Role of Novel Hole Technology in Fast Dissolving Tablets
Received: 10-Mar-2014 / Accepted Date: 29-Mar-2014 / Published Date: 05-Apr-2014 DOI: 10.4172/2329-9053.1000R1-001
Abstract
This review is concerning Fast dissolving tablets ready by Novel Hole technology. Once these Fast dissolving tablets contact with gastric fluids, the fluid can enters the hole formed within the tablet and immediate breaking of the tablet goes to takes place. This quick disintegration of tablets is additionally influenced by the formation of latest absolute space. This method is novel and most helpful for formulation into Fast dissolving tablets.
Keywords: Fast Dissolving Tablets; Hole technology
1818Introduction
A solid dosage form that dissolves or disintegrates speedily in oral fissure, leading to solution or suspension without the necessity of water is understood as quick dissolving tablet or oral dispersing indefinite quantity dosage [1]. The thought of Fast dissolving drug delivery system (FDDS) emerged from the will to supply patient with a lot of standard suggests that of taking their medication [2]. It's troublesome for several patients to swallow tablets and hard gelatin capsules. Hence, they are doing not fits prescription, which ends up in high incidence of non-compliance and ineffective medical care. In some cases like ailment, fulminant episodes of allergic attacks or coughing and inaccessibility of water, swallowing standard tablet is also troublesome. Significantly the problem is older by medical specialty and geriatric patients. Such issues will be resolved by suggests that of FDTs [3]. Bioavailability of a drug depends on absorption of the drug that is littered with solubility of the drug in canal fluid and permeableness of the drug across permeability membrane. The solubility of drug principally depends on physical-chemical characteristics of the drug. However, the speed of drug dissolution is greatly influenced by disintegration of the tablet. The drugs which dissolves at a slower rate from a non-disintegrating tablet attributable to exposure of limited surface area to the fluid.
When we place FDTs on tongue, this tablet disintegrates releasing, cathartic the drug that dissolves or disperses within the saliva. Some medications are absorbed from the mouth, pharynx and esophagus because the saliva passes down into the stomach. In such cases, bioavailability of drug is considerably bigger than those ascertained from standard tablet dosage form [4].
The fast-dissolving property of the tablet is because of a fast ingress of water into the tablet matrix leading to its fast disintegration. Hence, the essential approaches to developing FDTs embody maximizing the porous structure of the tablet matrix, incorporating the appropriate disintegrating agent and using extremely water- soluble excipients within the formulation [5].
Orally disintegrating tablets also are known as Orodispersible tablet [6], fast disintegrating tablet, mouth dissolving tablet, quick disintegrating tablet, quick dissolving tablet, fast dissolving tablet, porous tablets and repimelts. USP approved these dosage forms as ODTs. EP has used the term Orodispersible tablet for tablets that disperse speedily and within three min in mouth before swallowing. United States Food and Drug Administration outlined ODTs as “A solid dosage form containing medicative substances or active ingredient that disintegrates speedily typically inside a matter of seconds once placed upon a tongue.” The Disintegration time for ODTs usually ranges from many seconds to a few min.
Formulation Aspects In Developing FDTs
FDTs are formulated by utilizing various processes, which differ in their methodologies and the FDTs vary in various properties such as,Mechanical strength of tablet, Drug and dosage form stability, Taste and mouth feel, Rate of dissolution of drug formulation in saliva swallow ability, Rate of absorption from the saliva solution and Overall bioavailability [7-12].
Various technologies used in the manufacture of FDTs include
1. Freeze–drying or Lyophilization,
2.Molding
3. Spray drying
4. Direct compression
5. Compaction
6. Cotton candy process
7. Mass-extrusion
Freeze-drying or Lyophilization
Lyophilization may be used to prepare tablets that have terribly porous open matrix network into that saliva rapidly moves to disintegrate freeze-dried mass when it's placed in mouth. The drug is entrapped in an exceedingly water soluble matrix that is freeze dried to provide a unit that rapidly disperses in mouth.
Advantage: ODT prepared by Lyophilization, which are extremely porous and improved absorption and bioavailability.
Molding
In this technology, soluble ingredients are used, so that tablet disintegrates and dissolves fastly. The powder mix is moistened with a hydro alcoholic solvent and is shaped in to tablet by the use of compression pressure lower than that employed in comparison of conventional tablets. The solvent is then removed by air-drying. Molded tablets have a porous structure that enhances dissolution. Two issues unremarkably encountered are mechanical strength and poor taste masking characteristics.
Spray drying
Spray drying could be a method by those extremely porous, fine powders may be made. This method relies on a particulate support matrix that is ready by spray drying an aqueous composition containing support matrix and different components to make an extremely porous and fine powder. This is often then mixed with active ingredients and compressed into tablets.
Direct compression
In this methodology, tablets are compressed directly from the mixture of the drug and excipients with none preliminary treatment. The mixture to be compressed should have adequate flow properties. A sort of disintegrant and its proportion are of prime importance. The opposite factors to be thought-about are particle size distribution, contact angle, pore size distribution, the tablet hardness and water absorption capability.
Addition of disintegrants
In several FDT technologies supported direct compression, the disintegrants primarily have an effect on the speed of disintegration and hence the dissolution. Disintegrants like microcrystalline cellulose, cross linked carboxy methyl cellulose sodium, cross linked polyvinyl pyrrolidone and part substituted hydroxypropyl cellulose are water insoluble however absorb water and swell as a result of capillarity and are considered as effective disintegrants within the preparation of FDTs. FDT may also be achieved by incorporating effervescent disintegrating agents, that generates CO2.
Sugar-based excipients
Another approach to quick dissolving tablets by direct compression is that the use of sugar-based excipients (e.g., dextrose, fructose, isomalt, maltitok, maltose, mannitol, sorbitol, starch hydrolyse, polydextrose, and xylitol), that show high aqueous solubility and sweetness and hence, impart taste masking and a satisfying mouth feel.
Compaction
Melt granulation
In this method hydrophilic waxy binders are used. It not only acts as a binder and increases physical resistance of tablets, but also helps disintegration of tablets as it melts in mouth and solubilizes rapidly leaving no residue.
Phase transition process
Tablets were produced by compressing a powder containing two sugar alcohols with high and low melting points and subsequent heating at a temperature between their melting points. Before heating process, tablet does not have sufficient hardness because of low compatibility. The tablet hardness was increased after heating process, due to increase of inner particle bonds or the bonding surface area in tablets induced by phase transition of lower melting point sugar alcohol.
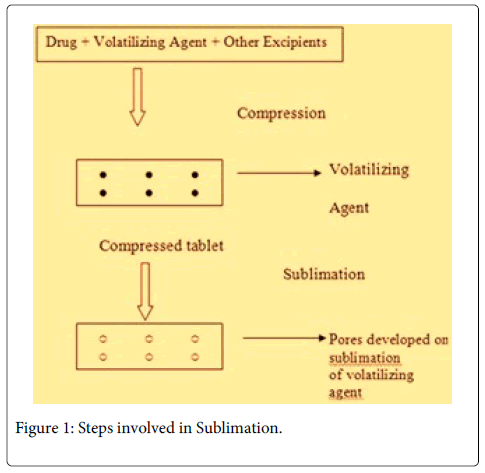
Sublimation
The basis of this technique is to add inert solid ingredients that volatilize readily, (e.g. camphor, ammonium bicarbonate, naphthalene, urea, urethane and pthalic anhydride etc.) to other tablet excipients and the mixture is then compressed into tablets. Volatile material is then removed via sublimation, which generate a porous structure (Figure 1) [13].
Cotton candy process
The cotton candy process is also known as the “candy floss” and forms the basis of the technologies such as Flash Dose (Fuisz technology). ODT is formed using a candy floss or shear form matrix; the matrix is formed from saccharides or polysaccharides processed into amorphous floss by a simultaneous action of flash melting and centrifugal force. The candy floss can then be milled and blended with active ingredients and other excipients and subsequently compressed into ODT.
Mass extrusion
This technology involves softening the active blend using the solvent mixture of water-soluble polyethylene glycol, by the use of methanol and expulsion of softened mass through the extruder or syringe to get a cylinder of product.
Role Of Novel Hole Technology In Fast Dissolving Tablets
The main objective of this technology is to design and development of Fast dissolving tablets by novel hole technology. It is a novel approach to decrease the disintegration time and increase the patient compliance. By using this technology absolute surface area of the tablet increase due to hole formation [14,15]. Immediate breaking of the tablet takes place due to the fluid enters into the hole formed in the tablet.
Several technologies were developed to enhance the disintegration time but the tablets prepared by hole technology [15] have increased surface area due to formation of hole and increased pore structure. The main principle involved in hole technology is sublimation [13]. Highly volatile ingredients like ammonium bicarbonate, ammonium carbonate, benzoic acid, camphor, naphthalene, urea, urethane and phthalic anhydride may be compressed along with other excipients into a tablet. This volatile material is then removed by sublimation leaving behind a highly porous matrix. Tablets manufactured by this technique have reported to usually disintegrate in 10-20 s.
The tablets prepared with hole technology showed all the parameters like hardness, friability, weight variation within the limits. All the formulations with increased concentrations of super disintegrants showed better drug release compared to the formulations with less concentration of super disintegrants.
Preparation of fast dissolving tablets by Direct compression method
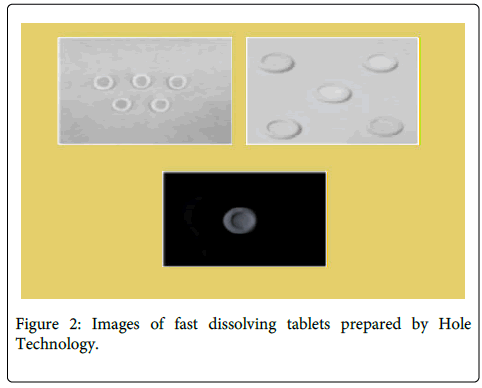
Preparation of FDTs by novel hole technology All the ingredients are weighed accurately and taken. Plain 100 mg camphor tablets were prepared by taking plain camphor granules and compressed into tablets. In the next step, Drug, excipient and super disintegrant were mixed in a plastic container. Magnesium stearate and Talc were passed through sieve # 60 mixed and blend with initial mixer in the plastic container. This mixer is then placed in the die cavity and at the center of the die cavity, previously compressed camphor tablets were kept then compressed into tablets. These tablets containing tablet in tablet. i.e. Camphor tablet is present in Drug containing tablet. After compression, these tablets were dried at 60°C by keeping the tablets in a hot air oven until complete removal of camphor to make tablets with hole at the center leading to formation of extra absolute surface area (Figure 2) [14-16].
Evaluation of Tablets
Pre-compression parameters Characteristics like tapped density, bulk density, carr’s index, hausner’s ratio should be studied for powder blend of formulations which are ready to compress in to tablets. Post compression parameters: All the prepared tablets were subjected to various physical characteristics like Crushing strength, Friability, Thickness, Diameter, Hole depth, Disintegration time, Wetting time, Weight variation, Drug content [17-35].
Weight variation test: Weight variation test has to be done by weighing 20 tablets individually, by using electronic balance. Calculating the average weight and comparing the individual tablet weight to the average weight [21-23].
Tablet thickness: The thickness has to be measured by placing tablet between two arms of the Vernier calipers. 5 tablets were taken and their thickness has to be measured.
Tablet hardness: The tablet hardness, which is the force required to break a tablet in a diametric compression force. The hardness tester used in the study will be Monsanto hardness tester, which applies force to the tablet diametrically with the help of an inbuilt spring.
Tablet friability: The friability of the tablets has to be measured in a Roche friabilator (M/s. Elite Scientific & Equipment’s.). Tablets of a known weight (Wo) or a sample of 20 tablets are dedusted in a drum for a fixed time (100 revolutions) and weighed (W) again. Percentage friability was calculated from the loss in weight as given in equation as below.
The weight loss should not be more than 1%. Determination was made in triplicate. % Friability = 100 (Wo -W) / Wo.
In-vitro disintegration time: In the disintegration time study, the tablets are taken and introduced in each tube of disintegration apparatus, and the tablet rack of the disintegration apparatus has to be positioned into a 1 litre beaker containing 900 ml of distilled water and time of disintegration was recorded at 37 ± 2°C [23-28].
Wetting time study: In wetting time study a piece of tissue paper folded twice has to be placed in a petridish (with internal diameter 6.5 cm) containing 5 ml of distilled water. A tablet has to be placed on the paper and the time for complete wetting of the tablet is measured in seconds.
Drug content analysis: Total 10 tablets are weighed and powdered. The powder equivalent to Drug has to be taken and dissolved in Buffer. After that an aliquot of the filtrate has to be diluted and analyzed spectrophotometrically (Elite UV- 150 double beam spectrophotometer).
In-vitro characterization of prepared tablets: In-vitro disintegration time: In the disintegration time study, the tablets has to be taken and introduced in each tube of disintegration apparatus, and the tablet rack of the disintegration apparatus has to be positioned into a 1 litre beaker containing 900 ml of distilled water and time of disintegration has to be recorded at 37 ± 2°C.
In the wetting time study: In wetting time study a piece of tissue paper folded twice was placed in a petridish (with internal diameter 6.5 cm) containing 5 ml of distilled water. A tablet was placed on the paper and the time for complete wetting of the tablet was measured in seconds [36-44].
Drug content analysis: Total 10 tablets have to be weighed and powdered. The powder equivalent to drug has to be taken and dissolved in medium. After that an aliquot of the filtrate was diluted and analyzed spectrophotometrically (Elite UV- 150 double beam spectrophotometer) at predefined nm [38-40].
In-vitro release studies: The in-vitro dissolution study has to be carried out in the USP dissolution test apparatus (M/s Lab India (Model – DS 8000) type 2 (paddle). 900 ml of the dissolution medium has to be taken in vessel and the temperature was maintained at 37 ± 0.5°C. The speed of the paddle is set at 50 rpm. 5 ml of the dissolution medium has to be withdrawn and the same amount of fresh medium is replenished to the dissolution medium. The sample withdrawn has to be filtered and diluted with buffer prior to analysis in the UV Spectrophotometer (Elite UV- 150 double beam spectrophotometer) at 285 nm [40-44].
Calculation of dissolution parameters: The dissolution parameters like Dissolution Efficiency, T50 and T90 should be calculated. The drug release profiles of the tablets should studied includes zero order, First order, Higuchi square root of time model, Korsmeyer-peppas model, and Hixson-Crowell model [45-47].
Conclusion
The tablets prepared with Hole technology have all the parameters like hardness, friability, weight variation within the limits. All the formulations with increased concentrations of super disintegrants showing better drug release compared to the formulations with less concentration of super disintegrants. The formulations with Novel Hole Technology will increase the surface of tablets by hole formation. When these fast dissolving tablets contact with gastro intestinal fluids, the fluid will enters the hole present in the tablet and immediate breaking of the tablet is going to takes place.
References
- Prakash V, Maan S, Deepika, Yadav SK, Hemalatha et al. (2011) Fast disintegrating tablets: Opportunity in drug delivery system. J Adv Pharm Technol Res 2: 223-235.
- Ishikawa T, Watanabe Y, Utoguchi N, Matsumoto M (1999) Preparation and evaluation of tablets rapidly disintegrating in saliva containing bitter-taste-masked granules by the compression method. Chem Pharm Bull (Tokyo) 47: 1451-4.
- Sammour OA, Hammad MA, Megrab NA, Zidan AS (2006) Formulation and optimization of mouth dissolve tablets containing rofecoxib solid dispersion. AAPS PharmSciTech 7: E167-E175.
- Bhowmik D, Chiranjib B, Krishnakanth, Pankaj, Chandira RM, et al. (2009) Fast Dissolving Tablet: An Overview. Journal of Chemical and Pharmaceutical Research 1: 163-177.
- Siddiqui MN, Garg G, Sharma PK (2010) Fast Dissolving Tablets: Preparation, Characterization and Evaluation: An Overview. International Journal of Pharmaceutical Sciences Review and Research 4: 015.
- Schiermeier S1, Schmidt PC (2002) Fast dispersible ibuprofen tablets. Eur J Pharm Sci. 15: 295-305.
- Shrivastava M, Chourasiya D, Soni S, Patidar D, Jatav R, et al. (2012) Formulation and In-Vitro Evaluation of Mouth Dissolving Tablets of Phenytoin Sodium Using Different Disintegrating Agents. Int J Nov Drug Deliv Tech 2: 249-255.
- Prateek S, Ramdayal G, Kumar SU, Ashwani C, Ashwini G, et al. (2012) Fast Dissolving Tablets: A New Venture in Drug Delivery. Am. J. PharmTech Res 2: 4.
- Deepak Sharma (2013) Formulation Development and Evaluation of Fast Disintegrating Tablets of Salbutamol Sulphate for Respiratory Disorders. ISRN Pharmaceutics Volume 2013, Article ID 674507, 8 pages.
- Sharma D, Chopra R, Bedi N (2012) Development and Evaluation of Paracetamol Taste Masked Orally Disintegrating Tablets Using Polymer Coating Technique. International Journal of Pharmacy and Pharmaceutical Sciences 4: Suppl 3.
- Shukla, Dali, Chakraborty, Subhashism, Sanjay S, et al. (2009) Mouth Dissolving Tablets I: An Overview of Formulation Technology. Scientia Pharmaceutica 77: 309-326.
- Siddiqui, Nehal M, Garg G, Sharma PK (2010) Fast Dissolving Tablets: Preparation, Characterization And Evaluation: An Overview. International Journal of Pharmaceutical Sciences Review & Research 4: 87-96.
- Bhardwaj V, Bansal M, Sharma PK (2010) Formulation and evaluation of fast dissolving tablets of amlodipine besylate using different super disintegrants and camphor as sublimating agent. American-Eurasian Journal of Scientific Research 5: 264-269.
- Movva B, Kumar DL, Kumar MR (2013) Formulation and Evaluation of Fast Dissolving Tablets of Ranitidine Hydrochloride by Hole Technology. Asian J Pharm Clin Res 6: 143-147.
- Upendra K, Rao, Raghavendra NG (2011) Design and Development of Aceclofenac Fast Dissolving Tablets by Novel Hole Technology: A Novel Approach to Decrease the Disintegration Time and Increase the patient compliance. Drug Invention Today 3: 91-94.
- Peddi MG (2013) Novel Drug Delivery System: Liquid Solid Compacts. J Mol Pharm Org Process Res 1: 108.
- Madhavi BR, Murthy VSN, Rani AP, Kumar GD (2013) Buccal Film Drug Delivery System-An Innovative and Emerging Technology. J Mol Pharm Org Process Res 1: 107.
- Babu B, Meyyanathan SN, Gowramma B, Muralidharan S, Elango K, et al. (2012) Pharmacokinetic Evaluation of Newly Developed Oral Immediate Release and Sustained Release Dosage Forms of Losartan Potassium. J Bioequiv Availab 4: 121-127.
- Shakeel F, Mohammed SF, Shafiq S (2009) Comparative Pharmacokinetic Profile of Aceclofenac from Oral and Transdermal Application. J Bioequiv Availab 1: 013-017.
- Zheng C, Wang Y (2013) Prediction of Oral Bioavailability: Challenges and Strategies. J Bioequiv Availab 6: 10000e47
- Dutta AK, Ikiki E (2013) Novel Drug Delivery Systems to Improve Bioavailability of Curcumin. J Bioequiv Availab 6:1000172
- Balderas-Acata JI, RÃos-RogrÃguezBuenoEP, del Castillo-GarcÃa S, Espinosa-MartÃnez C , Burke-Fraga V, et al. (2011) Bioavailability of Two Coated-Tablet Formulations of a Single Dose of Pantoprazole 40 mg: An Open-Label, Randomized, Two-Period Crossover, Comparison in Healthy Mexican Adult Volunteers. J Bioequiv Availab 3: 1000063
- Valenzuela F, Davila G, Ibañez Y, Garcia L, Crownover P, et al. (2011) Relative Bioavailability of Chewable and Conventional Film-Coated Tablet Formulations of Sildenafil 100 mg in Healthy Volunteers Under Fasting Conditions. J Bioequiv Availab 3: 1000087
- Bragatto MS, dos Santos MB, Pugens Pinto AM, Gomes E, Angonese NT, et al. (2011) Comparison between Pharmacokinetic and Pharmacodynamic of Single-Doses of Furosemide 40 mg Tablets. J Bioequiv Availab 3: 191-197.
- Feleder EC, Yerino GA, Halabe EK, Carla S, Soledad G, et al. (2011) Single-Dose Bioequivalence of a New Fixed-Dose Combination Tablet Containing TenofovirDisoproxilFumarate and Lamivudine. J Bioequiv Availab 3: 236-243.
- Pechenkin MA, Balabushevich NG, Zorov IN, Staroseltseva LK, Mikhalchik EV, et al. (2011) Design, In Vitro and In Vivo Characterization of Chitosan-Dextran Sulfate Microparticles for Oral Delivery of Insulin. J Bioequiv Availab 3: 244-250.
- Palma-Aguirre JA, Mireya LG, de Jesus CST, Mariel PG, Elisa ZB, et al. (2010) Bioavailability of Two Oral Tablet Formulations of citalopram 20 mg: Single-Dose, Open-Label, Randomized, Two-Period Crossover Comparison in Healthy Mexican Adult Subjects. J Bioequiv Availab 2: 018-022
- Bapuji AT, VenkataRavikiran HL, Nagesh M, Syedba S, Ramaraju D, et al. (2010) Bioequivalence Testing - Industry Perspective. J Bioequiv Availab 2: 098-101.
- Kanfer I (2010) Strategies for the Bioequivalence Assessment of Topical Dermatological Dosage Forms. J Bioequiv Availab 2: 102-110.
- Junior EA, Duarte LF, PirasolVanunci ML, Teixeira ML (2010) Bioequivalence of Two Oral Contraceptive Drugs Containing Ethinylestradiol and Gestodene in Healthy Female Volunteers. J Bioequiv Availab 2: 125-130.
- Khattak S, Malik F, Hameed A, Ahmad S, Rizwan M, et al. (2010) Comparative Bioavailability Assessment of Newly Developed Flurbiprofen Matrix Tablets and Froben SR® Tablets in Healthy Pakistani Volunteers. J Bioequiv Availab 2: 139-144.
- Shakeel F, Ramadan W, Shafiq S (2009) Solubility and Dissolution Improvement of Aceclofenac using Different Nanocarriers. J Bioequiv Availab 1: 039-043.
- Roda A, Simoni P, Roda G, Caponi A, Pastorini E, et al. (2009) Pharmacokinetics and Safety of a New 1200 mg Single-Dose Delayed Release MesalazineMicrogranule Formulation. J Bioequiv Availab 1: 044-051.
- Lima R, Vasconcelos T, Cerdeira R, Lefebvre M, Sicard E, et al. (2009) Bioequivalence of Final Tablet Formulation and Research Tablet Formulation of Eslicarbazepine Acetate in Healthy Volunteers. J Bioequiv Availab 1: 093-098.
- Moreira RF, Rigato HM, Borges BC, Sverdloff CE, Oliveira RA, et al. (2009) Effect of Hyperlipemic Food on the ComparativeBioavailability of Two Bupropion Formulations after Administration of a Single Oral Dose of 150 mg in Healthy Human Volunteers. J Bioequiv Availab 1: 103-111.
- Teksin ZS, Agabeyoglu I, Yamac K (2009) Bioavailability of Pentoxifylline-Chitosan Oral Matrix Tablet in Healthy Subjects. J Bioequiv Availab 1: 115-120.
- Dewan B, Sahu N (2010) Bioequivalence Study of Troxipide Tablet Formulations. J Bioequiv Availab 2: 050-054.
- Zhang CL, Jiao JJ, Wu YN, Song JQ, Gao WZ, et al. (2011) Study on Pharmacokinetics and Bioequivalence of Cefdinir Dispersible Tablet in Healthy Chinese Volunteers. J Bioequiv Availab 3: 114-117.
- Feng Y, Wang N (2013) The New Generation of Drug Discovery and its Analytical Technologies. J Bioequiv Availab 5: e42.
- Al-Ghananaeem A (2013) Sublingual and Nasal Trans mucosal Drug Delivery for Breakthrough Pain: A Frontier in Cancer Therapy. J Bioequiv Availab 5: e29.
- Harahap Y, Budi Prasaja MM, Lusthom W, Hardiyanti, Azmi F, et al. (2013) Bioequivalence of Trimetazidine Modified Release Tablet Formulations Assessed in Indonesian Subjects. J Bioequiv Availab 5: 117-120.
- Nichols AI, Richards LS, Behrle JA, Posener JA, Fruncillo R, et al. (2013) Effects of Age and Sex on the Pharmacokinetics, Safety, and Tolerability of Oral Desvenlafaxine in Healthy Adults. J Bioequiv Availab 5: 088-094.
- Mascoli V, Kuruganti U, Bapuji AT, Wang R, Damle B (2013) Pharmacokinetics of a Novel Orodispersible Tablet of Amlodipine in Healthy Subjects. J Bioequiv Availab 5: 076-079.
- Nishant T, Kumar DS, Kumar A, Phaneendra M (2011) Role of Pharmacokinetic Studies in Drug Discovery. J Bioequiv Availab 3: 263-267.
- Ruiz A, Cuesta F, Parra S, Montoya B, Restrepo M, et al. (2012) Bioequivalence Evaluation of Two Formulations of Lamotrigine Tablets in Healthy Volunteers. J Bioequiv Availab 4: 030-034.
- Parthasarathi D, Gajendra C, Dattatreya A, Sree Venkatesh Y (2011) Analysis of Pharmacokinetic & Pharmacodynamic Models in Oral and Transdermal Dosage Forms. J Bioequiv Availab 3: 268-276.
- Wagh N, Gayakwad NJ, Christina AJM, Bhople A, Thakre A (2013) A Bioequivalence Study of Two Finofibrate Tablet Formulations in Indian Healthy Subjects. J Bioequiv Availab 5: 016-021.
Citation: Damodar R, Movva B, Vinay CV (2014) Role of Novel Hole Technology in Fast Dissolving Tablets. J Mol Pharm Org Process Res 2: R1-001. DOI: 10.4172/2329-9053.1000R1-001
Copyright: ©2014 Damodar R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17989
- [From(publication date): 4-2014 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 13235
- PDF downloads: 4754