Role of Inorganic Polyphosphate in Promoting Persistence of Mycobacteria
Received: 12-Dec-2023 / Manuscript No. JIDT-23-116613 / Editor assigned: 16-Dec-2023 / PreQC No. JIDT-23-116613(PQ) / Reviewed: 30-Dec-2023 / QC No. JIDT-23-116613 / Revised: 06-Dec-2023 / Manuscript No. JIDT-23-116613(R) / Published Date: 13-Dec-2023 DOI: 10.4172/2332-0877.1000570
Abstract
In recent paper, He, et al., elegantly demonstrate the role of inorganic plyphospahte (polyP) in promoting persistence of Mycbacteria. Firstly, polyP affects the formation, morphology and ultramicrostructure of biofilms of Mycobacterium smegmatis by affecting the synthesis of short chain fatty acids. Furthermore, polyP regulates inflammatory factors expression through transition of macrophages from M1 to M2 type and promotes the survival of M. smegmatis in cells. Tuberculosis is still the main threat to human health. Persistence is an important cause of drug resistance and recurrence for M. tuberculosis.
Keywords: Polyphosphate; Mycobacteria; Hydrophobicity; Mutation
Introduction
One of the important reasons for persistence is the formation of biofilm. In fact, M. tuberculosis grows in the form of biofilm in vivo and involves toxicity and drug resistance [1]. In addition, the formation of biofilm is closely related to the occurrence of infection, tissue necrosis, cavity formation, and the recurrence of tuberculosis [2].
Polyphosphate (polyP) is a linear molecule formed by high-energy phosphate bonds of phosphate radicals, widely present in the biological world [3]. The polyP kinases 1 (PPK1) is primarily responsible for maintaining bacterial polyP synthesis and Exopolyphosphatease (PPX) is responsible for the hydrolysis of polyP to inorganic phosphate [4]. They collectively regulate the dynamic homeostasis of intracellular polyP.
The dynamic homeostasis of polyP affects the formation of bacterial biofilm. In previous studies, we found that over-expression of PPX reduced intracellular polyP and affected the biofilm formation of M. smegmatis [5]. In He, et al., article, we found that the absence of polyP not only changed the morphology and ultramicrostructure of the biofilm, but also significantly reduced the survival ability of bacteria in macrophages [6].
Methodology
To investigate the effect of polyP deficiency on the biofilm formation of M. smegmatis, He, et al., constructed ppk1 mutant strains and complemented strains, and tested their intracellular polyP levels [6]. The results showed that the polyP levels in ppk1 mutant strains were significantly lower than those in wild and complemented strains [6]. ppk1 mutant strains not only exhibited attenuated biofilm formation ability, but also showed significant differences in biofilm morphology and ultramicrostructure compared to wild strains [6]. The initiation of biofilm formation involving interactions between cells or solid surfaces, once sufficient cells aggregate, extracellular matrix is generated to form biofilms. Mycobacteria does not have a special cell surface adsorption structure, so the components on the cell wall surface mediate the interaction process between bacteria or solid surfaces [5]. The lipids outside the cell wall endow the cell surface with hydrophobicity, thereby promoting the interaction between bacteria or solid surfaces and determining the initial stage of biofilm formation. Therefore, He, et al., analyzed the lipids (fatty acids) levels of ppk1 mutant strains and found that the levels of various short chain fatty acids in the mutant strains were significantly reduced, indicating that changes in fatty acid levels are related to biofilm formation, morphology, and ultramicrostructure [6]. Consistent with the changes in the lipid composition of the cell wall, ppk1 mutant strains also showed elevated sensitivity to antibiotics targeting the cell wall such as vancomycin.
The afore mentioned experimental evidences indicate that polyP deficiency leads to impaired biofilm formation, and its alteration in the morphology and ultramicrostructure of biofilm is related to changes in lipid composition on cell walls. How and what induce the formation of mycobacterial biofilms remains an important issue. The reasons for its impact on biofilm formation are not only related to the lipid composition of the cell wall surface, but also to the following two factors [7-14]. Firstly, the second messenger (p) ppGpp plays a very important role in biofilm formation. In Mycobacteria, (p) ppGpp is synthesized by rel, which promotes bacterial growth at low levels and biofilm formation at high levels [12]. Secondly, the metabolic state affects biofilm formation. The reduction of NADH induced biofilm formation, however, the aggregation of intracellular NADH could lead to significant changes in cell surface hydrophobicity and delayed biofilm formation [8,15]. Recently, Chakraborty, et al., elegantly reviewed the roles of lipids, (p)ppGpp and metabolic states in the biofilm formation of M. smegmatis [7]. However, it is currently unclear what the link between lipids, (p)ppGpp and metabolic state is in regulating the formation of biofilms.
Results and Discussion
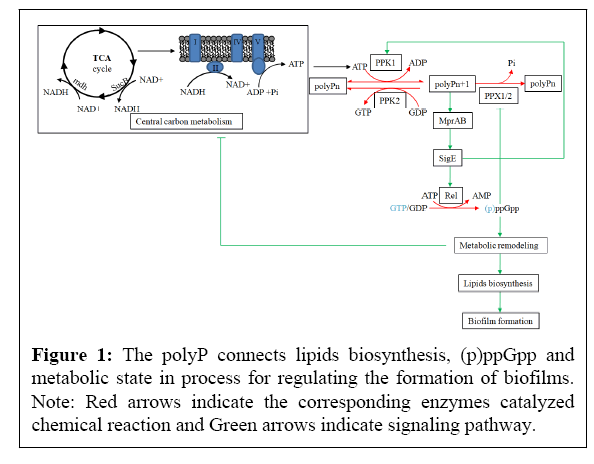
We propose that the metabolic state and (p)ppGpp are connected through polyP, thereby altering the biosynthesis of fatty acids and ultimately affecting the formation of biofilms (Figure 1). The reasons are as follows:
(1) PolyP regulates the expression of rel gene, leading to the synthesis of (p)ppGpp and ultimately affecting lipid metabolism. As shown in the Figure 1, polyP is located at a key node in the PPK1-polyP-MprAB-SigE-Rel signaling pathway. Phosphorylation and activation of the two component system MprAB by polyP induces the expression of sigE and rel genes in Mycobacteria, leading to an increase in (p)ppGpp [9,10]. (p)ppGpp regulates genes transcription and translation, involving various metabolic processes including lipid biosynthesis [11]. Although the specific mechanism is unknown, there are many examples for (p)ppGpp regulating lipid biosynthesis and affecting biofilm formation. For example, the inability to synthesize (p)ppGpp leads to changes in surface-related characteristics of M. smegmatis, such as impaired biofilm formation [12]. Moreover, the rel mutation of M. smegmatis reducd the biosynthesis of glycopeptides and polar lipids, resulting in altered cell surface characteristics [13]. Interestingly, The rpoZ gene deletion mutation of M. smegmatis resulted in attenuated biofilm formation and the inability to synthesize short chain fatty acids [14]. ω factor encoded by the rpoZ gene is the smallest subunit of RNA polymerase and can bind to ppGpp to regulate gene transcription [15]. Surprisingly, the biofilm morphology exhibited by the rpoZ mutant strains is very similar to that of the ppk1 mutant strains. Scanning electron microscopy shows that the biofilm formed by the two mutant strains all lacks extracellular matrix, indicating that they may both affect biofilm formation through the (p) ppGpp [6,14].
The NADH generated by the TCA cycle enters into an electron transfer chain to produce ATP. PPK1 uses ATP to synthesize polyP, which activates the two component system MprAB, thereby inducing the expression of sigE. Resultant SigE then induces the expression of rel, which catalyzes the synthesis of pppGpp and ppGpp with ATP and GTP/GDP. (p)ppGpp is a stringent response molecule that can lead to metabolic remodeling. Through this pathway, polyP connects metabolic states, (p)ppGpp, and NADH, thereby regulating the formation of biofilms.
(2) PolyP may regulate metabolic status of cells and alter the intracellular NADH/NAD+ ratio. The absence or aggregation of polyP in M. tuberculosis could lead to an increase or decrease in its sensitivity to isoniazid, and the aggregation of polyP in M. smegmatis also showed a decrease in sensitivity to isoniazid [16-20]. PolyP changes the sensitivity of Mycobacteria to INH, which may be related to alteration in the intracellular NADH/NAD+ ratio. A widely recognized superoxide production is the transfer of electrons from NADH through the electron transfer chain to oxygen [21]. The recent viewpoint is that antibiotics kill bacteria by producing superoxide through promoting NADH to quickly enter the electron transfer chain, accompanied with a decrease in the NADH/NAD+ ratio [22]. NADH is produced through the TCA cycle, so intermediate metabolites or related enzymes in the TCA cycle participate in the regulation of NADH. For example, M. tuberculosis up-regulated the expression of icl1 gene coding isocitrate lyase to increase the activity of the glyoxylate shunt and reduce the production of NADH in the TCA cycle, thereby reducing the production of reactive oxygen intermediates in the respiratory chain and then increasing resistance to the anti-tuberculosis drug such as INH [23]. Therefore, M. tuberculosis can increase resistance to anti-tuberculosis drugs through metabolic regulation such as remodeling of the TCA cycle (manifested as the aggregation of pyruvate, succinate, malate, and fumarate, while reduction of α-ketoglutarate) [23]. Interestingly, metabolomics analysis revealed that NAD+ levels and a large number of metabolites such as the acetyl-CoA, malate, and succinate significantly elevated in polyP-aggregated M. tuberculosis [24]. The changes in these metabolites in polyP-aggregated M. tuberculosis are highly consistent with those in M. tuberculosis induced by isoniazid, indicating that the aggregation of polyP may lead to remodeling of the TCA cycle to reduce the production of reactive oxygen species caused by NADH, and then leading to resistance to INH. The possible mechanism underlying polyP regulates metabolic status may be through the PPK1-polyP-MprAB-SigE-Rel signaling pathway. For example, the energy metabolism and redox homeostasis of the sigE mutation of M. tuberculosis are imbalanced, with central metabolic remodeling and reduced electron transfer chain activity [25]. Furthermore, polyP also affects intracellular ATP/GTP ratio. PolyP is a linear molecule formed by high-energy phosphate bonds between more than ten to hundreds of phosphate radicals. In the prokaryotic system, the synthesis and utilization of polyP are accomplished by PPK1 and PPK2, respectively. PPK1 is primarily responsible for reversible polyP synthesis with ATP, while PPK2 primarily utilizes polyP to produce GTP [26]. A rapid accumulation of polyP resulted in a drop in the intracellular ATP levels [27]. Moreover, GTP is required for the synthesis of (p)ppGpp and cell wall components of Mycobacteria.
Interestingly, polyP also affects the survival of M. smegmatis in macrophages, which is highly correlated with the persistence of Mycobacteria. He, et al., found that the survival of polyP-deficient mutant strains in vitro was not affected, but their survival ability in macrophages was significantly reduced [6]. By analyzing the expression levels of inflammatory factors in macrophages infected with ppk1 mutant strains, it was found that the expression levels of anti-inflammatory factor IL-10 in macrophages infected with ppk1 mutant strains were significantly lower than those in macrophages infected with wild strains and complemented strains after 2 and 4 hours of infection [6]. After 24 hours of infection, the expression levels of pro-inflammatory factors IL-6 and TNF-α were significantly increased in macrophages infected with ppk1 mutant strains, while the anti-inflammatory factor PPAR-γ was significantly reduced compared to macrophages infected wild strains [6]. The fact that the absence of polyP alters the expression of inflammatory factors in macrophages infected with M. smegmatis suggests that polyP may alter the transition of macrophages from M1 to M2, thereby promoting bacterial survival within macrophages. Similarly, Rijal et al. reported that polyP secreted by M. tuberculosis increased the survival ability of its in human macrophages [28]. Moreover, the aggregation of polyP by M. tuberculosis increased the expression of IL-2 and IL-10 in infected macrophages. These results indicate that the polyP of Mycobacteria regulates the transition of macrophages from M1 to M2, thereby increasing the persistence of bacteria in macrophages.
Conclusion
In summary, polyP affects the persistence of Mycobacteria. The polyP synthase PPK may serve as a potential drug target to reduce bacterial persistence, thereby reducing treatment time for tuberculosis and reducing the development of drug resistance.
Acknowledgment
This review was written as a follow-up to the “Innovation and Entrepreneurship Training Program for College Students (202310517008)” that I received. I would like to thank Dr. Tingyu SHI for recommending me for this award as well as the many students (Taibin Huang, Jiangquan Chen, zhengqing Zhao) that assisted me in these studies.
References
- Chakraborty P, Bajeli S, Kaushal D, Radotra BD, Kumar A (2021) Biofilm formation in the lung contributes to virulence and drug tolerance of Mycobacterium tuberculosis. Nat Commun 12: 1606.
[Crossref] [Google Scholar] [PubMed]
- Basaraba RJ, Ojha AK (2017) Mycobacterial biofilms: revisiting tuberculosis bacilli in extracellular necrotizing lesions. Microbiol Spectr 5.
[Crossref] [Google Scholar] [PubMed]
- Brown MR, Kornberg A (2008) The long and short of it - polyphosphate, PPK and bacterial survival. Trends Biochem Sci 33: 284-290.
[Crossref] [Google Scholar] [PubMed]
- Zhu Y, Huang W, Lee SSK, Xu W (2005) Crystal structure of a polyphosphate kinase and its implications for polyphosphate synthesis. EMBO Rep 6: 681-687.
[Crossref] [Google Scholar] [PubMed]
- Shi T, Fu T, Xe J (2011) Polyphosphate deficiency affects the sliding motility and biofilm formation of Mycobacterium smegmatis. Curr Microbiol 63: 470-476.
[Crossref] [Google Scholar] [PubMed]
- He C, Li B, Gong Z, Huang S, Liu X, et al. (2023) Polyphosphate kinase 1 is involved in formation, the morphology and ultramicrostructure of biofilm of Mycobacterium smegmatis and its survivability in macrophage. Heliyon 9: e14513.
[Crossref] [Google Scholar] [PubMed]
- Chakraborty P, Kumar A (2019) The extracellular matrix of mycobacterial biofilms: Could we shorten the treatment of mycobacterial infections? Microb Cell 6: 105-122.
[Crossref] [Google Scholar] [PubMed]
- Singh R, Mailloux RJ, Dao SP, Appanna VD (2007) Oxidative stress evokes a metabolic adaptation that favors increased NADPH synthesis and decreased NADH production in Pseudomonas fluorescens. J Bacteriol 189: 6665-6675.
[Crossref] [Google Scholar] [PubMed]
- Sanyal S, Banerjee SK, Banerjee R, Mukhopadhyay J, Kundu M (2013) Polyphosphate kinase 1, a central node in the stress response network of Mycobacterium tuberculosis, connects the two-component systems MprAB and SenX3-RegX3 and the extracytoplasmic function sigma factor, sigma E. Microbiology 159: 2074-2086.
[Crossref] [Google Scholar] [PubMed]
- Sureka K, Dey S, Datta P, Singh AK, Dasgupta A, et al. (2007) Polyphosphate kinase is involved in stress-induced mprAB-sigE-rel signalling in mycobacteria. Mol Microbiol 65: 261-276.
[Crossref] [Google Scholar] [PubMed]
- Traxler MF, Summers SM, Nguyen HT, Zacharia VM, Hightower GA, et al. (2008) The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol Microbiol 68: 1128-1148.
[Crossref] [Google Scholar] [PubMed]
- Gupta KR, Baloni P, Indi SS, Chatterji D (2016) regulation of growth, cell shape, cell division, and gene expression by second messengers (p)ppGpp and cyclic Di-GMP in Mycobacterium smegmatis. J Bacteriol 198: 1414-1422.
[Crossref] [Google Scholar] [PubMed]
- Gupta KR, Kasetty S, Chatterji D (2015) Novel functions of (p)ppGpp and Cyclic di-GMP in mycobacterial physiology revealed by phenotype microarray analysis of wild-type and isogenic strains of Mycobacterium smegmatis. Appl Environ Microbiol 81: 2571-2578.
[Crossref] [Google Scholar] [PubMed]
- Mathew R, Mukherjee R, Balachandar, Chatterji D (2006) Deletion of the rpoZ gene, encoding the omega subunit of RNA polymerase, results in pleiotropic surface-related phenotypes in Mycobacterium smegmatis. Microbiology 152: 1741-1750.
[Crossref] [Google Scholar] [PubMed]
- Vrentas CE, Gaal T, Ross W, Ebright RH, Gourse RL (2005) Response of RNA polymerase to ppGpp: requirement for the omega subunit and relief of this requirement by DksA. Genes Dev 19: 2378-2387.
[Crossref] [Google Scholar] [PubMed]
- Singh R, Singh M, Arora G, Kumar S, Tiwari P, et al. (2013) Polyphosphate deficiency in Mycobacterium tuberculosis is associated with enhanced drug susceptibility and impaired growth in guinea pigs. J Bacteriol 195: 2839-2851.
[Crossref] [Google Scholar] [PubMed]
- Thayil SM, Morrison N, Schechter N, Robuin H, Karakousis PC (2011) The role of the novel exopolyphosphatase MT0516 in Mycobacterium tuberculosis drug tolerance and persistence. PLoS One 6: e28076.
[Crossref] [Google Scholar] [PubMed]
- Chuang YM, Belchis DA, Karakousis PC (2013) The polyphosphate kinase gene ppk2 is required for Mycobacterium tuberculosis inorganic polyphosphate regulation and virulence. MBio 4: e00039-e00113.
[Crossref] [Google Scholar] [PubMed]
- Chuang YM, Bandyopadhyay N, Rifat D, Rubin H, Bader JS, et al. (2015) Deficiency of the novel exopolyphosphatase Rv1026/PPX2 leads to metabolic downshift and altered cell wall permeability in Mycobacterium tuberculosis. MBio 6: e02428.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Cumming BM, Mao C, Zhu Y, Lu P, et al. (2018) RbpA and sigma(B) association regulates polyphosphate levels to modulate mycobacterial isoniazid-tolerance. Mol Microbiol 108: 627-640.
[Crossref] [Google Scholar] [PubMed]
- Imlay JA (2013) The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11: 443-454.
[Crossref] [Google Scholar] [PubMed]
- Kohanski MA, Dwyer D, Hayete B, Lawrence CA, Collins JJ (2007) A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130: 797-810.
[Crossref] [Google Scholar] [PubMed]
- Nandakumar M, Nathan C, Rhee KY (2014) Isocitrate lyase mediates broad antibiotic tolerance in Mycobacterium tuberculosis. Nat Commun 5: 4306.
[Crossref] [Google Scholar] [PubMed]
- Chuang YM, Dutta NK, Hung C, Wu T, Rubin H, et al. (2016) Stringent response factors ppx1 and ppk2 play an important role in Mycobacterium tuberculosis metabolism, biofilm formation, and sensitivity to isoniazid in vivo. Antimicrob Agents Chemother 60: 6460-6470.
[Crossref] [Google Scholar] [PubMed]
- Baruzzo G, Serafini A, Finotello F, Sanavia T, Mazzabo LC, et al. (2023) Role of the extracytoplasmic function sigma factor SigE in the stringent response of Mycobacterium tuberculosis. Microbiol Spectr 11: e0294422.
[Crossref] [Google Scholar] [PubMed]
- Sureka K, Sanyal S, Basu J, Kundu M (2009) Polyphosphate kinase 2: a modulator of nucleoside diphosphate kinase activity in mycobacteria. Mol Microbiol 74: 1187-1197.
[Crossref] [Google Scholar] [PubMed]
- Gray MJ, Wholey W, Wagner NO, Cremers CM, Schickert AM, et al. (2014) Polyphosphate is a primordial chaperone. Mol Cell 53: 689-699.
[Crossref] [Google Scholar] [PubMed]
- Rijal R, Cadena LA, Smith MR, Carr JF, Gomer RH (2020) Polyphosphate is an extracellular signal that can facilitate bacterial survival in eukaryotic cells. Proc Natl Acad Sci U S A 117: 31923-31934.
[Crossref] [Google Scholar] [PubMed]
Citation: Gong H, Wang J, Shi T (2023) Role of Inorganic Polyphosphate in Promoting Persistence of Mycobacteria. J Infect Dis Ther 11:570. DOI: 10.4172/2332-0877.1000570
Copyright: © 2023 Gong H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1567
- [From(publication date): 0-2023 - Oct 21, 2025]
- Breakdown by view type
- HTML page views: 1260
- PDF downloads: 307