariate analysis.
To determine the threshold for I/N with data from this study, we performed Receiver Operating Characteristic (ROC) curves and calculated the Area Under the ROC Curve (AUROCC). Predictive values are presented with sensitivity, specificity and positive and Negative Predictive Values (PPV and NPV). The statistical analysis was carried out through the statistical program SPSS ® (Statistical Package for the Social Sciences) 26.0 version.
Results
Of 112 patients included, 55 patients were excluded. 41 were managed conservatively, 13 had sepsis and/or received antibiotics and one was due to poor processing of the PCT sample, leaving a study sample of 57 patients. 38 patients (66%) were male and 19 (33%) were females. The infant group got the highest prevalence with 33% and the main cause of intestinal obstruction was secondary to ASBO (66%) followed by intussusception (17%). All patients were followed up until discharged.
In 36 patients (63%) the intestine was considered normal, in 7 (12%) there was ischemia and in 14 (24%) necrosis corroborated by histopathology, with an I/N incidence of 36%. There were no differences between sex or age (p=0.70 and p=0.79 respectively).
Through a univariate analysis of variables associated with I/N, we found that elevated PCT was the variable that had the most association with I/N (p=<0.001, RR 16.28 (95% CI, 4.20-63.07). Other variables associated with I/N were acute abdomen and bandemia at admission (Table 1). In the multivariate analysis, only PCT level >1.0 ng/ml was statistically associated with I/N (OR=144.24 (95% CI, 12.65-1643.67), p<0.001).
| Variable | Sub variable | I/N (n=21) | Normal intestine (n=36) | P value | RR | CI 95% |
|---|---|---|---|---|---|---|
| Abdominal pain at physical exam* | Yes | 17 (81%) | 27 (75%) | 0.60 | 1.25 | 0.51-3.07 |
| No | 4 (19%) | 9 (25%) | ||||
| Fever* | Yes | 4 (19%) | 5 (14%) | 0.60 | 1.25 | 0.55-2.86 |
| No | 17 (81%) | 31 (86%) | ||||
| Acute abdomen* | Yes | 10 (48%) | 3 (8%) | <0.001 | 3.07 | 1.70-5.56 |
| No | 11 (52%) | 33 (92%) | ||||
| 20 ml/kg fluid bolus given** | Yes | 11 (52%) | 18 (50%) | 0.86 | 1.06 | 0.53-2.09 |
| No | 10 (48%) | 18 (50%) | ||||
| Vasopressor therapy | Yes | 1 (5%) | 2 (5%) | 0.89 | 0.90 | 0.17-4.62 |
| No | 20 (95%) | 34 (95%) | ||||
| Metabolic acidosis° | Yes | 5 (24%) | 6 (17%) | 0.51 | 1.30 | 0.61-2.79 |
| No | 16 (76%) | 30 (83%) | ||||
| Leukocytosis | Yes | 10 (48%) | 18 (50%) | 0.86 | 0.94 | 0.47-1.86 |
| No | 11 (52%) | 18 (50%) | ||||
| Bandemia*** | Yes | 11 (52%) | 7 (19%) | 0.01 | 2.38 | 1.24-4.56 |
| No | 10 (48%) | 29 (81%) | ||||
| Hyperlactatemia° | Yes | 7 (33%) | 10 (28%) | 0.65 | 1.17 | 0.58-2.38 |
| No | 14 (67%) | 26 (72%) | ||||
| PCT>1.0 ng/ml | Yes | 19 (90%) | 2 (6%) | <0.001 | 16.28 | 4.20-63.07 |
| No | 2 (10%) | 34 (94%) | ||||
| Note: *After the placement of a nasogastric tube and intravenous fluids administration for more than 8 hours, **Crystalloid solution, *** >10% of the leukocytes count, °Lactic acid >2.0 mmol/L in a blood sample, RR=Relative Risk, CI=Confidence Interval. | ||||||
Table 1: Physical, laboratory data and treatment need during hospitalization for IO and their association with Ischemia or Necrosis of the intestine (I/N).
Serum PCT level
Regarding PCT values, the median was statistically higher in patients with I/N compared to those patients with normal intestine: 4.13 (13.9) vs. 0.11 (0.28) ng/ml, p=<0.001. Likewise, it was also higher in patients with necrosis, compared with patients with ischemia: 8.76 (19.67) vs. 1.50 (1.02) ng/ml, p=0.01. Additionally, the median of the ORPCT was statistically higher than the previous one, but only in the patients with I/N: 4.14 (13.9) vs. 0.5 (0.47) ng/ml, p=0.008.
Taking the PCT threshold >1.0 ng/ml as positive for the last measurement taken before surgery and relating it to I/N, we obtained a sensitivity of 90%, specificity of 94%, PPV of 90% and NPV of 94%.
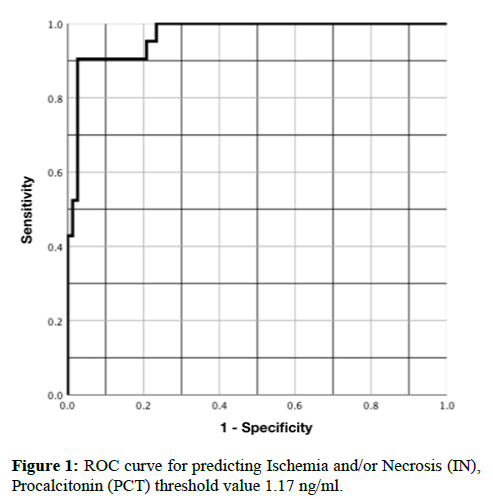
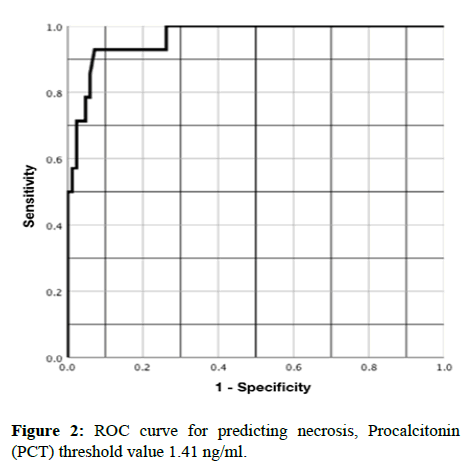
Finally, using a ROC curve, the AUROCC for PCT and I/N was 0.96 (95% CI, 0.91-1.00), with a PCT threshold >1.17 ng/ml and yielded a sensitivity of 90%, specificity of 97%, PPV of 95% and NPV of 94%, p=<0.001, RR 17.57 (95% CI, 4.54-67.90) (Figure 1). In the same way, using a ROC curve only for the patients with necrosis, the AUROCC was 0.94 (95% CI, 0.89-1.00), with a PCT threshold >1.41 ng/ml and yielded a sensitivity of 92%, specificity of 88, PPV 72% and NPV of 97%, p=<0.001, RR 28.16 (95% CI, 3.98-119.12) (Figure 2 and Table 2).
| Final state of the intestine | PCT threshold ng/ml | Sensitivity | Specificity | PPV | NPV | p value | RR | IC 95% RR |
|---|---|---|---|---|---|---|---|---|
| Ischemia or Necrosis (I/N) | 1.17 | 90% | 97% | 95% | 94% | <0.001 | 17.57 | 4.54-67.90 |
| Only necrosis | 1.41 | 92% | 88% | 72% | 97% | <0.001 | 28.16 | 3.98-119.12 |
Table 2: Predictive values of PCT threshold for I/N or only necrosis.
Discussion
I/N are serious conditions that can occur in any patient with IO and timely identification is fundamental when deciding to do surgery and its timing, all affecting the treatment and prognosis of these patients. As of this moment, there is not a gold standard for the early identification of I/N, however, elevated PCT has shown a clear association with this complication.
There are several studies that show many factors such as pain, fever, acute abdomen, leukocytosis, bandemia or lactic acidosis associated with I/N. In our univariate analysis, we found an association of elevated PCT, acute abdomen or bandemia with I/N. The highest RR was found when using only elevated PCT. In the multivariate analysis, only PCT level >1.0 ng/ml was statistically associated with I/N.
The increase in PCT has shown significant value in the identification of I/N, mainly in patients with ASBO, reporting sensitivities of 72%-100%, specificities of 73%-91%, PPV of 40%-89% and NPV of 80%-92%, although the reported PCT positive threshold ranges from 0.25 ng/ml to 2 ng/ml. In the present study, we only included patients who required surgical management, which gives more accuracy because we could observe macroscopically and microscopically (in patients who had undergone bowel resection the real status of the bowel, differing from our previous study where patients who responded successfully to medical treatment were qualified as with the absence of I/N which can be a source of bias. The prevalence of I/N was 36%, which is like those reported in other series, which makes our diagnostic and predictive values reliable [29-32].
We demonstrated, like Cosse, that serum PCT value in patients with I/N is significantly higher compared to those with normal intestine and even gets higher in patients with necrosis compared to those with ischemia. Because of this, we believe we can argue that the greater the degree of intestinal suffering will raise the serum PCT value.
We observed that the ORPCT increased significantly compared to the last one taken longer than 12 hours before surgery and this was even more pronounced in patients with I/N, so it may be necessary to take PCT levels more frequently than every 24 hours, as suggested by Cosse who recommends measuring PCT every 6 hours. At our hospital, due to financial reasons, we are not able to take PCT as often.
Relative to the threshold of PCT to predict I/N, the ROC curve showed a level of 1.17 ng/ml, slightly higher than the one in our previous study, but with higher diagnostic values. In relation to the threshold to predict intestinal necrosis, we found a PCT value of >1.41 ng/ml to be the best one (p=<0.001 and RR 28.16).
The most important fact for a surgeon confronting the patient with IO is to suspect I/N since it may indicate surgery and avoid utter complications, we think that the threshold of 1.17 ng/ml should prevail with this intention. These values differ from the threshold of 0.57 ng/ml reported by Cosse to predict the need for surgical treatment in adults with ASBO. In another study, the same author reported a threshold for intestinal necrosis ≥ 2.47 ng/ml, higher than in our study, with similar PPV and lower NPV. We believe that, as more studies in children with IO are reported, it will be possible to advance in the knowledge of the best threshold to predict I/N or anticipate necrosis in these patients.
Fair to say, the only false positive case in the present study was a 14 year old patient with a history of gastroschisis who presented to the emergency room with acute abdomen and a PCT value of 3.0 ng/ml, at surgery a midgut volvulus was found and the bowel condition was referred to as congestive, but without ischemia. A detorsion of the volvulus was achieved. The patient did well, being discharged without incidents. We have no explanation for the reason for the false positive result, although the subjective appreciation of “congestive bowel without ischemia” in a patient with midgut volvulus could have been skewed.
We had 2 false negatives cases. The first was a patient with ASBO and a normal PCT value at admission, 24 and 48 hours later (0.05, 0.24 and 0.30 ng/ml respectively) who was taken to the operating room at 48 hours secondary to fever, and intestinal necrosis was found. The last PCT was considered ORPCT since it was taken 11 hours before the surgery. Given the progressive increase in the PCT level, conceivably if we had taken another PCT close to the surgery it could have been above the study threshold. The second patient, also with ASBO and normal PCT at diagnosis, 24 and 48 hours (0.3, 0.01, 0.01 ng/ml respectively) and ORPCT of 0.24 ng/ml, had intestinal ischemia that resolved with lysis of adhesions, without obvious explanation of the normal value of PCT in the presence of ischemia, which reflects that the level of PCT is not infallible.
One advantage of our study is that we included only patients who were operated on and the presence/absence of I/N was qualified by the surgeon in the operating room which improves the evaluation of the condition of the intestine. The limitations of this study are that the sample is still small, which explains the width in the confidence intervals reported, the last measure of PCT level (ORPCT) varied in relation to the time before surgery and the age and etiology of the intestinal obstruction groups are heterogeneous.
Conclusion
This study authenticates similar findings about the use of PCT, corroborates its association with I/N in pediatric patients with IO and expands the evidence of its use in this field and can be reproduced in any pediatric center that has the possibility of measuring the level of PCT. We suggest a PCT threshold of >1.17 ng/ml and >1.41 for predicting I/N and intestinal necrosis respectively. The best frequency with which to take PCT for these purposes should be investigated in children.
Acknowledgments
We hereby acknowledge “Dicipa®” Laboratories for collaborating in the study by supplying the necessary reagents for the measurements of PCT in patients’ serum.
Ethical Considerations
All parents of patients who required surgery were informed about the need for an extra sample for PCT in the operating room and written consent was obtained if they agreed. Those that did not consent were eliminated from the study.
Conflicts of Interest
The authors have no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not for profit sectors.
References
- Cappell MS, Batke M (2008) Mechanical obstruction of the small bowel and colon. Med Clin North Am 92: 575-597. [Crossref] [Google Scholar] [PubMed]
- Wilson H, Hardy JD, Farringer JL (1955) Intestinal obstruction. I. causes and management in infants and children. Ann Surg 141: 778-791. [Crossref] [Google Scholar] [PubMed]
- Adamou H, Magagi IA, Habou O (2017) Acute mechanical intestinal obstruction in children at zinder national hospital, Niger: Aetiologies and prognosis. Afr J Paediatr Surg 14: 49-52. [Crossref] [Google Scholar] [PubMed]
- Stehr W, Gingalewski CA (2012) Other causes of intestinal obstruction. 7th edition, Elsevier, Philadelphia, 1127-1134.
- Ikeda H, Matsuyama S, Suzuki N (1993) Small bowel obstruction in children: Review of 10 years’ experience. Acta Paediatr Jpn 35: 504-507. [Crossref] [Google Scholar] [PubMed]
- Hyak J, Campagna G, Johnson B (2019) Management of pediatric adhesive small bowel obstruction: Do timing of surgery and age matter? J Surg Res 243: 384-390. [Crossref] [Google Scholar] [PubMed]
- Chang YJ, Yan DC, Lai JY (2017) Strangulated small bowel obstruction in children. J Pediatr Surg 52: 1313-1317. [Crossref] [Google Scholar] [PubMed]
- Zielinski MD, Eiken PW, Bannon MP (2010) Small bowel obstruction who needs an operation? A multivariate prediction model. World J Surg 34: 910-919. [Crossref] [Google Scholar] [PubMed]
- Montagnana M, Danese E, Lippi G (2018) Biochemical markers of acute intestinal ischemia: Possibilities and limitations. Ann Transl Med 6: 341. [Crossref] [Google Scholar] [PubMed]
- Di Saverio S, Coccolini F, Galati M (2013) Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2013 update of the evidence based guidelines from the world society of emergency surgery ASBO working group. World J Emerg Surg 8: 42. [Crossref] [Google Scholar] [PubMed]
- Maung AA, Johnson DC, Piper GL (2012) Evaluation and management of small bowel obstruction: An Eastern association for the surgery of trauma practice management guideline. J Trauma Acute Care Surg 73: S362-S369. [Crossref] [Google Scholar] [PubMed]
- Moya F, Nieto A, R-Candela JL (1975) Calcitonin biosynthesis: Evidence for a precursor. Eur J Biochem 55: 407-413. [Crossref] [Google Scholar] [PubMed]
- Picariello C, Lazzeri C, Valente S (2011) Procalcitonin in acute cardiac patients. Intern Emerg Med 6: 245-252. [Crossref] [Google Scholar] [PubMed]
- Meisner M (2014) Update on procalcitonin measurements. Ann Lab Med 34: 263-273. [Crossref] [Google Scholar] [PubMed]
- Blijlevens NM, Donnelly JP, Meis JF (2000) Procalcitonin does not discriminate infection from inflammation after allogeneic bone marrow transplantation. Clin Diagn Lab Immunol 7: 889-892. [Crossref] [Google Scholar] [PubMed]
- Assicot M, Gendrel D, Carsin H (1993) High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341: 515-518. [Crossref] [Google Scholar] [PubMed]
- Stocker M, Van Herk W, El Helou S (2017) Procalcitonin guided decision making for duration of antibiotic therapy in neonates with suspected early onset sepsis: A multicentre, randomised controlled trial (NeoPIns). Lancet 390: 871-881. [Crossref] [Google Scholar] [PubMed]
- Wacker C, Prkno A, Brunkhorst FM, Schlattmann P (2013) Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect Dis 13: 426-435. [Crossref] [Google Scholar]
- Vouloumanou EK, Plessa E, Karageorgopoulos DE, Mantadakis E, Falagas ME (2011) Serum procalcitonin as a diagnostic marker for neonatal sepsis: A systematic review and meta-analysis. Intensive Care Med 37: 747-762. [Crossref] [Google Scholar] [PubMed]
- Ayten R, Dogru O, Camci C (2005) Predictive value of procalcitonin for the diagnosis of bowel strangulation. World J Surg 29: 187-189. [Crossref] [Google Scholar] [PubMed]
- Markogiannakis H, Memos N, Messaris E (2011) Predictive value of procalcitonin for bowel ischemia and necrosis in bowel obstruction. Surgery 149: 394-403. [Crossref] [Google Scholar] [PubMed]
- Cosse C, Regimbeau JM, Fuks D (2013) Serum procalcitonin for predicting the failure of conservative management and the need for bowel resection in patients with small bowel obstruction. J Am Coll Surg 216: 997-1004. [Crossref] [Google Scholar] [PubMed]
- Cosse C, Sabbagh C, Rebibo L (2014) Kinetics of procalcitonin in the management of small bowel obstruction: A preliminary report. Surgery Curr Res 4: 184. [Crossref] [Google Scholar]
- Cosse C, Sabbagh C, Browet F (2015) Serum value of procalcitonin as a marker of intestinal damages: Type, extension and prognosis. Surg Endosc 29: 3132-3139. [Crossref] [Google Scholar] [PubMed]
- Cosse C, Sabbagh C, Carroni V (2017) Impact of a procalcitonin based algorithm on the management of adhesion related small bowel obstruction. J Visc Surg 154: 231-237. [Crossref] [Google Scholar] [PubMed]
- Evaristo-Mendez G, Gallegos-Sierra C, Cruz-Temores S (2019) Predictive value of procalcitonin for the need of surgery and the presence of ischemia and necrosis in patients with small bowel obstruction. Cir Cir 87: 45-52. [Google Scholar]
- Bracho-Blanchet E, Dominguez-Muñoz A, Fernandez-Portilla E (2017) Predictive value of procalcitonin for intestinal ischemia and/or necrosis in pediatric patients with Adhesive Small Bowel Obstruction (ASBO). J Pediatr Surg 52: 1616-1620. [Crossref] [Google Scholar] [PubMed]
- Baer G, Baumann P, Buettcher M (2013) Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): A randomized controlled trial. PLoS One 8: e68419. [Crossref] [Google Scholar] [PubMed]
- Stocker M, Van Herk W, El Helou S, Dutta S (2017) Procalcitonin guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: A multicenter randomized controlled trial (NeoPIns). Lancet 390: 871-881. [Crossref] [Google Scholar] [PubMed]
- Gürleyik E, Gürleyik G (1998) Small bowel volvulus: A common cause of mechanical intestinal obstruction in our region. Eur J Surg 164: 51-55. [Crossref] [Google Scholar] [PubMed]
- Tanaka S, Yamamoto T, Kubota D (2008) Predictive factors for surgical indication in adhesive small bowel obstruction. Am J Surg 196: 23-27. [Crossref] [Google Scholar] [PubMed]
- Kuehn F, Weinrich M, Ehmann S (2017) Defining the need for surgery in small bowel obstruction. J Gastrointest Surg 21: 1136-1141. [Crossref] [Google Scholar] [PubMed]
Citation: Dominguez A, Perez R, Fernandez E, Ortega I, Lopez A, et al. (2023) Role of Procalcitonin in the Timely Detection of Ischemia and Necrosis in Children with Intestinal Obstruction. J Gastrointest Dig Syst 13:731
Copyright: © 2023 Dominguez A, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2483
- [From(publication date): 0-2023 - Oct 31, 2025]
- Breakdown by view type
- HTML page views: 2120
- PDF downloads: 363