Research Article Open Access
Role of GPR40 for Fish Oil PUFA-mediated BDNF Synthesis in the Monkey Hippocampus
Arumugam Mathivanan1,2, Yoshio Minabe2, Tsuguhito Ota3, Naoto Nagata3, Kosuke R. Shima3 and Tetsumori Yamashima*
1Departments of Restorative Neurosurgery, Brain/Liver Interface Medicine Research Center, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan
2Departments of Restorative Neurosurgery and Psychiatry and Neurobiology, Brain/Liver Interface Medicine Research Center, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan
3Department of Cell Metabolism and Nutrition, Brain/Liver Interface Medicine Research Center, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan
- Corresponding Author:
- Tetsumori Yamashima
Department of Restorative Neurosurgery
and Psychiatry, Kanazawa University Graduate
School of Medical Science, Kanazawa, Japan
Tel: +81(90)2129-1429
E-mail: yamashima215@gmail.com
Received date: January 25, 2016; Accepted date: February 29, 2016; Published date: March 07, 2016
Citation: Mathivanan A, Minabe Y, Ota T, Nagata N, Shima KR, et al. (2016) Role of GPR40 for Fish Oil PUFA-mediated BDNF Synthesis in the Monkey Hippocampus. J Alzheimers Dis Parkinsonism 6:213. doi:10.4172/2161-0460.1000213
Copyright: © 2016 Mathivanan A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Background: Polyunsaturated fatty acids (PUFA) are known to be crucial for learning and memory. However, the detailed mechanism of PUFA effects upon neuronal functions remains almost unknown except for the possible facilitation of membrane fluidity. G-protein coupled receptor 40 (GPR40) was found to induce Ca2+ mobilization in response to diverse PUFA. Thereafter, the authors found GPR40 expression in the newborn neurons of the monkey hippocampus after ischemia. This suggested implications of PUFA-mediated GPR40 signaling for adult neurogenesis underlying learning and memory.
Objective: This study aims at evaluating whether PUFA-mediated GPR40 activation can affect synthesis of brain-derived neurotrophic factor (BDNF) with the aid of its proteolytic enzyme furin.
Methods: Monkeys underwent 20 min transient whole brain ischemia by clamping both the innominate and left subclavian arteries. On days 7 and 15 after ischemia/reperfusion, when adult neurogenesis was shown to be maximal previously by the authors, the brain samples were resected. By the Western blotting analysis of mature-BDNF (m-BDNF), pro-BDNF and furin, syntheses of BDNF in response to two GPR40 agonists as well as selective GPR40 antagonist GW1100, were studied using normal and post-ischemic monkey dentate gyrus (DG) tissue extracts.
Results: Both up-regulation of m-BDNF synthesis in response to two GPR40 agonists; fish oil PUFA and docosahexaenoic acid (DHA) and its down-regulation in response to GW1100, were observed. GPR40 antagonist inhibited m-BDNF synthesis, whereas two GPR40 agonists stimulated m-BDNF synthesis conceivably via furin activation. Cleavage of p-BDNF to m-BDNF by furin as well as syntheses of m-BDNF and furin in the DG tissues, occurred immediately after incubation with fish oil PUFA or DHA. Dynamic changes of GPR40, m-BDNF synthesis, and furin occurred simultaneously.
Conclusions: These data, although correlative, suggested that m-BDNF may be synthesized by the cleavage of pre-stocked pro-BDNF and/or released from the cell store in response to PUFA. By activating GPR40 and furin, PUFA may be related to adult neurogenesis and the concomitant synaptic plasticity for learning and memory. To the best of our knowledge, this is the first report suggesting a role of GPR40 in PUFA-mediated m-BDNF synthesis.
Keywords
Cerebral ischemia; Adult neurogenesis; PUFA; Furin; BDNF; Fish oil
Abbreviations
Akt: Protein Kinase B; BDNF: Brain-derived Neurotrophic Factor; BMP: Bone Morphogenic Protein; CaMKIV: Ca2+/ Calmodulin-dependent Protein Kinase Type IV; CREB: cAMP Response Element-Binding Protein; DG: Dentate Gyrus; DHA: Docosahexaenoic Acid; ER: Endoplasmic Reticulum; ERK1/2: Extracellular Signalregulated Kinase; GPR40: G-Protein Coupled Receptor 40; HIF-1: Hypoxia-inducible Factor-1; LTD: Long-term Depression; LTP: Longterm Potentiation; MAPK: Mitogen Activated Protein Kinase; m-BDNF: Mature-Brain-Derived Neurotrophic Factor; NGF: Nerve Growth Factor; p-BDNF: Pro-Brain-Derived Neurotrophic Factor; pCREB: Phosphorylated Camp Response Element-Binding Protein; PUFA: Polyunsaturated Fatty Acids; TGFβ-1: Transforming Growth Factor Beta 1.
Introduction
Brain-derived neurotrophic factor (BDNF) was first purified from the mammalian brain [1], and it’s the second member of the neurotrophin family next to the nerve growth factor (NGF) [2]. BDNF is widely accepted to regulate neuronal survival, differentiation, dendritic morphology, and synaptic plasticity [3,4]. It has been implicated for the differentiation and survival of the CNS neurons, because of the most abundant and widespread expression not only in the developing brain but also in the adult mammalian brain [5]. Furthermore, BDNF has a critical role in long-term potentiation (LTP) [6-8], and recently emerged as a regulator of adult neurogenesis and the concomitant synaptogenesis indispensable for learning and memory [9].
BDNF protein is synthesized intracellularly as a precursor, 32 kDa pro-BDNF (p-BDNF) and rapidly converted as the 14 kDa m-BDNF after the intracellular cleavage, and the converted m-BDNF, but not p-BDNF, accumulates/stored in the neuronal cells which is called as pre-stocked BDNF and is released upon stimulation [10]. Or, p-BDNF is secreted extracellularly, then cleaved to m-BDNF in the synaptic cleft [11]. The extent of the intra- versus extra-cellular processing of p-BDNF is unclear, but the secretion of p-BDNF predominates [12,13]. p-BDNF and m-BDNF may have opposite biological effects; p-BDNF induces cell death whereas m-BDNF supports cell survival [14,15]. Once released into the synaptic cleft, p-BDNF binds preferentially to pan neurotrophin receptor p75NTR for facilitating pro-apoptotic effects and long-term depression (LTD), while m-BDNF binds preferentially to both pre- and post-synaptic TrkB receptors for facilitating prosurvival effects and LTP [8,15]. p-BDNF and m-BDNF activate different intracellular messenger cascades and affect distinct cellular responses.
Lack of precise knowledge about the mechanisms by which PUFA influence BDNF synthesis, may constitute a severe limitation in elucidating the mechanism of PUFA effects upon the higher cognitive functions and complex behaviours. Since deorphanization of GPR40 in 2003 [16,17], however, our understanding of the basic molecular and cellular mechanisms of PUFA underlying learning and memory has considerably advanced. In primates GPR40 mRNA was found to be abundantly expressed in both the brain and the pancreas, while in rodents it was expressed mainly in the pancreas, being negligible in the brain. Accordingly, for the following decade most studies using rodent experimental paradigms were done to clarify the molecular mechanism of the insulin secretion, so until recently there were very few studies focusing the rodent brain [18].
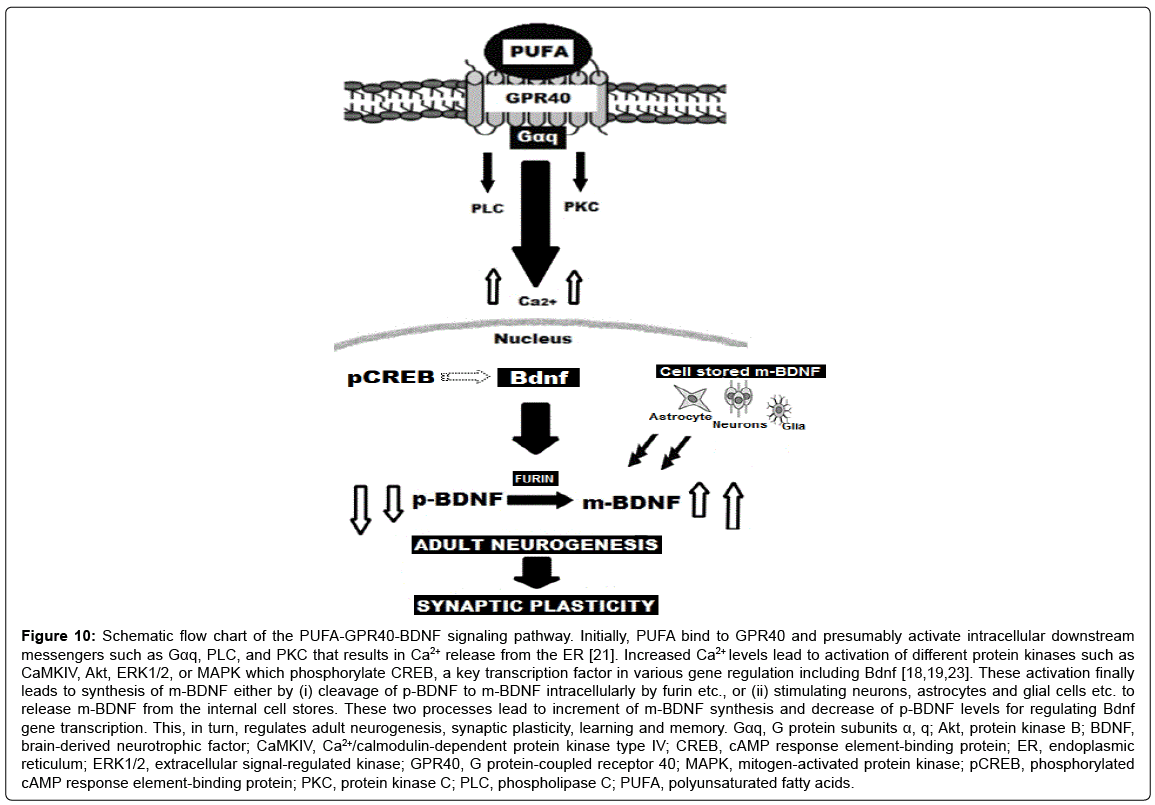
Recently, Boneva and Yamashima [19] suggested in the ischemic monkey experimental paradigm that PUFA, GPR40, phosphorylated cAMP response element-binding protein (pCREB), and BDNF may be engaged in the same signaling pathway for promoting neurogenesis in the adult hippocampus. They speculated that PUFA initially bind to GPR40 and activate intracellular downstream messengers such as Gαq, PLC, and PKC. The resultant Ca2+ release from the endoplasmic reticulum (ER) may lead to activation of different protein kinases such as Ca2+/calmodulin-dependent protein kinase type IV (CaMKIV), protein kinase B (Akt), extracellular signal-regulated kinase (ERK1/2), or mitogen-activated protein kinase (MAPK), which phosphorylate CREB, a key transcription factor in various gene regulation including Bdnf. Accordingly, it is indispensable to elucidate the signaling mechanism of intracellular Ca2+ mobilization necessary for the synthesis of BDNF that is facilitated by PUFA.
In this study, we focused implication of GPR40 for regulating BDNF synthesis in the monkey hippocampus. It was focused because of the two reasons; 1) Since the extent of GPR40 protein expression in the brain was much larger in primates compared to rodents [16,17,19-22], the monkey hippocampus was appropriate for studying dynamic changes at the protein level, and 2) it showed a remarkable up-regulation of adult neurogenesis on the 2nd week after ischemia, being in deep association with BDNF synthesis [19-23]. Since the PUFA-GPR40-CREB-BDNF signaling was already indicated in our ischemic monkey experimental paradigms, the monkey hippocampal tissues before and after the ischemic insult were focused for the present in vitro analysis to compare both. The effects of GPR40 activation and inhibition upon BDNF synthesis as well as cleavage of p-BDNF by its proteolytic enzyme furin, were studied by comparing the normal (non-ischemic) and post-ischemic dentate gyrus (DG) tissues after incubation with GPR40 agonists such as fish oil PUFA and DHA as well as its selective antagonist GW1100.
Materials and Methods
Animals
Surgical procedures and postoperative care of experimental animals were done with proper approval of Animal Care and Ethics Committee of Kanazawa University (Approval No.: AP-132874). They were performed in strict adherence with the guidelines of both the Animal Care and Ethics Committee of Kanazawa University and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Maximum efforts were done to reduce animal suffering from the pain throughout the experiments with reference to the guidelines of the NIH and Kanazawa University’s Animal Care and Ethics Committee. We decided to use the monkey samples for this experiment, because of the two reasons mentioned above. Furthermore, the amino acid sequence of the macaque monkeys are approximately 95% homologous with humans, being appropriate for both elucidating common molecular cascade with humans and utilizing various antihuman antibodies. A total of 10 young adult (4–6 years of age) Japanese monkeys (Macaca fuscata) with body weight of 5–8 kg, being supplied by National Bio-Resource Project ‘‘Japanese Monkeys’’ (Approval No.: Okazaki No.1-117, Japan) were bred in air-conditioned cages under standard conditions of humidity and temperature with 12 h light: 12 h dark cycle, and were allowed free daily access to food and water.
Ischemic operation and sampling/tissue preparation
Ten monkeys were divided into control animals (n=4) undergoing sham-operation and ischemic animals (n=3 for each post-ischemic days 7 and 15). Under general anesthesia, transient global brain ischemia was carried out as described previously [24-26]. Briefly, by the mediastinal approach after resecting the sternum, both the innominate and left subclavian arteries were clamped for 20 min under general anesthesia, according to the surgical procedure previously described [24,25]. A laser Doppler (Vasamedics, St. Paul MN) was used to demonstrate the effectiveness of clamping. Body temperature, pupil size, arterial blood pressure, and cerebral blood flow were monitored. Thereafter, the blood circulation of the brain was resumed. Monkeys were sacrificed on different time points after the ischemic insult/reperfusion; days 7 and 15, intracardial perfusion was done with 500 ml of ice-cold saline under general anesthesia, and the brain was quickly removed and cut into small samples after craniectomy. Dissected fresh samples of the hippocampal DG were immediately put into the liquid nitrogen and stored at -80°C until use.
GPR40 agonists and antagonist
Extracted fish oil (PUFA No.3; Menhaden Fish Oil containing mixer of PUFAs; Table 1) was obtained from Supelco (Bellefonte, PA, USA), while DHA (cis -4,7,10,13,16,19- Docosahexaenoic acid) was obtained from Sigma (St Louis, Missouri, MO, U.S.A.). GPR40 selective antagonist, GW1100 (ethyl 4-[5-{[2-(ethyloxy)-5pyrimidinyl] methyl}-2- {[(4-fluorophenyl) methyl]thio}-4-oxo1(4H)- pyrimidinyl] benzoate), was obtained from Cayman Chemical (Ann Arbor, MI, USA). Both agonists and antagonist were dissolved in 99.5% DMSO. Working solution was prepared with radio-immunoprecipitation assay (RIPA) buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% NaDOC, 0.1% SDS) (Sigma) at the concentration of 10 μM in all in vitro studies.
| Sl.No. | Name of the PUFA |
|---|---|
| 1. | cis-4,7,10,13,16,19-Docosahexaenoic acid methyl ester |
| 2. | cis-11,14,17-Eicosatrienoic acid methyl ester |
| 3. | Methyl arachidonate |
| 4. | Methyl all-cis-7,10,13,16,19-docosapentaenoate |
| 5. | Methyl all-cis-5,8,11,14,17-eicosapentaenoate |
| 6. | Methyl cis-11-eicosenoate |
| 7. | Methyl linoleate |
| 8. | Methyl linolenate |
| 9. | Methyl myristate |
| 10. | Methyl oleate |
| 11. | Methyl palmitate |
| 12. | Methyl palmitoleate |
| 13. | Methyl stearate |
| 14. | Methyl stearidonate |
| 15. | 11,14-Octadecadienoic acid methyl ester |
| 16. | 9,11,14-Octadecatrienoic acid methyl ester |
| 17. | cis-11-Octadecenoic methyl ester |
Table 1: PUFA composition of extracted fish oil (PUFA No.3; Menhaden Fish Oil).
Diverse PUFA including DHA are well known endogenous in vitro agonists/ligands for GPR40 receptor [17-19,27], which are highly abundant in Fish oil compared to other animal sources. GW1100 is very highly selective antagonist of GPR40 being 1000 time specific for the GPR40 receptor [18,27]. These are the reason for the selection of these agonists/antagonist for this in vitro study.
Incubation of tissue extracts with agonists or antagonist
The hippocampal DG tissues stored at -80°C were thawed/lysed on ice for 45 min and then homogenized by ultrasonic disruption in RIPA buffer without addition of any protease inhibitors, and centrifuged at 120 x 100 g at 4°C for 20 min to obtain whole tissue lysates. The supernatant was collected and protein concentration was measured by the Bradford assay. Incubation of the tissue extracts was done according to the method previously described by Ho et al. [28] with slight modification. Briefly, 10 μL of agonists/antagonist at the concentration of 10 μM were added to 10 μg total protein of tissue extracts, mixed well, and incubated for 30 min, 60 min, 90 min at 37°C. 1 M DTT was added to the samples for 30 min on ice to stop the reaction, then SDSloading buffer was added and boiled for 5-10 min at 99°C, mixed well, and served for the Western blotting analysis. Control samples were not incubated with agonist/antagonist; they were diluted 1:1 with SDSloading buffer containing 1M DTT and boiled for 5-10 min at 99°C, mixed well, and served for the Western blotting analysis.
Different incubation time points were fixed based on our previous study results [21], showing that 10 μM of PUFA/DHA induced PLC/ PKC/PI3 signaling pathway and increases Ca2+ mobilization in rat neural stem cells transfected with GPR40 gene. Here, we tested various concentration/dose levels (5, 10 and 20 μM) of agonists/antagonist and then optimized to 10 μM throughout the study.
Antibodies
For the Western blotting analysis, the following antibodies were used as a primary antibody: rabbit anti-human BDNF (Sigma-Aldrich, St. Louis, MO) (1:1,000); rabbit anti-human pro-BDNF (Sigma, St. Louis, MO) (1:500); rabbit anti-human furin (Sigma-Aldrich, St. Louis, MO) (1:5,000); mouse anti-β Actin (Sigma, St. Louis, MO) (1:10,000). The following horseradish peroxidase-conjugated antibodies were used as a secondary antibody: goat anti-rabbit IgG (Sigma, St. Louis, MO) (1:3,000 and 1:5,000), and goat anti-mouse IgG (Santa Cruz, Santa Cruz, CA) (1:10,000).
We have cross checked the antibodies for their specific expression. The rabbit anti-human BDNF antibody used in this study is more specific and detected only m-BDNF protein and not cross-reacted with p-BDNF protein, likewise rabbit anti-human pro-BDNF antibody detected only p-BDNF protein and not cross-reacted with m-BDNF protein in the tested samples.
Western blotting
For the electrophoresis, 10 μg total protein of each samples were run on 12% SDS acrylamide gel for 2 h, and 30 min at the constant voltage condition. The protein was transferred to hybond polyvinylidene fluoride (PVDF) nitrocellulose membrane using Trans-Blot Turbo Transfer pack and Trans-Blot Turbo Blotting System according to the manufacturer’s instructions (Bio-Rad, USA). Then, the membrane was blocked with 10% (w/v) non-fat skim milk in TBS-T (Tris-buffered saline/0.5% Tween 20) at room temperature (22°C) for 1h. Primary antibody was diluted in the blocking solution (5% (w/v) non-fat skim milk in TBS-T) and incubated overnight at 4°C. On the following day, the membrane was rinsed three times in TBS-T. The respective horseradish peroxidase-conjugated secondary antibody was added and incubated at room temperature for 1 h. The membrane was rinsed, and Amersham ECL select Western blotting detection reagent (GE Healthcare Limited, Buckinghamshire, UK) was added for 5 min to visualize the immunoreactive bands. The signal development was performed on a film (Amersham Hyperfilm ECL; GE Healthcare Limited, UK). Using specific antibodies, m-BDNF was detected at 14 kDa, p-BDNF at 32 kDa, while furin was detected at 87-88 kDa.
Image acquisition and statistical analysis
Densities of the protein bands were measured by ImageJ software. Results were shown as mean ± SEM of at least three independent experiments with 4 replicates each. Using GraphPad Prism 6 (Oberlin, San Deiego, CA, USA), the statistical significance was determined by one-way ANOVA with Bonferroni’s post hoc test to compare the agonists/antagonist-incubated samples with the control, whereas two– way ANOVA with Bonferroni’s post-test to compare the data between the two agonists and antagonist. Differences were considered significant when P<0.05.
Results
Two endogenous GPR40 agonists such as fish oil PUFA and DHA as well as selective GPR40 antagonist GW1100, were utilized to elucidate PUFA-GPR40 signaling for BDNF synthesis by comparing the non-ischemic and post-ischemic DG tissues. Based on our previous ischemic monkey experimental paradigms studies, we hypothesized that the post-ischemic DG tissues concomitant with up-regulation of hippocampal neurogenesis [19-23] might show more BDNF synthesis in vitro in response to agonists, compared to the non-ischemic DG tissues.
Effects of GPR40 agonists/antagonist upon BDNF synthesis in the non-ischemic DG tissues
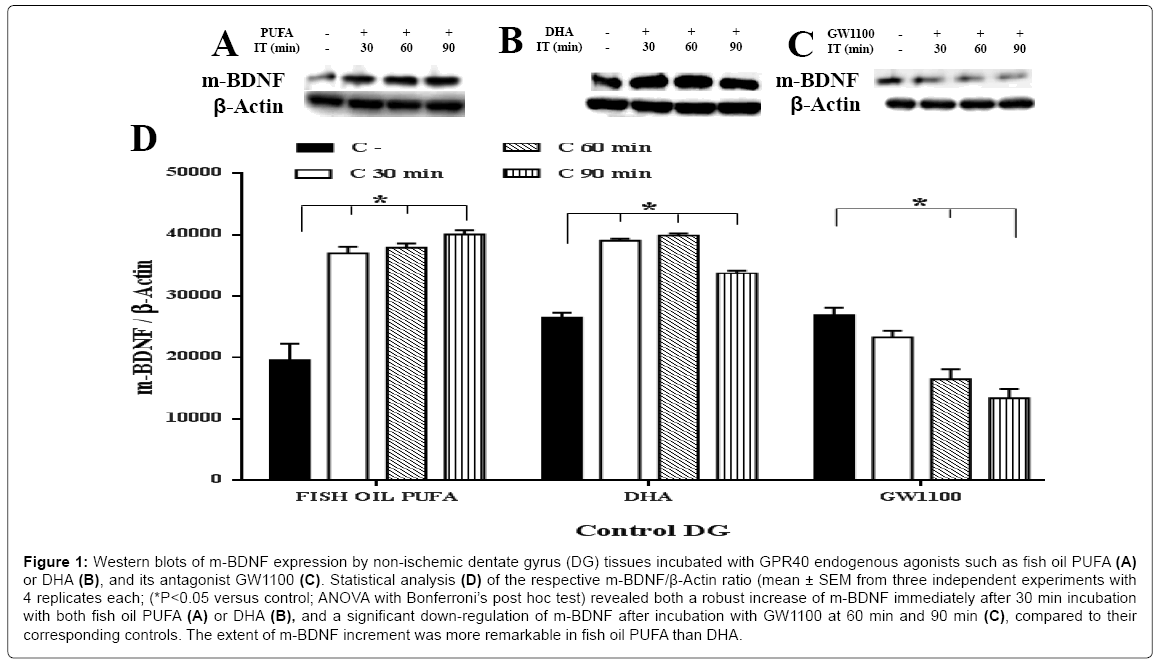
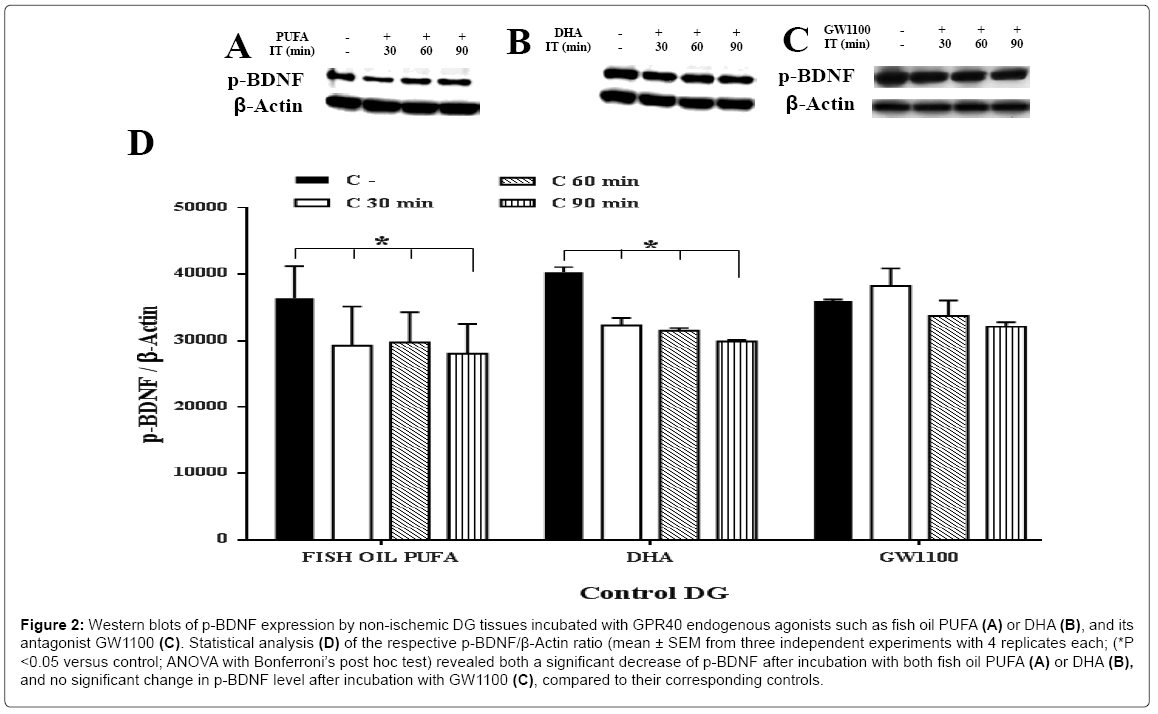
To elucidate the potential link between GPR40 activation/inhibition and BDNF synthesis, dynamic changes in the protein levels of both m-BDNF (14 kDa) and p-BDNF (32 kDa) were studied, using specific antibodies. The m-BDNF protein showed a robust increase immediately after 30 min incubation in response to the two putative endogenous agonists of GPR40 receptors such as fish oil PUFA or DHA, compared to their corresponding controls (Figure 1A, 1B and 1D). The extent of increment of m-BDNF synthesis was higher in fish oil PUFA (> 2 fold at all incubation time points) than DHA (approximately 1.6 fold at 30 min and 60 min), compared to their corresponding controls (ANOVA with Bonferroni’s post hoc test; P<0.05). These data indicate that synthesis of m-BDNF might not be dependent on the incubation time in in vitro . On the contrary, p-BDNF showed a significant (ANOVA with Bonferroni’s post hoc test; P<0.05) decrease after incubation with fish oil PUFA or DHA at all time points (Figure 2A, 2B and 2D).
Figure 1: Western blots of m-BDNF expression by non-ischemic dentate gyrus (DG) tissues incubated with GPR40 endogenous agonists such as fish oil PUFA (A) or DHA (B), and its antagonist GW1100 b. Statistical analysis (D) of the respective m-BDNF/β-Actin ratio (mean ± SEM from three independent experiments with 4 replicates each; (*P<0.05 versus control; ANOVA with Bonferroni’s post hoc test) revealed both a robust increase of m-BDNF immediately after 30 min incubation with both fish oil PUFA (A) or DHA (B), and a significant down-regulation of m-BDNF after incubation with GW1100 at 60 min and 90 min (C), compared to their corresponding controls. The extent of m-BDNF increment was more remarkable in fish oil PUFA than DHA.
Figure 2: Western blots of p-BDNF expression by non-ischemic DG tissues incubated with GPR40 endogenous agonists such as fish oil PUFA (A) or DHA (B), and its antagonist GW1100 (C). Statistical analysis (D) of the respective p-BDNF/β-Actin ratio (mean ± SEM from three independent experiments with 4 replicates each; (*P <0.05 versus control; ANOVA with Bonferroni’s post hoc test) revealed both a significant decrease of p-BDNF after incubation with both fish oil PUFA (A) or DHA (B), and no significant change in p-BDNF level after incubation with GW1100 (C), compared to their corresponding controls.
Next, to confirm that the BDNF synthesis was mediated by GPR40, its selective antagonist GW1100 for the GPR40 receptor [18,27], was used to inhibit the GPR40 function/activation. The m-BDNF synthesis showed a significant down-regulation at 60 min (approximately 0.6 fold) and 90 min (approximately 0.5 fold) after incubation, compared to the control (Figure 1C and 1D). On the contrary, p-BDNF showed no significant changes (Figure 2C and 2D) (ANOVA with Bonferroni’s post hoc test; P<0.05). These data indicate that the selective antagonist completely inhibited GPR40 function which in-turn prevented the synthesis of m-BDNF but not p-BDNF. The m-BDNF synthesis might be mediated by GPR40 while p-BDNF synthesis was unrelated to the GPR40 receptors or might not be mediated by GPR40 receptors.
Effects of GPR40 agonists/antagonist upon BDNF synthesis in the post-ischemic DG tissues
Since the m-BDNF synthesis was shown to be mediated by GPR40 in the non-ischemic DG tissues, the same experiments were done using the post-ischemic DG tissues to clarify whether the in vitro BDNF synthesis increases concomitant with in-vivo up-regulation of adult neurogenesis (based on our previous studies) after ischemia [19-23]. We focused on the post-ischemic day 7 and day 15 samples for this purpose, because newborn neurons in the monkey hippocampus showed a remarkable increase on the 2nd week after ischemia in our previous experimental paradigm studies [19,20].
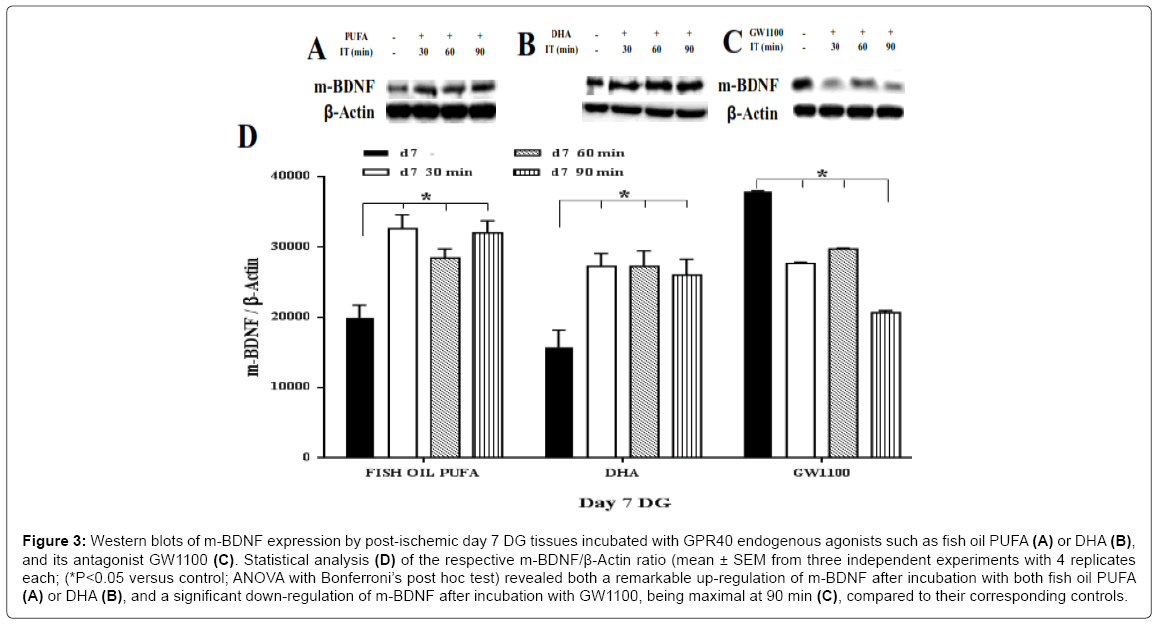
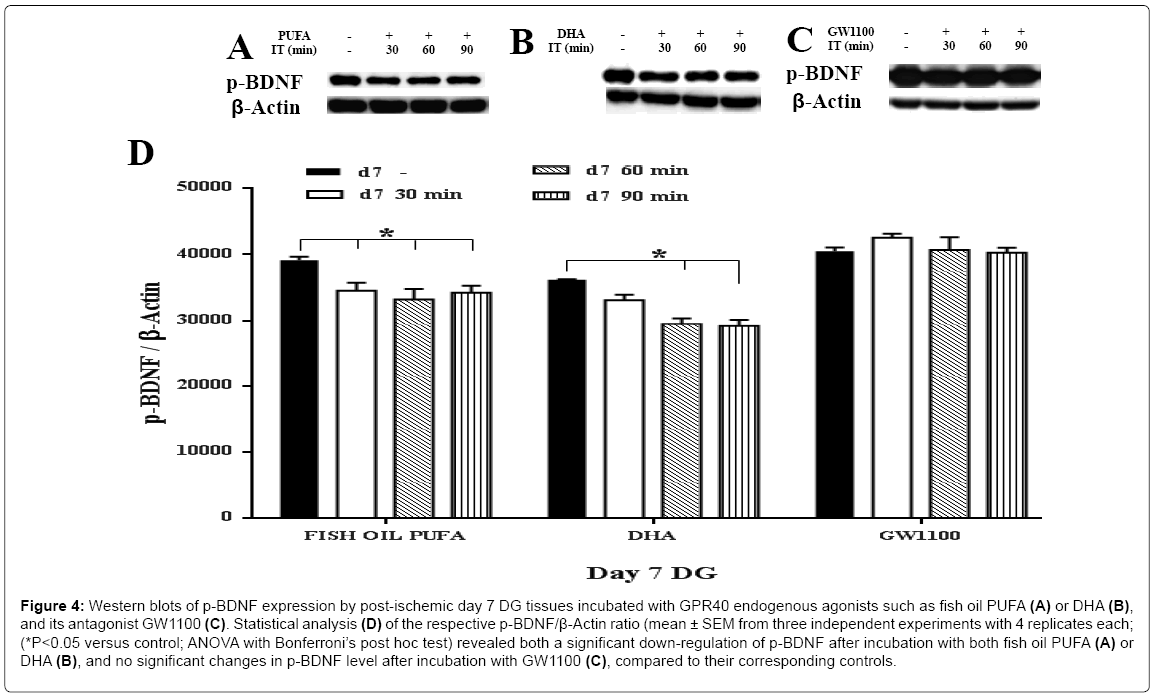
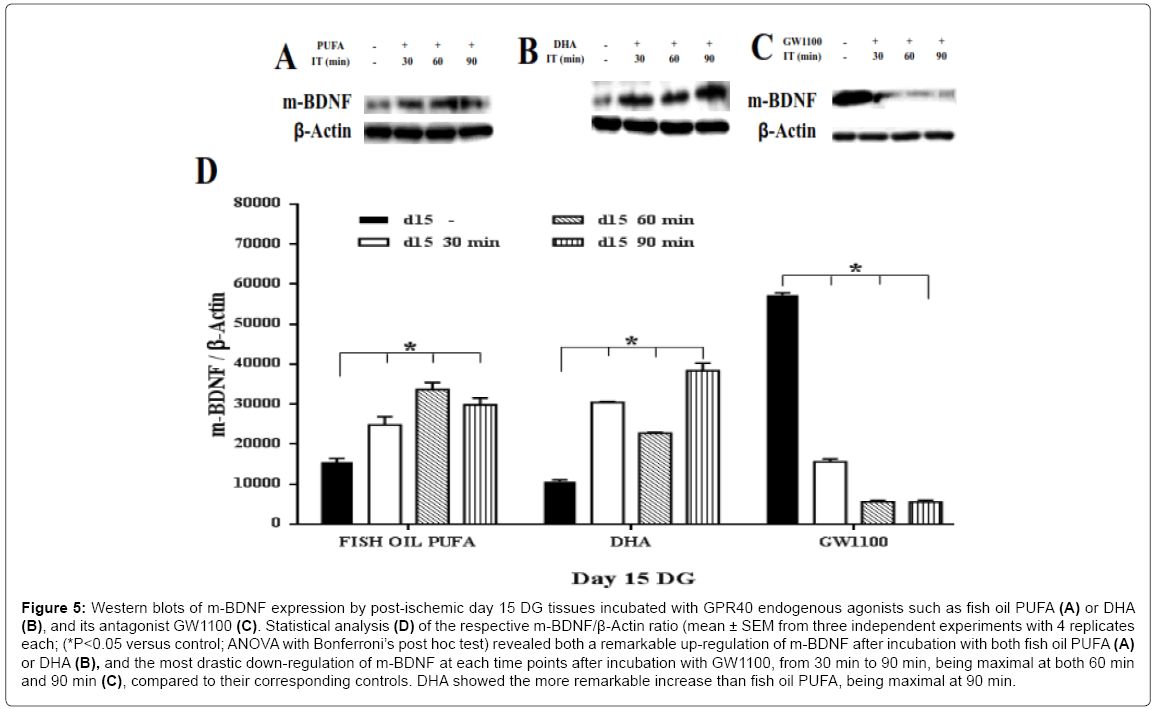
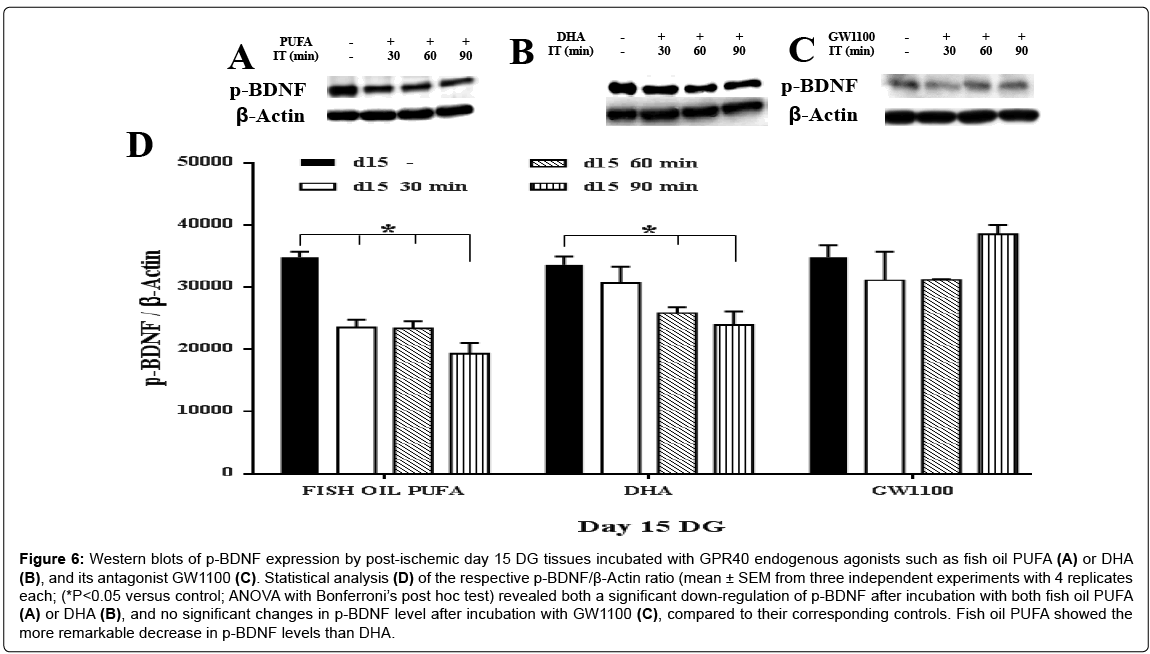
In response to two agonists of fish oil PUFA and DHA, a remarkable up-regulation of the m-BDNF synthesis was found on the both postischemic day 7 (approximately 1.7 fold for both agonists) (Figure 3A, 3B and 3D) and post-ischemic day 15 (Figure 5A, 5B and 5D ), compared to the controls (ANOVA with Bonferroni’s post hoc test; P < 0.05). On day 15, DHA showed a higher increment of m-BDNF synthesis (approximately 3 fold at 30 min; 4 fold at 90 min) then fish oil PUFA (approximately 1.7 fold at 30 min; > 2 fold at both 60, 90 min) compared to the corresponding controls. On the contrary, p-BDNF showed a significant (ANOVA with Bonferroni’s post hoc test; P<0.05) down-regulation on both post-ischemic day 7 (Figure 4A, 4B and 4D) and day 15 (Figure 6A, 6B and 6D), compared to the corresponding controls. On day 15, fish oil PUFA showed remarkable decrease (almost 0.5 fold) in the p-BDNF levels compared to DHA. The extent of downregulation was stronger on day 15 than day 7 (by comparing their fold decrements). These results indicate that PUFA may act as GPR40 receptor agonist to mediate synthesis of m-BDNF with consumption of p-BDNF especially in the post-ischemic DG tissues.
Figure 3: Western blots of m-BDNF expression by post-ischemic day 7 DG tissues incubated with GPR40 endogenous agonists such as fish oil PUFA Western blots of m-BDNF expression by post-ischemic day 7 DG tissues incubated with GPR40 endogenous agonists such as fish oil PUFA (A) or DHA (B), and its antagonist GW1100 (C). Statistical analysis (D) of the respective m-BDNF/β-Actin ratio (mean ± SEM from three independent experiments with 4 replicates each; (*P<0.05 versus control; ANOVA with Bonferroni’s post hoc test) revealed both a remarkable up-regulation of m-BDNF after incubation with both fish oil PUFA (A) or DHA (B), and a significant down-regulation of m-BDNF after incubation with GW1100, being maximal at 90 min (C), compared to their corresponding controls.
Figure 4: Western blots of p-BDNF expression by post-ischemic day 7 DG tissues incubated with GPR40 endogenous agonists such as fish oil PUFA (A) or DHA (B), and its antagonist GW1100 (C). Statistical analysis (D) of the respective p-BDNF/β-Actin ratio (mean ± SEM from three independent experiments with 4 replicates each; (*P<0.05 versus control; ANOVA with Bonferroni’s post hoc test) revealed both a significant down-regulation of p-BDNF after incubation with both fish oil PUFA (A) or DHA (B), and no significant changes in p-BDNF level after incubation with GW1100 (C), compared to their corresponding controls.
Figure 5: Western blots of m-BDNF expression by post-ischemic day 15 DG tissues incubated with GPR40 endogenous agonists such as fish oil PUFA (A) or DHA (B), and its antagonist GW1100 (C). Statistical analysis (D) of the respective m-BDNF/β-Actin ratio (mean ± SEM from three independent experiments with 4 replicates each; (*P<0.05 versus control; ANOVA with Bonferroni’s post hoc test) revealed both a remarkable up-regulation of m-BDNF after incubation with both fish oil PUFA (A) or DHA (B), and the most drastic down-regulation of m-BDNF at each time points after incubation with GW1100, from 30 min to 90 min, being maximal at both 60 min and 90 min (C), compared to their corresponding controls. DHA showed the more remarkable increase than fish oil PUFA, being maximal at 90 min.
Figure 6: Western blots of p-BDNF expression by post-ischemic day 15 DG tissues incubated with GPR40 endogenous agonists such as fish oil PUFA (A) or DHA (B), and its antagonist GW1100 (C). Statistical analysis (D) of the respective p-BDNF/β-Actin ratio (mean ± SEM from three independent experiments with 4 replicates each; (*P<0.05 versus control; ANOVA with Bonferroni’s post hoc test) revealed both a significant down-regulation of p-BDNF after incubation with both fish oil PUFA (A) or DHA (B), and no significant changes in p-BDNF level after incubation with GW1100 (C), compared to their corresponding controls. Fish oil PUFA showed the more remarkable decrease in p-BDNF levels than DHA.
To confirm that the BDNF synthesis was mediated by GPR40 also in the post-ischemic tissues, the inhibition experiment using GW1100 was done. The m-BDNF protein showed a significant down-regulation on the post-ischemic day 7 (approximately 0.5 fold at 90 min), compared to the control (Figure 3C and 3D). In contrast, the post-ischemic day 15 showed the most drastic down-regulation of m-BDNF at each time points (0.3 fold at 30 min; 0.1 fold at both 60, 90 min) compared to the control (ANOVA with Bonferroni’s post hoc test; P<0.05) (Figure 5C and 5D). Interestingly, p-BDNF showed no significant changes on both the post-ischemic day 7 (Figure 4C and 4D) and day 15 (ANOVA with Bonferroni’s post hoc test; P<0.05) (Figure 6C and 6D), presumably because absence of its consumption. These data indicate that GPR40 antagonist completely inhibited activation of GPR40 in the ischemic DG tissues also to prevent synthesis of m-BDNF but not p-BDNF. The m-BDNF synthesis, might be mediated by GPR40 in both the normal and post-ischemic DG tissues, but p-BDNF synthesis, might not be mediated by GPR40 or unrelated to the GPR40 receptors in both the normal and post-ischemic DG tissues.
While comparing fold increment or decrements, day 15 postischemic tissues showed more drastic synthesis of m-BDNF or strong decrease of p-BDNF compared to the day 7 and non-ischemic tissues.
Effects of GPR40 agonists/antagonist upon synthesis/ expression of furin in the non-ischemic DG tissues
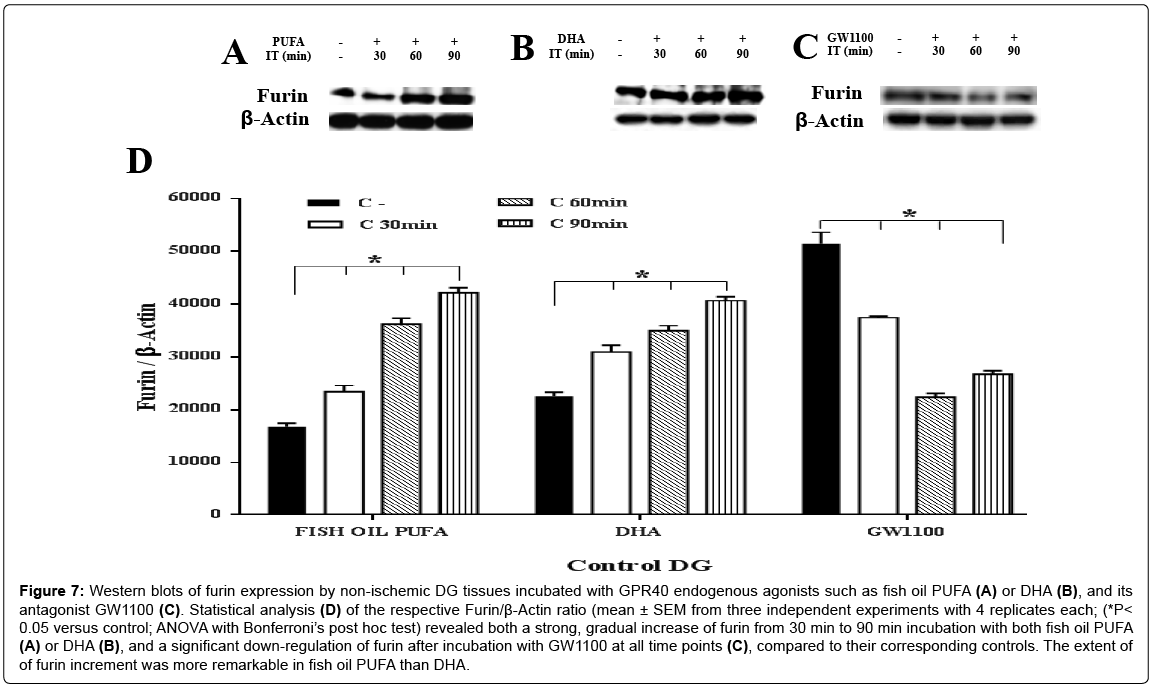
To confirm whether the synthesis of m-BDNF protein occurred via cleavage of p-BDNF by furin in response to the GPR40 signaling, dynamic changes in the levels of furin protein (87-88 kDa) were studied in the non-ischemic DG tissues, using a specific antibody. Furin showed a strong and gradual increase after 30~90 min incubation with the two GPR40 agonists such as fish oil PUFA or DHA, compared to their corresponding controls (ANOVA with Bonferroni’s post hoc test; P<0.05) (Figure 7A, 7B and 7D). Fish oil PUFA showed a very strong and gradual increase of furin (approximately 1.5 fold at 30 min; 2.5 fold at 90 min) than DHA (approximately 1.4 fold at 30 min; 1.8 fold at 90 min), compared to their corresponding controls.
Figure 7: Western blots of furin expression by non-ischemic DG tissues incubated with GPR40 endogenous agonists such as fish oil PUFA (A) or DHA (B), and its antagonist GW1100 (C). Statistical analysis (D) of the respective Furin/β-Actin ratio (mean ± SEM from three independent experiments with 4 replicates each; (*P< 0.05 versus control; ANOVA with Bonferroni’s post hoc test) revealed both a strong, gradual increase of furin from 30 min to 90 min incubation with both fish oil PUFA (A) or DHA (B), and a significant down-regulation of furin after incubation with GW1100 at all time points (C), compared to their corresponding controls. The extent of of furin increment was more remarkable in fish oil PUFA than DHA.
Next, to confirm that the BDNF synthesis by furin was mediated by GPR40, its selective antagonist GW1100 was used to inhibit the GPR40 function/activation. Furin showed a significant downregulation immediately after 30 min (0.7 fold) and 60 min and 90 min (approximately 0.5 fold) after incubation, compared to the control (ANOVA with Bonferroni’s post hoc test; P<0.05) (Figure 7C and 7D). These data indicate that the selective antagonist completely inhibited GPR40 function to down-regulate furin or reduce the furin protein levels, and that furin may be related to the BDNF synthesis via GPR40 signaling.
Effects of GPR40 agonists/antagonist upon synthesis/ expression of furin in the post-ischemic DG tissues
Since the m-BDNF synthesis by furin was shown to be mediated by GPR40 in the normal DG tissues, the same experiments were done using the post-ischemic DG tissues (day 7 and day 15) to clarify whether increment of the BDNF synthesis occur concomitant with upregulation of furin after ischemia.
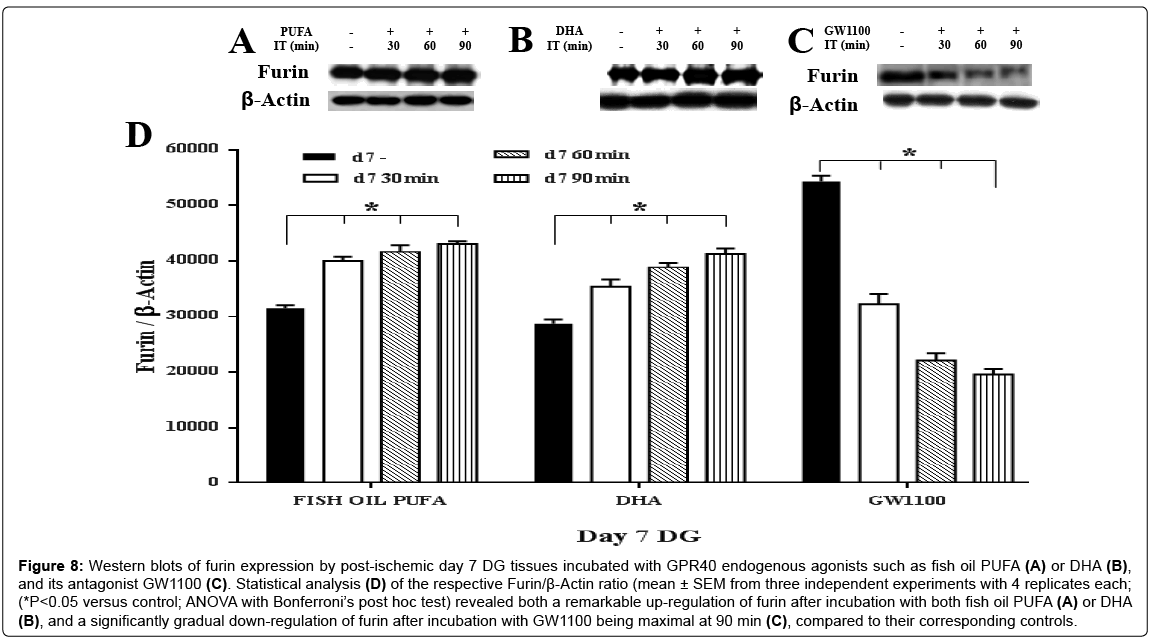
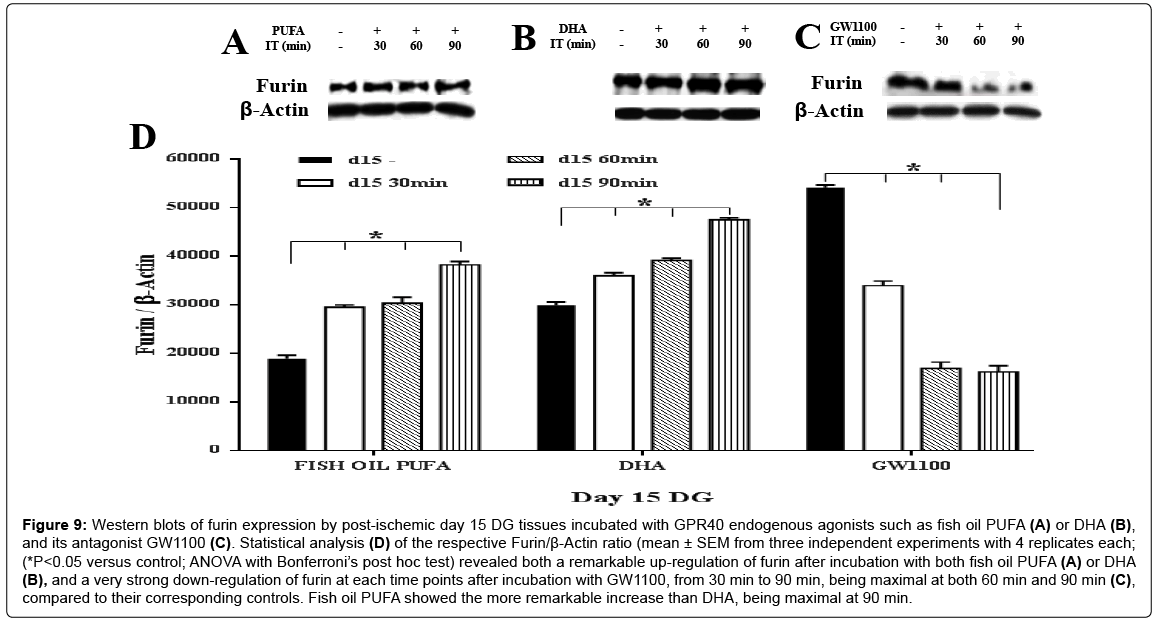
Remarkable up-regulation of furin was found on both postischemic day 7 (approximately 1.3 fold at 30 min; 1.5 fold at 90 min in response to the two agonists) (Figure 8A, 8B and 8D) and postischemic day 15 (fish oil PUFA showed a higher increment of furin (approximately 1.6 fold at 30 min; 2.1 fold at 90 min) then DHA (1.2 fold at 30 min; approximately 1.6 fold at 90 min)), compared to the controls (ANOVA with Bonferroni’s post hoc test; P<0.05) (Figure 9A, 9B and 9D). The extent of up-regulation was stronger on day 15 than day 7 (by comparing their fold increment). These results suggest that PUFA may act as GPR40 receptor agonist to mediate synthesis of m-BDNF with the aid of furin especially in the post-ischemic tissues.
Figure 8: Western blots of furin expression by post-ischemic day 7 DG tissues incubated with GPR40 endogenous agonists such as fish oil PUFA (A) or DHA (B), and its antagonist GW1100 (C). Statistical analysis (D) of the respective Furin/β-Actin ratio (mean ± SEM from three independent experiments with 4 replicates each; (*P<0.05 versus control; ANOVA with Bonferroni’s post hoc test) revealed both a remarkable up-regulation of furin after incubation with both fish oil PUFA (A) or DHA (B), and a significantly gradual down-regulation of furin after incubation with GW1100 being maximal at 90 min (C), compared to their corresponding controls.
Figure 9: Western blots of furin expression by post-ischemic day 15 DG tissues incubated with GPR40 endogenous agonists such as fish oil PUFA (A) or DHA (B), and its antagonist GW1100 (C). Statistical analysis (D) of the respective Furin/β-Actin ratio (mean ± SEM from three independent experiments with 4 replicates each; (*P<0.05 versus control; ANOVA with Bonferroni’s post hoc test) revealed both a remarkable up-regulation of furin after incubation with both fish oil PUFA (A) or DHA (B), and a very strong down-regulation of furin at each time points after incubation with GW1100, from 30 min to 90 min, being maximal at both 60 min and 90 min (C), compared to their corresponding controls. Fish oil PUFA showed the more remarkable increase than DHA, being maximal at 90 min.
Next, to confirm that m-BDNF synthesis by furin was mediated by GPR40 in the post-ischemic tissues also, the antagonist experiment was done. Furin showed a significant down-regulation on the post-ischemic day 7 (approximately 0.6 fold (30 min), 0.4 fold (60 min), and 0.35 fold (90 min)), compared to the control (ANOVA with Bonferroni’s post hoc test; P<0.05) (Figure 8C and 8D). In contrast, the post-ischemic day 15 showed very strong down-regulation of furin at each incubation time (0.6 fold at 30 min; 0.3 fold at both 60 and 90 min) (ANOVA with Bonferroni’s post hoc test; P<0.05) (Figure 9C and 9D), compared to the control. These data indicate that GPR40 antagonist completely inhibited GPR40 function which in-turn prevented the furin protein to cleave the stock p-BDNF into m-BDNF in the post-ischemic DG tissues also. The m-BDNF synthesis by furin might be mediated by GPR40 receptors in both the normal and post-ischemic DG tissues.
While comparing fold increment, both day 15 post-ischemic tissues and the non-ischemic tissues showed more drastic expression of furin compared to the day 7 post-ischemic tissues.
Discussion
In this study, we demonstrated that (1) the m-BDNF synthesis was mediated by GPR40 in both the normal (non-ischemic) and post-ischemic DG tissues, (2) the m-BDNF synthesis occurred more drastically in the day 15 post-ischemic tissues, compared to the day 7 and non-ischemic tissues while comparing their fold increment/upregulation, (3) the furin expression/synthesis was also mediated by GPR40 in both the normal and post-ischemic DG tissues, (4) the furin expression/synthesis also occurred more drastically in both the day 15 post-ischemic tissues and the non-ischemic tissues, compared to the day 7 post-ischemic tissues, (5) similarly, the decrease (consumption/ cleavage) of p-BDNF was the most remarkable on day 15, (6) cleavage of p-BDNF to m-BDNF by furin as well as syntheses of m-BDNF and furin in the DG tissues occurred immediately, within 30 minutes, after incubation with fish oil PUFA or DHA, and (7) the syntheses of both m-BDNF and furin were significantly inhibited by the selective GPR40 antagonist, which was maximal on day 15.
These findings suggest that (i) there may be a close relation between activation of GPR40 receptors and m-BDNF synthesis, but not with p-BDNF synthesis, and that (ii) the proteolytic cleavage of p-BDNF to m-BDNF occurs presumably by furin in the DG tissues in response to the PUFA signals via GPR40 receptors. It is reasonable that both the p-BDNF decrease and the concomitant increment of both m-BDNF and furin were the most remarkable on day 15 after ischemia. Although later time point (90 min) showing slight decrease in synthesis of m-BDNF with fish oil PUFA, but considering overall synthesis of both m-BDNF and furin with concomitant cleavage/decrease of p-BDNF were highly remarkable on day 15 after ischemia in response to the two agonists, fish oil PUFA and DHA while comparing to the day 7 postischemic and normal (non-ischemic) DG tissues. This is presumably/ probably because adult hippocampal neurogenesis peaked on day 15 which is shown by our previous monkey experimental paradigms studies [19,20,22], and m-BDNF was the most necessary for the neuronal differentiation at this time point.
The exact production/release sites of BDNF (either mature- or pro- BDNF) are still unknown, because of both (i) the extremely low level of BDNF expression in most of the neuronal tissues, and (ii) lack of evidence regarding the subcellular localization of endogenous BDNF in neurons. However, endogenously-expressed BDNF were found [29,30] at highest levels in the presynaptic neuronal processes such as cortical axons projecting to the striatum, and in the hippocampal mossy fibers projecting to CA3 pyramidal cells; whereas relatively low/ negligible levels were found [11] in the postsynaptic neuronal processes (hippocampal CA1 and cortical pyramidal neurons are few). It remains unelucidated whether endogenously-expressed BDNF is mainly located in axons (presynaptic) or dendrites (postsynaptic), or whether release of endogenous BDNF occurs from both. Accordingly, the ultimate answer to the production/release site of endogenously-expressed BDNF would not be available until an appropriate staining procedure can detect a single BDNF vesicle at the endogenous expression level in the living tissue [31].
BDNF is synthesized as a precursor 32 kDa p-BDNF protein, proteolytically cleaved by enzymes like furin or pro-convertases intracellularly, and released as the 14 kDa m-BDNF [31,32]. It is possible that other proteases except for furin, may be activated by the internal Ca2+ mobilization and implicated for the p-BDNF cleavage. Since the present data showed that increases of m-BDNF and furin concomitant with a decrease of p-BDNF occurred in both the normal and post-ischemic DG tissues in response to the two agonists, it is probable that GPR40 activation stimulated furin to cleave p-BDNF to m-BDNF intracellularly. Likewise, p-BDNF might be secreted extracellularly and might be cleaved by metalloproteinases and/ or plasmin to m-BDNF [11,33]. Among four possible proteolytic enzymes that can be involved in p-BDNF cleavage including furin, proconvertases, metalloproteinases and plasmin, the strongest candidate that can be activated by PUFA-GPR40 signaling may be furin. However, altered levels in furin protein concentrations as shown here, but not actual increase of the enzyme activity, are not a conclusive evidence of GPR40-mediated furin enzyme activity. Additional experiments would strengthen role of furin, by demonstrating that specific inhibition of its enzyme activity leads to a loss of agonist-mediated increase in m-BDNF levels.
It is well-known that furin cleaves p-BDNF to m-BDNF in the trans-Golgi network to release m-BDNF there [10,31,34]. The present results using the DG tissues are in complete agreement with the previous reports. In the normal tissues, furin is expressed at very low levels, however, its up-regulation occurs in a variety of human cancers, because furin expression is enhanced during hypoxia in cancer cells [35,36]. In agreement with this, ischemia might be also a common pathological state in the brain that can enhance furin expression. For example, Yokota et al. [37] first reported that up-regulation of furin gene occurs twice in the rat hippocampus after transient global cerebral ischemia, compared to the control. Chen et al. [38] very recently analysed furin expression in the cultured astrocytes being exposed to the ischemia-like oxygen and glucose-deprivation, and observed a significant up-regulation of furin. They reported that furin-mediated BDNF up-regulation occurs after oxygen and glucose-deprivation. These reports [37,38] are consistant with the present results. Accordingly, it is likely that furin-mediated m-BDNF synthesis occurs via GPR40 activation in the post-ischemic DG tissues especially on the post-ischemic day 15 which is shown highest up-regulation of both in this study. Here, the two putative endogenous agonists of the GPR40 receptors such as fish oil PUFA and DHA remarkably activated furin and m-BDNF syntheses, while GPR40 selective antagonist GW1100 completely inhibited syntheses of both. It may be reasonable to speculate that the PUFA-GPR40 molecular signaling acts as a trigger for activation and up-regulation of furin to synthesize m-BDNF from p-BDNF. Then, such a question emerges which molecular cascade was induced by the PUFA-GPR40 signaling as a trigger of furin up-regulation and activation?
Furin promoters are known to have putative binding sites for hypoxia-inducible factor-1 (HIF-1), a transcription complex that plays a pivotal role in the cellular adaptation to hypoxia. McMahon et al. [39] demonstrated that the levels of fur mRNA, encoding furin, were remarkably increased upon the hypoxic challenge, and that the hypoxic/ HIF-1 regulation of furin correlated with an increased proteolytic activation. The authors speculate that GPR40 activation at the in-vivo ischemic insult induced a rapid and sustained phosphorylation of endogenous p42/p44 MAPK. The downstream MAPK cascade elements enhanced nuclear translocation of mothers against decapentaplegic homolog 2 (Smad2), where it may interact with DNA-binding proteins and direct transcription of the fur gene [40]. In parallel, PUFA bind to GPR40 in vitro and trigger an intracellular cascade that results in Ca2+ release form the ER [19,21]. 1) Pre-stocked BDNF is reported by Matsumoto et al. [10] that p-BDNF is rapidly converted intracellularly to m-BDNF, and the converted m-BDNF, but not p-BDNF, accumulates in the neuronal cells and is released upon stimulation. 2) Since GPR40 is also expressed in the glial cells in the monkey brain [20], it is also probable GPR40 stimulated also glial cell stores. It is coceivable that GPR40 receptors may not only stimulated furin to synthesize m-BDNF by p-BDNF cleavage, but also stimulated internal cell stores such as neurons, astrocytes and glial cell etc.. to release the pre-stocked m-BDNF. This are the two probable reasons in this in vitro study why some DG samples did not show a concomitant decrease in the p-BDNF protein level, despite the drastic/double fold increase of m-BDNF synthesis.
In this study, m-BDNF synthesis from the cleavage of pre-stocked p-BDNF by furin and release of m-BDNF from the cell stores [10], occurred more drastically on the post-ischemic day 15 DG, compared to the non-ischemic controls and day 7 post-ischemic samples. This is probably because GPR40 up-regulation occurred most dramatically on day 15 after ischemia which is shown by previous studies [20], and up-regulated GPR40 being involved in the day 15 homogenized tissues could efficiently respond to the two ligands of fish oil PUFA or DHA for inducing internal Ca2+ mobilization [22] necessary for both activating furin [37] and stimulating neurons, astrocytes and glial cell etc.. to release the stored m-BDNF. In-vivo up-regulation of furin and GPR40 in the day 15 DG, presumably occurred in response to the rapid in vitro Ca2+ mobilization from the remaining internal Ca2+ stores such as endoplasmic reticulum and mitochondria. These might facilitate furin-mediated cleavage of p-BDNF to m-BDNF and also release the pre-stocked m-BDNF.
By activating GPR40 and furin, PUFA may be related to adult neurogenesis, neuronal development and the concomitant synaptic plasticity for learning and memory. The present data provide one of the possible molecular mechanisms by which m-BDNF synthesis increases in the post-ischemic DG tissues, and thereby impacts bioavailability of GPR40 for synaptogenesis and synaptic plasticity in the newborn neurons. However, in response to conjugated linoleic acids or trans isomers of arachidonic acid, the non-esterified fatty acid receptor GPR40 was recently demonstrated in rodents to mediate lipotoxicity to neurons, microvessels, or β-cells [41]. Since non-esterified fatty acids in the blood are not infrequently elevated and/or oxidized excessively in the human subjects suffering from metabolic syndrome, type 2 diabetes, Alzheimer’s disease, Parkinson’s disease etc., the pharmacological effects of PUFA binding with GPR40 are extremely complex. It is likely that according to the extent of GPR40 activation, PUFA effects may be beneficial or detrimental. We must always keep in mind ‘dual effects of GPR40 for human health’ in response to diverse fatty acids. Since our understanding of the pharmacology of GPR40 in the brain is still incomplete, not only its physiological effects but also its pathological effects should be studied further (Figure 10).
Figure 10: Schematic flow chart of the PUFA-GPR40-BDNF signaling pathway. Initially, PUFA bind to GPR40 and presumably activate intracellular downstream messengers such as Gαq, PLC, and PKC that results in Ca2+ release from the ER [21]. Increased Ca2+ levels lead to activation of different protein kinases such as CaMKIV, Akt, ERK1/2, or MAPK which phosphorylate CREB, a key transcription factor in various gene regulation including Bdnf [18,19,23]. These activation finally leads to synthesis of m-BDNF either by (i) cleavage of p-BDNF to m-BDNF intracellularly by furin etc., or (ii) stimulating neurons, astrocytes and glial cells etc. to release m-BDNF from the internal cell stores. These two processes lead to increment of m-BDNF synthesis and decrease of p-BDNF levels for regulating Bdnf gene transcription. This, in turn, regulates adult neurogenesis, synaptic plasticity, learning and memory. Gαq, G protein subunits α, q; Akt, protein kinase B; BDNF, brain-derived neurotrophic factor; CaMKIV, Ca2+/calmodulin-dependent protein kinase type IV; CREB, cAMP response element-binding protein; ER, endoplasmic reticulum; ERK1/2, extracellular signal-regulated kinase; GPR40, G protein-coupled receptor 40; MAPK, mitogen-activated protein kinase; pCREB, phosphorylated cAMP response element-binding protein; PKC, protein kinase C; PLC, phospholipase C; PUFA, polyunsaturated fatty acids.
Conclusion and Future Perspectives
In summary, we have demonstrated up-regulation of m-BDNF synthesis in response to two GPR40 agonists such as fish oil PUFA and DHA and its down-regulation in response to GPR40 selective antagonist GW1100 by using the normal and post-ischemic dentate gyrus (DG) tissues of monkey hippocampus. GPR40 antagonist inhibited m-BDNF synthesis but not p-BDNF, whereas both two GPR40 agonists stimulated m-BDNF synthesis conceivably via the enzyme furin. As simultaneous changes of GPR40, m-BDNF synthesis, and furin were observed, GPR40 might be related to m-BDNF synthesis; however, implication of furin should be confirmed by further studies. Considering our previous studies along with the present data, we can conclude that 1) fish oil PUFA or DHA may be responsible for m-BDNF synthesis which is essential for regulating synaptogenesis and adult neurogenesis, and 2) this effect may be dependent on furin-dependent cleavage of p-BDNF to generate m-BDNF.
Acknowledgements
This work was supported by the grant from Indian Council of Agricultural Research, New Delhi, India under ICAR International Fellowship 2010-11, No: 29- 1/2009-EQR/Edn. and co-financed by (Kiban-Kennkyu (B): 18390392, 22390273) Japanese Ministry of Education, Culture, Sports, Science and Technology. The authors have no conflict of interest to declare.
References
- Barde YA, Edgar D, Thoenen H (1982) Purification of a new neurotrophic factor from mammalian brain. EMBO J 1: 549-553.
- Cohen S, Levi-Montalcini R, Hamburger V (1954) A nerve growth-stimulating factor isolated from sarcom as 37 and 180.ProcNatlAcadSci U S A 40: 1014-1018.
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, et al. (1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389: 856-860.
- Butowt R, von Bartheld CS (2001) Sorting of internalized neurotrophins into an endocytictranscytosis pathway via the Golgi system: ultrastructural analysis in retinal ganglion cells J Neurosci 21: 8915-8930.
- Murer MG, Yan Q, Raisman-Vozari R (2001) Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. ProgNeurobiol 63: 71-124.
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31-39.
- Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23: 649-711.
- Cunha C, Brambilla R, Thomas KL (2010) A simple role for BDNF in learning and memory? Front MolNeurosci 3: 1.
- He C, Qu X, Cui L, Wang J, Kang JX (2009) Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. ProcNatlAcadSci U S A 106: 11370-11375.
- Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, et al. (2008) Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci 11: 131-133.
- Lessmann V, Gottmann K, Malcangio M (2003) Neurotrophin secretion: current facts and future prospects. ProgNeurobiol 69: 341-374.
- Barker PA (2009) WhitherproBDNF? Nat Neurosci 12: 105-106.
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, et al. (2009) Neuronal release of proBDNF. Nat Neurosci 12: 113-115.
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, et al. (2009) Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation, and cell survival. Mol Brain 2: 27.
- Yang J, Hargrove CL, Siao CJ, Marinic T, Clarke R, et al. (2014) ProBDNF negatively regulates neuronal remodeling, synaptic transmission and synaptic plasticity in hippocampus. Cell Rep 7: 796-806.
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, et al. (2003) The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J BiolChem 278: 11303-11311.
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, et al. (2003) Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173-176.
- Zamarbide M, Etayo-Labiano I, Ricobaraza A, Martínez-Pinilla E, Aymerich MS, et al. (2014) GPR40 activation leads to CREB and ERK phosphorylation in primary cultures of neurons from the mouse CNS and in human neuroblastoma cells. Hippocampus 24: 733-739.
- Boneva NB, Yamashima T (2012) New insights into "GPR40-CREB interaction in adult neurogenesis" specific for primates. Hippocampus 22: 896-905.
- Ma D, Lu L, Boneva NB, Warashina S, Kaplamadzhiev DB, et al. (2008) Expression of free fatty acid receptor GPR40 in the neurogenic niche of adult monkey hippocampus. Hippocampus 18: 326-333.
- Ma D, Zhang M, Larsen CP, Xu F, Hua W, et al. (2010) DHA promotes the neuronal differentiation of rat neural stem cells transfected with GPR40 gene. Brain Res 1330: 1-8.
- Yamashima T (2008) A putative link of PUFA, GPR40 and adult-born hippocampal neurons for memory. ProgNeurobiol 84: 105-115.
- Yamashima T (2012) 'PUFA-GPR40-CREB signaling' hypothesis for the adult primate neurogenesis. Prog Lipid Res 51: 221-231.
- Yamashima T, Saido TC, Takita M, Miyazawa A, Nishijyo H, et al. (1996) Transient brain ischemia provokes Ca2+ , PIP2 and calpain responses prior to delayed neuronal death in monkeys. Eur J Neurosci 8: 1932-1944.
- Yamashima T, Kohda Y, Tsuchiya K, Ueno T, Yamashita J, et al. (1998) Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: A novel strategy for neuroprotection based on ‘calpain-cathepsin hypothesis’. Eur J Neurosci 10: 1723-1733.
- Yamashima T (2000) Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. ProgNeurobiol 62: 273-295.
- Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, et al. (2006) Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol 148: 619-628.
- Ho Y, Logue E, Callaway CW, DeFranco DB (2007) Different mechanisms account for ERK activation in distinct brain regions following global ischemia and reperfusion. Neuroscience 145: 248-255.
- Smith MA, Zhang LX, Lyons WE, Mamounas LA (1997) Anterograde transport of endogenous brain-derived neurotrophic factor in hippocampal mossy fibers. Neuroreport 8: 1829-1834.
- Danzer SC, McNamara JO (2004) Localization of brain-derived neurotrophic factor to distinct terminals of mossy fiber axons implies regulation of both excitation and feed forward inhibition of CA3 pyramidal cells. J Neurosci 24: 11346-11355.
- Lessmann V, Brigadski T (2009) Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res 65: 11-22.
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, et al. (2001) Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J BiolChem 276: 12660-12666.
- Mizoguchi H, Nakade J, Tachibana M, Ibi D, Someya E, et al. (2011) Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J Neurosci 31: 12963-12971.
- Seidah NG, Benjannet S, Pareek S, Chrétien M, Murphy RA (1996) Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proproteinconvertases. FEBS Lett 379: 247-250.
- Bassi DE, Mahloogi H, Al-Saleem L, Lopez De Cicco R, Ridge JA, et al. (2001) Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. MolCarcinog 31: 224-232.
- Mercapide J, Lopez De Cicco R, Bassi DE, Castresana JS, Thomas G, et al. (2002) Inhibition of furin-mediated processing results in suppression of astrocytoma cell growth and invasiveness. Clin Cancer Res 8: 1740-1746.
- Yokota N, Uchijima M, Nishizawa S, Namba H, Koide Y (2001) Identification of differentially expressed genes in rat hippocampus after transient global cerebral ischemia using subtractive cDNA cloning based on polymerase chain reaction. Stroke 32: 168-174.
- Chen Y, Zhang J, Deng M (2015) Furin mediates brain-derived neurotrophic factor upregulation in cultured rat astrocytes exposed to oxygen–glucose deprivation. J Neurosci Res 93: 189-194.
- McMahon S, Grondin F, McDonald PP, Richard DE, Dubois CM (2005) Hypoxia-enhanced expression of the proproteinconvertasefurin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J BiolChem 280: 6561-6569.
- Blanchette F, Rivard N, Rudd P, Grondin F, Attisano L, et al. (2001) Cross-talk between the p42/p44 MAP kinase and Smad pathways in transforming growth factor beta 1-induced furin gene transactivation. J BiolChem 276: 33986-33994.
- Yamashima T (2015) Dual effects of the non-esterified fatty acid receptor 'GPR40' for human health. Prog Lipid Res 58: 40-50.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 11940
- [From(publication date):
March-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11034
- PDF downloads : 906