Role of Nitric Oxide in the Paranasal Sinuses- "Demystifying the Aerocrine Organ"
Received: 07-May-2020 / Accepted Date: 07-Jul-2020 / Published Date: 14-Jul-2020 DOI: 10.4172/2161-119X.1000398
Abstract
The functions of paranasal sinuses have recently undergone a conceptual change in literature, with emerging clinical data highlighting the sinus cavity as an ‘Aerocrine Organ’. Nitric oxide (NO) is an important mediator and
inflammatory marker in human upper airways. High concentration of Nitric oxide produced within the paranasal sinuses by the respiratory epithelium, has proved to be protective against infection or allergy induced airway
inflammation. The term ‘ aerocrine ’ illustrates the airborne transport of nitric oxide as a protective biological messenger in the human respiratory tract from the nasal passages to the respiratory alveoli. NO plays the role of a
messenger between the upper and lower respiratory tracts, reducing pulmonary vascular resistance and facilitating alveolar oxygen transfer into the bloodstream. This has revolutionized the concept of nasal breathing controlling the entire pulmonary function and oxygen uptake in the lungs. This concept has found references in many of the breathing patterns described in ancient Indian yogic practices like the ‘Praanayama and Jal-Neethi'. Nitric oxide level in the paranasal sinuses, has been established today as a bio-marker of well being of the respiratory system and has found numerous clinical applications in the diagnosis and management of upper airway inflammation. Present day research is focused on prophylactic and therapeutic administration of NO in patients with chronic respiratory diseases.
Keywords: Paranasal Sinuses; Aerocrine Organ; Nitric Oxide; Chemiluminiscence
Keywords
Paranasal Sinuses; Aerocrine Organ; Nitric Oxide; Chemiluminiscence
Introduction
The paranasal sinuses (PNS) seem to develop after regression of the erythropoietic marrow in the maxillary, frontal and sphenoid bones and its replacement by cavities filled with gas, which escapes into the nasal fossae through the ostia. The first precise anatomic description of the maxillary sinus or “antrum” was made by Highmore in 1651 [1]. Traditionally, the paranasal sinuses have been described to have the physiological functions of aiding in respiration, nasal humidification, airway lubrication, providing resonation inputs during phonation and reducing weight of the human skull. The nasal airway and choncha / turbinales create an aerodynamic effect, favoring air entry into the sinuses during inhalation. But in general, the sinuses have an anatomically unfavorable position, wherein they lie in close proximity to the nasal cavity, which is heavily colonized by a myriad of potentially pathogenic bacteria and viruses. Although the nose can clear mucous freely in both directions, the sinuses are left with tiny ostia through which mucus and invading bacteria and viruses need to be drained. Position of the ostium in the maxillary sinus being at the top of the cavity, forces the drainage system to work against the laws of gravity.
The presence of NO in exhaled air was discovered in 1991by Gustafsson et al. [2]. It seemed logical to suppose a pulmonary alveolar origin associated with respiratory gas exchange (oxygen, carbondioxide), but the quantity exhaled in nasal respiration turned out to be far greater than in oral respiration, suggesting large-scale NO production within the nasal cavities [3]. The role of the upper airways in NO production was demonstrated by measuring exhaled NO in tracheotomized subjects at the cannula, mouth and nose: the levels were low in the cannula, intermediate in the mouth and high in the nose [4]. NO is thus proven to be mainly produced in the upper airway. The physiological role of NO was discovered in 1998 and its discoverers were awarded the Nobel Prize for Medicine. Previously, it had been seen as merely an atmospheric pollutant, with no biological role, but was then shown to be a powerful vasodilator, produced by blood vessel endothelial cells, that relaxes the smooth muscle fibers of the vascular wall. In fact, its physiological role extends to many other cell and tissue functions, notably in the respiratory, nervous and immune systems as well [4, 5].
Research has provided a new insight into the function of paranasal sinuses. Today the PNS is believed to play a major role as a reservoir of NO, a highly bio-active inflammatory signal molecule. Nitric oxide is an important mediator and inflammatory marker in human upper airways. Enzymes responsible for NO production (Nitric Oxide Synthetase Type 1 & 2) have been demonstrated both in the nose and in the paranasal sinuses. NO levels in the PNS are reported to be several times higher than those in the nose. It has been proved by invitro studies, that PNS are the primary sites for NO production in the upper respiratory tract [6].
Role of the Aerocrine Messenger
The discovery of NO gas production in the paranasal sinuses happened as early as 1995, at the research laboratory in Karolinska Institue, Stockholm, Sweden. Further research on the Aerocrine function of PNS, has altered the traditional explanations of sinus physiology, creating an impact both in basic science and clinical arenas. Extensive clinical study has been performed by non-invasively calibrating NO levels in the maxillary sinus and nose, using a continuous gas chemiluminescence technique in healthy volunteers and in patients with upper airway inflammation. NO was sampled, via a drainage tube inserted into the maxillary sinus and the peaks in NO level objectively recorded. The data confirmed extremely high NO levels in the paranasal sinuses suggesting that PNS function as reservoirs for NO [6].
Healthy paranasal sinus epithelium expresses an inducible NO synthase enzyme that continuously generates large amounts of NO, a pluripotent gaseous messenger with potent vasodilating, and antimicrobial activity. This NO can be measured noninvasively in nasally exhaled breath. The role of NO in the sinuses is likely to enhance local host defense mechanisms via direct inhibition of pathogen growth and stimulation of muco-ciliary activity [7]. The NO concentration in a healthy sinus exceeds those that are needed for antibacterial effects in-vitro. In patients with primary ciliary dyskinesia (PCD) and in cystic fibrosis, nasal NO is found to be extremely low. This defective NO generation, most likely contributes to the great susceptibility to chronic sinusitis in patients. In addition, the low-nasal NO is of diagnostic value especially in PCD, where nasal NO is very low or absent.
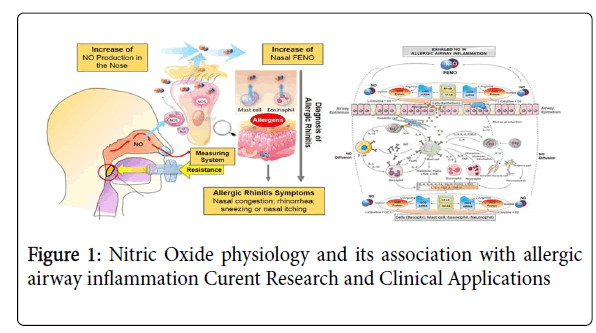
Intriguingly, NO gas from the nose and sinuses is also inhaled with every breath and reaches the lungs in a more diluted form to enhance pulmonary oxygen uptake via local vasodilation. Sinus NO thus appears to be a regulator of respiratory exchange and lung physiology, modulating the ventilation / perfusion ratio (VPR). The sinuses may be NO reservoirs releasing intermittent bolus according to ostial permeability [8]. If so, the rhythm, regulatory mechanisms and physiological role of such intermittent release remain to be determined. In this sense NO may be regarded as an "Aerocrine Hormone" that is produced in the sinuses and transported to a distal site of action with every inhalation [9] (Figure 1).
Current Research and Clinical Applications
NO has important functions in a variety of physiological and pathophysiological processes in the body, including vasoregulation, haemostasis, neurotransmission, immunity and respiration. The discovery of high concentrations of NO in the nasal airway and paranasal sinuses has important implications for the understanding of airway physiology. The high NO levels in the paranasal airways contribute to the first line of defense against microorganisms. Furthermore, auto-inhalation of NO may improve pulmonary function and other remote physiological processes. This airborne messenger system represents a new physiological concept of potential clinical importance. It has been found that nasal NO is altered in several airway disorders, including allergic rhinitis, PCD, cystic fibrosis, and sinusitis.
A multitude of investigations are currently underway to understand the nuances of the aerocrine system especially in cases of airway allergy, acute and chronic respiratory inflammation, and also in migraineous prodromes where allergy produces a tandem effect along with the vascular spasm in the sinuses. However, NO, like several other mediators, has a dualistic function. Airway NO levels are increased in airway inflammations, such as asthma and allergic rhinitis, but is reduced in cystic fibrosis and other conditions with ciliary dysfunction, sinusitis and after exposure to tobacco and alcohol. Consequently, NO may prove valuable as a non-invasive marker in the diagnosis and monitoring of numerous airway pathologies [10].
During quiet exhalation, mean output of nasal NO is estimated to be about 180 - 200 nL/min, while in patients with sinusitis it drastically falls to around 20 nL/min. An interesting fact is that 'humming' causes a more than tenfold increase in NO levels in the nasal cavity. This form of continuous phonation causes the air to oscillate, which in turn increases the exchange of air between the sinuses and nasal cavity [11]. Although the extent of such gas exchange in humans is largely unexplored, it is known that the oscillation of air occurring normally within the respiratory cycle facilitates the exchange of gases between the sinuses and the nasal cavity[12].
In conventional speech therapy, humming is generated by vibrating both arytenoids at the vocal apophysis with the mouth closed, the resulting sound resembles a cat purring, and should be produced without effort, at low flow-rate and pressure. Hence humming can be advocated as a mode of therapy for patients with partially occluded ostia and mild symptoms of sinusitis. But, in severe forms of sinusitis, there is total occlusion of ostia and humming does not cause any change in gas exchange within sinuses and the nitric oxide levels remain completely unchanged in such patients[13]. NO gas in inhaled air dilates blood vessels in the lung, leading to increased oxygen uptake and a reduction in pulmonary vascular resistance [14]. This may be used in providing respiratory therapy for long term ventilation patients. Currently, inhaled NO therapy is clinically approved for use in newborn children with persistent pulmonary hypertension [15].
Glucocorticoids, popularly used for a wide range of airway diseases, due to their potent anti-allergic & anti-inflammatory property, strongly down-regulate the Nitric Oxide Synthetase enzyme which is responsible for NO production in PNS. This possibly explains the reason behind the high incidence of mycotic infections in patients inadvertently on chronic steroid therapy, due to loss of protective function of high levels of NO within the sinuses. Thus, steroid therapy in upper airway inflammation has become a ‘double-edged sword’, since it provides immediate relief from airway hyperactivity, but in the long run predisposes to secondary airway infections.
Conclusion
Nitric Oxide produced in the paranasal sinuses is described as the outpost of all pulmonary anti-infectious defense mechanisms and has been established as the guardian and protector of upper airways. Inflammation of the airway reduces NO production within sinuses. Nitric Oxide estimation can be used as a sensitive diagnostic tool for confirmation of upper airway hyperactivity and inflammation. NO is also an ideal prognosticator to meticulously judge the response of upper airway to anti-inflammatory therapy. NO levels are of significant clinical value in predicting patients who are likely to benefit from antiinflammatory therapy with inhaled corticosteroids.
Expanding clinical applications have resulted in the invention of the exhaled nitric oxide (NO) monitoring system “NIOX”, which has been cleared by the US Food and Drug Administration since 2003 & is presently popular among the pulmonologists worldwide. The fractional concentration of exhaled NO has been extensively researched as a marker of airway inflammation in asthma and other upper airway diseases. NO is now poised to enter clinical applications in the realm of Otorhinolaryngology with investigators keen to introduce NO therapy in the management of a variety of rhinological diseases like sinonasal polyposis, refractory rhinosinusitis, mucociliary dyskinesia as in kartageners syndrome, cystic fibrosis, atrophic rhinitis, empty nose syndrome, obstructive sleep apnoea etc. Time is not far when nitric oxide respirators will be commercially available to support the therapy and convalescence of such patients.
References
- Â Lundberg JO. (2008) Nitric oxide and the paranasal sinuses. Anat Rec (Hoboken) 291: 1479-1484.
- Andersson J , Cervin A ‌, Lindberg S, Uddman R, ‌ Cardell LO. (2002) The Paranasal Sinuses as Reservoirs for Nitric Oxide. Acta Oto-laryngologica 122: 861-865.
- Menzel L, Hess A, Bloch W, Michel O, Schuster KD. (2005) temporal nitric oxide dynamics in the paranasal sinuses during humming. J Appl Physiol 98: 2064–2071.
- Dweik RA, Boggs PB, Erzurum SC. (2011) American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for clinical applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 184: 602–615.
- Lundberg JO, Szallasi F, Weitzberg E, Rinder J, Lidholm A, et al. (1995) High nitric oxide production in human paranasal sinuses. Nature Med 1: 370 - 373.
- Ragab SM, Lund VJ, Saleh HA, Scadding G. (2006) Nasal nitric oxide in objective evaluation of chronic rhinosinusitis therapy. Allergy 61: 717–724.
- Duong-Quy S. (2019) Clinical utility of the exhaled Nitric Oxide measurement with portable devices in the management of allergic airway inflammation and asthma. J Asthma Allergy 12: 331–341.
- Spahn JD, Malka J, Szefler SJ. (2016) Current application of exhaled nitric oxide in clinical practice. J Allergy Clin Immunol 138: 1296–1298.
- Orvath I, Barnes PJ, Loukides S. (2017) A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J 49.
- Djupesland P G, Chatkin J M, Qian W, Haight J S. (1999) Nitric oxide in the nose and paranasal sinuses-respiratory tract physiology in a new perspective. Tidsskr Nor Laegeforen 119: 4070-4072.
- Weitzberg E, Lundberg JON. (2002) Humming greatly increases nasal nitric oxide. Am J Respir Crit Care Med 166: 144-145.
- Belvisi MG, Ward JK, Mitchell JA, Barnes PJ. (1995) Nitric oxide as a neuro transmitter in human airways. Arch Int Pharmacodyn Ther 329: 97.
- Aust R, Falck B, Svanholm H. (1984) The intrinsic functions of the paranasal sinuses in health and inflammation. Rhinology 22: 105–107.
- Arnal JF, Flores P, Rami J, Murris-Espin M, Bremont F, et al. (1999) Nasal nitric oxide concentration in paranasal sinus inflammatory diseases. Eur Respir J 13: 307–312.
- Silkoff PE, Carlson M, Bourke T, Katial R, Ogren E, et al. (2004) The Aerocrine exhaled nitric oxide monitoring system NIOX is cleared by the US Food and Drug Administration for monitoring therapy in asthma. J Allergy Clin Immunol 114: 1241-1256.
Citation: Nair G, Nandhan R, Kameswaran M (2020) Role of Nitric Oxide in the Paranasal Sinuses -"Demystifying the Aerocrine Organ". Otolaryngol 10: 1000398. DOI: 10.4172/2161-119X.1000398
Copyright: © 2020 Nair G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License; which permits unrestricted use; distribution; and Role of Nitric Oxide in the Paranasal Sinuses - "Demystifying the Aerocrine Organ".
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3012
- [From(publication date): 0-2020 - Dec 20, 2024]
- Breakdown by view type
- HTML page views: 2340
- PDF downloads: 672