Role of Nicotinic Acid in Mitigating Methomyl Induced Acute Toxicity in Albino Rats
Received: 27-Feb-2016 / Accepted Date: 13-Apr-2016 / Published Date: 17-Apr-2016 DOI: 10.4172/2161-0681.1000268
Abstract
Methomyl has a significant animal and public health problem. The present study was designed to evaluate the protective effect of nicotinic acid against the acute toxic effects induced by methomyl in albino rats. Four groups were divided into control, nicotinic acid (NA) (100 mg/kg b.wt./twice daily) for 7 successive days, methomyl (MET) (8 mg/kg b.wt.) and nicotinic acid plus methomyl (NA+MET) treated groups. Red blood cells-RBCs count; hemoglobin- Hb, packed cell volume-PCV and blood indices were measured. Hepatic marker enzymes (aspartate aminotransferase-AST, alanine aminotransferase-ALT and alkaline phosphatase-ALP), proteinogram (total protein, albumin, globulin and albumin/ globulin ratio), serum bilirubin (total, direct and indirect) and serum urea and creatinine were measured. Acute MET toxicity resulted in normocytic normochromic anemia with a significant (P<0.05) increase in the AST, ALT and ALP activities. Serum protein and globulin were significantly (P<0.05) decreased with an increase in albumin globulin ratio (A/G ratio) in MET treated rats. Bilirubin (total and direct) and kidney markers were significantly (P<0.05) increased. The NA pretreatment showed an improvement in the erythrocytic parameters. It restored the hepatic and renal markers. This is the first study investigating the effects of NA supplementation on the hematological and biochemical parameters of methomyl intoxicated rats. These findings suggest that the nicotinic acid could be used in amelioration of the methomyl toxicity.
Keywords: Kidney; Liver; Methomyl, Nicotinic acid; Rats; Toxicity
312993Introduction
In the recent years, the use of pesticides in agriculture has been increasing to enhance the food production by eradicating unwanted insects and controlling disease vectors. The wide spread use of pesticides carries more occupational exposure, to high levels of these compounds, of agricultural and industrial workers as well as more contamination of food with pesticide residues [1].
Methomyl (MET) (Lannate1), S-methyl N-(methylcarbamoyloxy) thioacetimidate (C5H10N2O2S), is one of the most common pesticides which are used in the control of insects. It is used worldwide in agriculture and health programs. Besides its advantages in the agriculture, it causes several toxic effects [2]. Animals and human exposure occurs during spraying of flies and ingestion of food contaminated with methomyl [3]. The exposure to methomyl exerts neurodegenerative disorders, beside toxic actions on the liver, kidney, muscle and eye. It is suspected to be carcinogens and mutagens with high mortality rates [4].
Nicotinic acid (NA) is a member of the vitamin B family. It is well known for its functions in the treatment and prevention of atherosclerosis and other lipidmetabolic disorders due to decreasing plasma levels of low-density lipoprotein cholesterol. In recent years, NA is considered one of the most effective agents that offer the protection against the risk of cardiovascular problems. Despite extensive studies in the field of lipid metabolism, the effects of NA on other aspects of cellular physiology remain elusive. Nicotinic acid (NA) is metabolized to nicotinamide adenine dinucleotide (NAD), is a metabolite essential for cell survival, through the nicotinic acid phosphoribosyltransferase domain containing 1 (NAPRT1)–dependent salvage pathway.
Based on the above information, we hypothesize that nitric acid can reduce MET-induced hepatic and renal injuries. Since there are no published studies on the protection of nicotinic acid against methomyl toxicity, the present study was designed to explore the possible protective effect of nicotinic acid on methomyl-induced toxicity in rats.
Materials and Methods
Experimental animals
Thirty two female albino rats (150-200 g), four months old, were obtained from the Laboratory Animal House, Helwan, Egypt. All animals were kept in an environmentally controlled room with clean hygienic metal cages and maintained under a uniform laboratory condition. All animals were given balanced ration free from medication and drinking water was allowed ad.libitum throughout the experimental period. Rats were kept under standardized hygienic conditions (12 h light/dark cycles, the temperature was 23 ± 2°C and a minimum relative humidity of 44%) throughout the experimental period. All animals were acclimatized for 2 weeks before starting the experiment. The study was complied with the Animal Welfare Act and the National Research Council guidelines.
Chemicals
Nicotinic acid (NA) was purchased from Sigma Aldrich Corporation, St. Louis, Missuria, USA. Rats were orally given 100mg/kg b.wt., twice daily for 7 successive days before induction of acute toxicity [5].
Methomyl (MET) (Lannate1), S-methyl N-(methylcarbamoyloxy) thioacetimidate (C5H10N2O2S), 90% purity) was obtained from Shandong Huayang Technology Ltd. Company, China. All other analytical grade chemicals were purchased from Sigma Chemical Co. (Louis, Missuria, USA) was used to induce acute toxicity. Rats were orally given a single methomyl dose in water at a dose level of 8 mg/kg b.wt. [2].
Experimental design
Rats were segregated into four groups (8 rats each). Group I, served as normal control and was given water ad libitum, Group II, (NA treated) were administered nicotinic acid orally (100 mg/kg b.wt.) twice daily for 7 days. Group III, (MET treated) rats were given a single methomyl dose orally in water (8 mg/kg b.wt.). Group IV, (NA+ MET) animals were given both nicotinic acid and methomyl. Nicotinic acid was given 7 days before methomyl treatment. By the end of the experiment, 24 h after the methomyl administration, at the 8th day, the rats were sacrificed. Clinical signs, post mortem lesions and mortalities were recorded.
Blood sampling
Blood samples from all rats were collected, after light ether anesthesia, from the retro-orbital venous plexus of rats under sterile septic condition using a sterilized fine capillary 24 h after methomyl treatment. Blood was collected on EDTA tubes (dipotassium salt of ethylene diamine tertra acetic acid, Sigma Chemical Co, St. Louis, USA) for hematological analysis. The red blood cell profile included: red blood cells (RBC) count, hemoglobin (Hb), hematocrit (PCV) and the red blood cell indices (mean corpuscular volume -MCV, mean corpuscular hemoglobin-MCH and mean corpuscular hemoglobin concentration-MCHC) were performed immediatley after the collection of the blood. For serum samples, blood was allowed to flow smoothly into the tubes, left to clot for 2 hours at room temperature then centrifuged at 3000 rpm for 15 min. The clear supernatant serum was collected using sterile Pasteur pipettes. The collected serum was transferred to dry, sterile labeled eppendorf tubes for chemical analysis.
Hematological analysis
Total RBCs count was determined by Hayem’s fluid. Hematological assay was done manually using an improved Neubauer-ruled hemacytometer. Briefly, the whole blood was diluted 1:200 in Hayem’s fluid. Erythrocytes were counted in 5 secondary squares of the center primary square. The number of RBCs counted in the secondary squares was multiplied by 10,000 to calculate the total RBC count per microliter. Hemoglobin (Hb) was determined using the cyanomethemoglobin method (J. T. Baker, London, England) following manufacturer’s instructions. PCV was determined by using the microhematocrit centrifugation. Microcapillary tubes were filled, plugged with clay, and centrifuged at 10,000 rpm for 5 minutes. The PCV was determined using the built-in reader. RBC indices (MCV, MCH & MCHC) were calculated using standard formulas.
Biochemical estimations
All parameters were colorimetric measured using commercial kits provided by Biomerieux, Durham, NC, USA. All analysis was done using spectrophotometer Spekol 11 for biochemical serum analysis.
Serum enzymes: serum aspartate aminotransferase (AST) and serum alanine aminotransferase (ALT) activity were determined according to [6], serum alkaline phosphatase (ALP) activity according to [7].
Serum proteins: Serum total protein was colometrically determined [8]. The protein formed a colored complex with cupric ions in an alkaline medium is measured. Serum albumin was colometrically determined [9], a buffered solution bromocresol green forms with albumin, a green colored complex whose intensity is proportional to the amount of albumin present in the sample. Serum globulin level was determined by subtracting the albumin from the total proteins. A/G ratio for each sample was estimated using albumin and globulin values.
Serum bilirubin: Serum total and direct bilirubin were measured according to [10]. Serum indirect bilirubin was obtained from subtracting the obtained direct bilirubin level from the obtained total bilirubin.
Renal function markers in serum: The level of urea and creatinine in serum were calorimetrically measured according to [11] and [12] respectively.
Statistical analysis
Data were collected and analyzed by one-way analysis of variance ANOVA followed by Dunnett′s post hoc test. Statistical Package of the Social Science software (SPSS Inc., Chicago, IL, USA) was used. Data were presented as mean ± SE and significance was declared at (P<0.05).
Results
Toxic symptoms and postmortem lesions
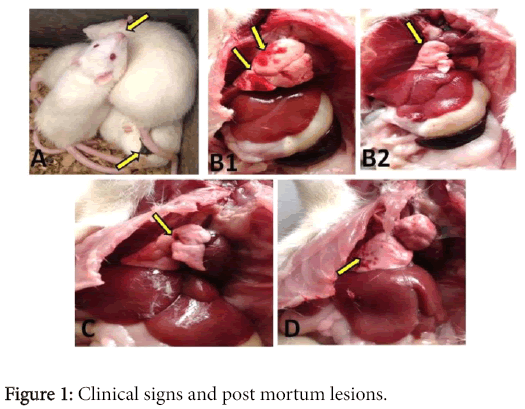
The toxic signs that were observed in group III, rats received MET showed respiratory distress, spontaneous defecation and rough hair coat (Figure 1).
A) Rats treated with MET showed gasping (thick arrow) and spontaneous defecation (thin arrow). B1&2) MET intoxicated rats showed congestion and haemorrhage (thick arrows) beside atrophy of the lung (thin arrow). C) Rats treated with NA showed normal lung tissue. D) Rats treated with NA+MET showed mild petechial haemorrhage.
Groups II and IV, the NA and NA+ MET did not show any sign of toxicity. No mortalities in all groups at 24 h post methomyl treatment. Post mortem lesions of sacrificed rats were congestion, hemorrhage and atrophy in lung of MET treated rats. These lesions were mild in NA+ MET treated rats (group IV). No lesion was observed in NA treated group.
Hematological assay
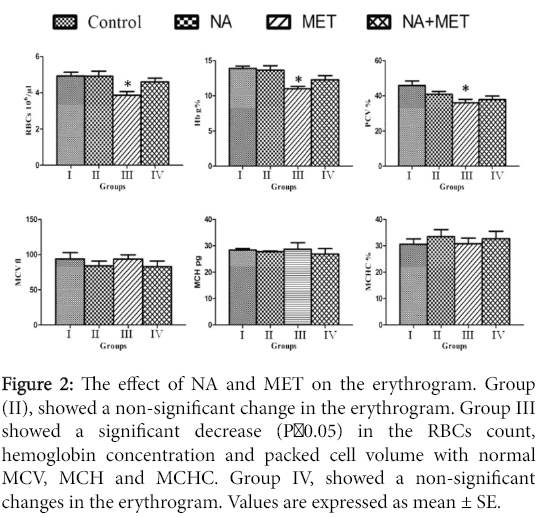
Nicotinic acid treated rats (group II), showed a non-significant change in the RBCs, Hb, PCV when compared with the control. Plus non-significant changes in the MCV, MCH and MCHC (Figure 2).
Figure 2: The effect of NA and MET on the erythrogram. Group (II), showed a non-significant change in the erythrogram. Group III showed a significant decrease (P<0.05) in the RBCs count, hemoglobin concentration and packed cell volume with normal MCV, MCH and MCHC. Group IV, showed a non-significant changes in the erythrogram. Values are expressed as mean ± SE.
MET intoxicated rats showed a significant (P<0.05) decrease in the RBCs, Hb and PCV with non-significant changes in the MCV, MCH and MCHC. The blood indices in these groups reflect normocytic normochromic anemia. Meanwhile the pretreated group IV, with NA +MET showed an improvement in the RBCs, Hb, PCV, MCV and MCHC when compared with the control group. The blood indices in this group were close to the normal.
Biochemical assay
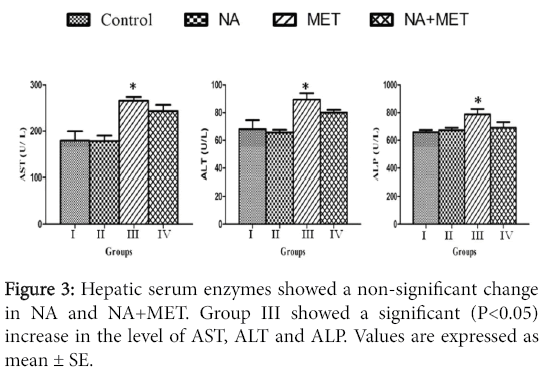
Enzymatic assay: The NA and NA+MET treated rats showed no significant change in the serum hepatic enzyme activities when compared with the control. The MET treated rats showed a significant (P<0.05) increase in the activities of AST, ALT and ALP compared with control (Figure 3).
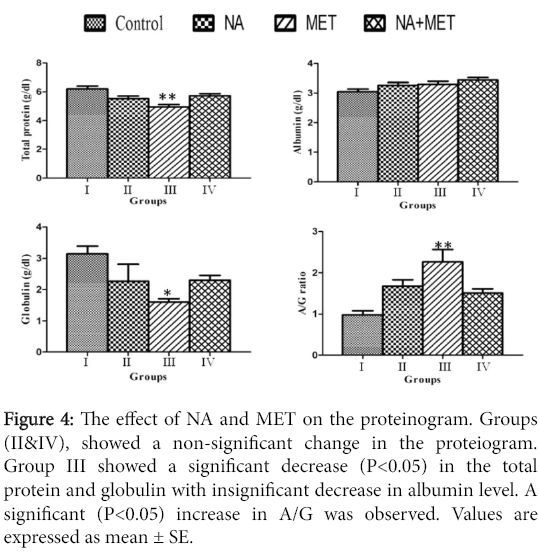
Proteinogram assay: The NA treated rats showed a non-significant change in the proteinogram when compared with control. MET treated rats showed a significant (P<0.05) decrease in the level of serum total protein and globulin, meanwhile the serum albumin level showed insignificant change. A/G ratio showed a significant (P<0.05) increase when compared with the control group. Supplementation of nitric acid of the MET-treated group produced recovery in the above mentioned biochemical variables (Figure 4).
Figure 4: The effect of NA and MET on the proteinogram. Groups (II&IV), showed a non-significant change in the proteiogram. Group III showed a significant decrease (P<0.05) in the total protein and globulin with insignificant decrease in albumin level. A significant (P<0.05) increase in A/G was observed. Values are expressed as mean ± SE.
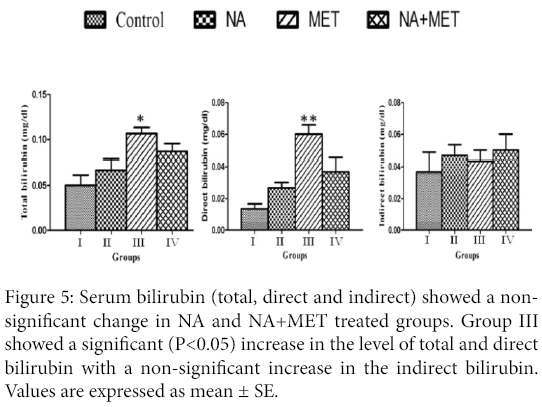
Serum bilirubin assay: Serum total, direct and indirect bilirubin showed a non-significant change in NA and NA+MET treated rats. MET treated rats showed a significant (P<0.05) increase in the level of serum total and direct bilirubin, meanwhile the serum indirect bilirubin showed a non-significant change when compared with control (Figure 5).
Figure 5: Serum bilirubin (total, direct and indirect) showed a nonsignificant change in NA and NA+MET treated groups. Group III showed a significant (P<0.05) increase in the level of total and direct bilirubin with a non-significant increase in the indirect bilirubin. Values are expressed as mean ± SE.
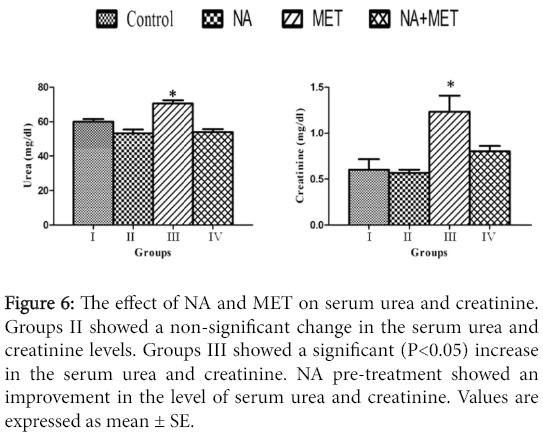
Renal markers assay: Serum urea and creatinine level was significantly (P<0.05) increased in MET treated group when compared with the control (Figure 6).
Figure 6: The effect of NA and MET on serum urea and creatinine. Groups II showed a non-significant change in the serum urea and creatinine levels. Groups III showed a significant (P<0.05) increase in the serum urea and creatinine. NA pre-treatment showed an improvement in the level of serum urea and creatinine. Values are expressed as mean ± SE.
This indicating an induction of nephrotoxicity in such group. The pre-treatment with NA ameliorate the increase in the level of serum urea and creatinine induced by MET treatment compared with the control group.
Discussion
The observed toxic signs in the acute toxicity study in the group exposed to methomyl alone were respiratory distress and spontaneous defecation. Methomyl is believed to act by inhibiting acetylcholine esterase (AChE) activity with accumulation of acetylcholine in the synapse leading to overstimulation of AChE receptors and respiratory distress. Similarly, respiratory distress and spontaneous defecation were documented [13,14]. The nicotinic acid pretreated groups did not manifest any toxic signs. Nicotinic acid decreased the occurrence of cholinergic signs of toxicosis through preventing the convulsions,gasping and death and so signifies the amelioration of the toxic signs [15] induced by acute methomyl exposure.
In the present study, the RBC’s count, Hb concentration and PCV were significantly decreased with normal MCV and MCHC in methomyl treated (group III). The acute exposure to methomyl may induce normocytic normochromic anemia. The anemia observed might have been due to the ability of the methomyl to cause acute extravascular hemolysis or it might be due to its ability to cause oxidative stress. It was thought that these changes were due to an increase rate of breakdown of red cells and/or the toxic effect of methomyl on bone marrow. The erythrocyte membrane was reported to be highly vulnerable in an oxidative stress condition because it contains high amounts of lipid, iron and is bathed in serum that has low antioxidant properties [16]. Also, the reduction in Hb content may be due to increased rate of breakdown of red cells and/or reduction in the rate of formation of RBC’s. The result is similar to that of [17] who observed a decrease in RBC’s count, Hb concentration and PCV in rats exposed to methomyl (1.70 mg/kg b.wt.) for 90 day. On the other hand, the rats that were pretreated with nicotinic acid showed an improvement in RBC’s count, Hb concentration and PCV. The increase in erythrocytic parameters previously observed were might be by the release of erythrocytes from blood depots and/or from haemopoetic tissues into the blood stream [18].
Liver is the first organ to face any foreign molecule that is carried through portal circulation and it is subjected to most damage. When liver cell plasma membrane is damaged, a variety of enzymes normally located in the cytosol are released into the blood stream. Their estimation in the serum is a useful quantitative marker of the extent and type of hepatocellular damage. One of the most sensitive and dramatic indicators of hepatocyte injury is the release of intracellular enzymes, such as AST, ALT and ALP in the circulation after methomyl treatment. The present results showed a significant increase in the activities of AST, ALT and ALP in the serum of treated rats suggesting that methomyl might cause critical injury to the liver. This increasing may be indicative of initial cell injury occurring associated with methomyl toxicity. Also due to changing in membrane permeability and loss of the functional integrity of the cell membranes in liver leading to cellular leakage with generalized release of these enzymes from the cell [14]. The increased levels of serum enzymes indicate an enhancement of permeability, hepatocytic damage or necrosis. However, pretreatment of nicotinic acid preventing the increase in the activities of these hepatic enzymes is the primary evidence of their hepatoprotective activity [19].
In this study, a significant decrease in the serum total protein and globulin levels with insignificant decrease in albumin was observed in the methomyl group. This might be due to the hepatocellular damage induced by methomyl intoxication. The total protein level may decrease due to the liver dysfunction where the imbalance between the rate of protein synthesis and the rate of its degradation in the liver. Also it might due to acute hemolytic anemia or severe loss through the urine in severe kidney disease. Destruction of the hepatic cells results in more hepatic releases to exacerbate hepatic dysfunction and causes decrease in the serum level of total protein. Toxic liver injury is associated with insignificant decrease in the serum level of albumin, meanwhile the hypoproteinemia due to hypoglobuleinemia could be due to the deteriorated hepatic activity [20]. Our observed decrease of these parameters due to methomyl intoxication is in consonance with the findings of [14]. The increased A/G ratio observed in the present study after in methomyl treated group might be due to the acute hepatic injury and these supported by the significant decrease in serum level of globulin with insignificant changes in albumin [21] .The present study has demonstrated the hepatoprotective potential of nicotinic acid. Pre-administration of nicotinic acid resulted in improvement of proteinogram against methomyl induced adverse effects on liver. It explained restoration of protein levels. The hepatoprotective ability of nicotinic acid may be connected with its anti-fibrotic potential for the liver injury [22].
Bilirubin is a metabolic breakdown product of blood heme with great diagnostic values. Estimating the activities of serum total, direct and indirect bilirubin can make assessment of liver function. Abnormal bilirubin concentrations usually signify the presence of a variety of diseases with liver dysfunctions, ranging from jaundice to infectious hepatitis [23]. The increased level of bilirubin is conventional indicator of the liver injury. In the present study, it is observed that administration of methomyl elevates the levels of serum total and direct bilirubin. The total bilirubin was increased due to the direct bilirubin was significantly increased, implying that hepatocellular injury may have occurred [24]. This is supported by the increased ALP levels in serum. Increases in ALP levels coupled with conjugated hyperbilirubinemia Furthermore, the bilirubin increase could indicate mild haemolysis and obstruction in the normal excretion of bile. The pre-treatment with NA was significantly decreases the total and direct bilirubin level. The stabilization of serum bilirubin level by NA is clear indication of the improvement of the functional status of the liver cells.
Kidney markers (urea and creatinine) were also estimated in the serum of experimental rats to confirm the renal damage. Methomyl treated rats exhibited a significant increase in urea and creatinine levels compared to control. Similar changes in creatinine and urea values were reported [2]. The elevation of serum urea and creatinine are considered as significant markers of renal dysfunction [25]. Elevated blood urea is correlated either with an increased protein catabolism or from a more efficient conversion of ammonia to urea as a result of increased synthesis of enzymes involved in urea production [2]. Creatinine is a metabolite of creatine and is excreted completely in urine via glomerular filtration. An elevation of its level in the blood is thus an indication of impaired kidney function [25]. The pretreatment of rats with nicotinic acid decreased serum urea and creatinine levels compared with the rats treated with methomyl alone. In our experimental conditions, nicotinic acid administrated to rats was found to inhibit the effects of methomyl toxicity on renal dysfunction markers. These findings are compatible with the results of other studies [26].
In conclusion, our finding suggests that the nicotinic acid affords protection in methomyl-induced acute toxicity in the rat. The data reported herein have important and direct implications in the clinical development of methomyl toxicity in terms of widening the protective index by NA and suggest that this strategy may be effective as initially proposed. Our work also underscores the need to fully assess the impact of the nicotinic acid in vivo metabolic macroenvironment when evaluating the efficacy of any compounds that target protection against methomyl toxicity. These novel findings suggest that nicotinic acid effectively prevents and causes the regression of experimental hematology, hepatic and renal toxicity. It may offer a very cost-effective opportunity for treating methomyl and other insecticides toxicity.
References
- Zeljezic D, Garaj-Vrhovac V (2001) Chromosomal aberration and single cell gel electrophoresis (Comet) assay in the longitudinal risk assessment of occupational exposure to pesticides. Mutagenesis 16:359-363.
- Djeffal A, Messarah M, Boumendjel A, Kadeche L, Feki AE (2015) Protective effects of vitamin C and selenium supplementation on methomyl-induced tissue oxidative stress in adult rats. Toxicology and Industrial Health 31: 31-43.
- Gil HW, Jeong MH, Park JS, Choi HW, Kim SY, et al. (2013) An outbreak of food borne illness due to methomyl pesticide intoxication in Korea. J Korean Med Sci 28: 1677-1681.
- Lee BK, Jeung KW, Lee HY, Jung YH (2011) Mortality rate and pattern following carbamatemethomyl poisoning. Comparison with organophosphate poisoning of comparable toxicity. Clinical Toxicology 49: 828-833.
- O'Brien T, Oeh J, Xiao Y, Liang X, Vanderbilt A, et al. (2013) Supplementation of nicotinic acid with NAMPT inhibitors results in loss of in vivo efficacy in NAPRT1-deficient tumor models. Neoplasia 15: 1314-1329.
- Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J ClinPathol 28: 56-63.
- Omene JA, Glew RH, Baig HA, Robinson DB, Brock W, et al. (1981) Determination of serum acid and alkaline phosphatase using 4-methylumbelliferyl phosphate. Afr J Med MedSci 10: 9-18.
- Krohn RI (2002) The colorimetric detection and quantitation of total protein. CurrProtoc Cell Biol, Mahin D Maines Appendix 3: A 3I 1-28
- Lukicheva TI, PivovarovaSIa, Doroguntseva II (1987) Determination of serum albumin by a colorimetric micromethod using bromcresol purple. Lab Delo : 3-6.
- Rutkowski RB, DEBaare L (1966) An ultramicro colorimetric method for determination of total and direct serum bilirubin. ClinChem 12: 432-438.
- Christian GD, KnoblockEC, Purdy WC (1965) ACoulometricDetermination of Urea Nitrogen in Blood and Urine. ClinC hem 11: 700-707.
- Heinegård D, Tiderström G (1973) Determination of serum creatinine by a direct colorimetric method. ClinChimActa 43: 305-310.
- Chaouali N, Amira D, Zitouni E, Gana I, Nouioui A, et al. (2014) Acute poisoning with anticholinesterase carbamate pesticides: methomyl-lannate. Annales de Biologie Clinique 72: 723-729.
- Patil JA, Patil AJ, Sontakke AV, Govindwar SP (2008) Effect of methomyl on hepatic mixed function oxidases in rats. Indian J Pharmacol 40: 158-163.
- Briend A, Nath SK, Heyman M, Desjeux JF (1993) Comparative effects of nicotinic acid and nicotinamide on cholera toxin-induced secretion in rabbit ileum. J Diarrhoeal Dis Res 11: 97-100.
- Garg DP, Kiran R, Bansal AK, Malhotra A, Dhawan DK (2008) Role of vitamin E in mitigating methomyl induced acute toxicity in blood of male Wistar rats. Drug ChemToxicol 31: 487-499.
- Mossa A-TH, Abbassy MA (2012) Adverse haematological and biochemical effects of certain formulated insecticides in male rats. Research J of Environmental Toxicology 6: 160.
- Tienken R, Kersten S, Frahm J, Hüther L, Meyer U, et al. (2015) Effects of Prepartum Dietary Energy Level and Nicotinic Acid Supplementation on Immunological, Hematological and Biochemical Parameters of Periparturient Dairy Cows Differing in Parity. Animals 5: 910-933.
- Zeb Shah T, Ali AB, Ahmad Jafri S, Qazi MH (2013) Effect of Nicotinic Acid (Vitamin B3 or Niacin) on the lipid profile of diabetic and non - diabetic rats. Pak J Med Sci 29: 1259-1264.
- Arbid MS, Koriem KM, Asaad GF, Megahed HA (2013) Effect of the antibiotic neomycin on the toxicity of the glycoside vicine in rats. J Toxicol 2013: 913128.
- Matsuzawa T, Hayashi Y, Nomura M, Unno T, Igarashi T, et al. (1997) A survey of the values of clinical chemistry parameters obtained for a common rat blood sample in ninety-eight Japanese laboratories. J ToxicolSci 22: 25-44.
- Arauz J, Rivera-Espinoza Y, Shibayama M, Favari L, Flores-Beltran RE, et al. (2015) Nicotinic acid prevents experimental liver fibrosis by attenuating the prooxidant process. IntImmunopharmacol 28:244-251.
- Jemnitz K, Lengyel G, Vereczkey L (2002) In vitro induction of bilirubin conjugation in primary rat hepatocyte culture. BiochemBiophys Res Commun 291: 29-33
- Robinson SH, Yannoni C, Nagasawa S (1971) Bilirubin excretion in rats with normal and impaired bilirubin conjugation: effect of phenobarbital. J Clin Invest 50: 2606-2613.
- Gowda S, Desai PB, Kulkarni SS, Hull VV, Math AA, et al. (2010) Markers of renal function tests. N Am J Med Sci 2: 170-173.
- Owada A, Suda S, Hata T (2003) Antiproteinuric effect of niceritrol, a nicotinic acid derivative, in chronic renal disease with hyperlipidemia: a randomized trial. Am J Med 114: 347-353.
Citation: Hashish EA, Elgaml SA (2016) Role of Nicotinic Acid in Mitigating Methomyl Induced Acute Toxicity in Albino Rats. J Clin Exp Pathol 6:268. DOI: 10.4172/2161-0681.1000268
Copyright: © 2016 Hashish EA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12815
- [From(publication date): 4-2016 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 11879
- PDF downloads: 936