Role of Ginger as Anti-inflammatory and Anti-apoptotic in Protection of Liver Damage Induced by Metalaxyl Fungicide in Male Albino Rats
Received: 04-May-2018 / Accepted Date: 26-May-2018 / Published Date: 02-Jun-2018 DOI: 10.4172/2161-0681.1000346
Keywords: Metalaxyl; Ginger; Oxidative stress; Pro-inflammatory cytokines; apoptosis
Introduction
Metalaxyl, N-(2, 6-Dimethylphenyl)-N-(methoxyacetyl)-DLalanine methyl ester, is a systemic benzenoid fungicide belonging to the most widely known member of the amide group. It is used in foliar spray mixtures for tropical and subtropical crops, as a soil treatment for the control of soil-borne pathogens, and as a seed treatment to control downy mildews, fungal diseases on fruits, peanuts, ornamentals, soybeans, cotton and grasses [1]. It is registered for use in many states worldwide including USA, European nations, Australia and India [2]. Recently, contamination of environmental with fungicide represents one of the problems of the region as well as worldwide importance. The presences of these toxic chemicals were recorded in water, air, house dust and in the tissues of non-occupationally exposed people, especially in the adipose tissue, blood and urine [3]. Several fungicides exert their biological effects mainly through electrophilic attack of cellular constituents with simultaneous generation of reactive oxygen species (ROS) [4]. ROS is a major cellular source of oxidative stress [5]. Metalaxyl is highly soluble in water and has the potential to reach groundwater and stable under when exposed to sunlight, with a half-life of 400 days (United States Environmental Protection Agency 1994). It has been recorded to have cytogenetic effects on animal chromosomes in vitro [6]. Also, metalaxyl exposure leads to abnormal haematological and biochemical activities induce oxidative stress and an observable toxicity [7]. It causes histological and biochemical changes in liver most probably through oxidative stress [8].
Over several years, herbal plants have been believed to be effective medicinal factor and recently remarkable amount of drugs have been prepared from expected sources, several among them based on their use in habitual medicine. Medicinal plants are of vast importance to the health of individuals and communities. Herbal medicines supply the health needs of almost 80% of the world’s population [9]. Ginger (Zingiber officinal Roscoe) is example of botanicals that gains popularity amongst modern physicians and its underground rhizomes are medicinally useful parts [10]. It relief the symptoms of vomiting and nausea associated with motion sickness, surgery and pregnancy [11]. Recently, ginger and some of its constituents are effective against cytokines synthesized and secreted at inflammation sites [12]. Ginger and its phytochemicals are also shown to decrease pro-inflammatory cytokines levels (TNF-α) and interlukin-6 (IL-6), and to reduce the elevated expression of NF-κB [13]. Many studies suggested the protective effects of ginger extracts against bromobenzene induced liver toxicity [14], and fungicide induced hepatotoxicity [15].
The aim of this study was to determine the protective effect of orally administered of ginger against oxidative damage induced by metalaxyl as proved by lipid peroxidation and histopathological changes in male albino rats.
Material and Methods
Materials
Metalaxyl and ginger were purchased from Zhejiang Heben Pesticide & Chemicals Co., Ltd. China., and Aktin Chemicals, Inc. company (Nature connecting health), Chengdu, China., respectively. Metalaxyl was dissolved in 430 μl DMSO and 5.577 ml Propylene glycol, freshly prepared and administered orally three times per week at a dose of 130 mg/kg b.wt (1/10 of LD50) [16]. Ginger was dissolved in distilled water and administered orally to rats at a dose level of (100 mg/kg b.wt) once daily for 8 weeks [17]. All the other chemicals and reagents were of the highest purified grades available purchased from El Gomhouria Company for Trading Chemicals and Medical Appliances, Egypt.
Methods
Male albino rats, 150 days of age and weighing 150-200 g, were purchased laboratory Animals Research Center, Faculty of Veterinary Medicine, Benha University. Animals were kept at room temperature (25°C) and relative humidity (50-65%) at a 12 h light/dark cycle with ad libitum access to standard rodent diet and water. Rats were allowed to acclimatize to the animal facility for at least 15 days before the start of the experiments.
In the study, a total of 42 male rats were randomly allocated into 3 groups, of 14 animals each. All animals were fasted overnight before the experiment. The first group (normal control group) received orally 1 ml distilled water. The second group (the metalaxyl exposed group) rats received metalaxyl at a dose level of 1/10 LD50 (130 mg/kg b.wt) orally three times per weeks for 8 weeks. The third group (metalaxyl+ginger group) rats received metalaxyl (130 mg/Kg b.wt) orally three times per week and treated daily with ginger (100 mg/kg b.wt/orally) for 8 weeks. The experimental protocols were also approved by the Animal Care and Use Committee at Benha University and are in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Blood collection
Random blood samples from each group were collected by ocular vein puncture into non-anticoagulant tubes under anesthesia at 4 and 8 weeks from the onset of experimental.
Preparation of liver homogenates for L-MDA
Animals were killed by Percussive blow to the head; liver tissues were excised and washed immediately with ice cold 0.9% NaCl. Liver tissues were cut, weighed and minced into small pieces, homogenized with a glass homogenizer in 9 volume of ice-cold 0.05 mM potassium phosphate buffer (pH7.4) to make 10% homogenates. The homogenates were centrifuged at 6000 r.p.m for 15 minutes at 4°C then the resultant supernatant were used.
Preparation of liver homogenates for molecular gene expression
About 0.5 of liver tissue put in eppendorf tubes and were immediately kept in liquid nitrogen and stored at -80°C till RNA extraction for determination of the following: NF-kB, TNF-α, PPARα, Caspase-8 and DNA fragmentation by comet assay.
Preparation of liver tissue for histopathological and immunohistochemical analysis
A sample of each tissue was excised and fixed in 10% neutral buffered formalin solution and subjected for histopathological examination according to the technique described by [18]. Immunohistochemical staining (BAX) examination according to the technique described by [19,20].
The severity of the microscopical lesions mainly in the liver were classified according to Ortatatli et al. [21] as following: degree 1, slight changes including congestion of blood vessels, mild hepatocellular swelling caused by hydropic degeneration; moderate (degree 2); clear hepatocellular swelling in centrilobular and midzonal areas, in association with leukocytic cellular aggregation; severe (degree 3); diffuse and severe hepatocellular swelling and necrotic areas.
Measurement of serum NO level
Serum NO level was colorimetrically determined according to method described by Mesbah at. Al [22]. Total nitrite was measured in acid medium. The formed nitrous acid diazotise sulphanilamide and the product are coupled with N-(1-naphthyl) ethylene-diamine. The resulting azo dye has a bright reddish-purple color which can be measured at 540 nm. NO is expressed as μmol/l.
Measurement of L-MDA in liver tissue homogenates
Liver L-MDA, as a marker for lipid peroxidation, was determined according to Mesbah et al. [23]. The principle of the methods is depends on determination of thiobarbituric acid reactive substance content. Thiobarbituric reacts with l-malondialdehyde in acidic medium at temperature of 100°C for 15 minutes to form thiobarbituric acid reactive products. The absorbance of the resultant color product can be measured at 532 nm. L-MDA is expressed as nmol/g tissue.
Measurement of NF-kB, TNF-α, PPAR-α and caspase-8 gene expression
The mRNA expression contents of NF-κB , TNF-α, PPARα and caspase-8 were determined by real-time quantitative polymerase chain reaction (real- time qPCR) analysis in liver of rats. β-actin was used as a loading control. Total RNA was isolated from liver using the High Pure RNA Isolation Kit (iNtRON Biotechnology, easy-REDTM Total RNA Extraction Kit) according to the manufacturers’ instructions. From each sample, cDNA was reversely transcribed using a RevertAidTM First Strand cDNA Synthesis Kit (Thermo Scientific, Fermentas, #EP0451, USA). Then, real-time quantitative PCR amplification carried out on Faststart Universal SYBR Green Master (Roche, GER). Target gene was was normalized with β-actin by used the 2-ΔΔCt method [24].
Measurement of DNA fragmentation by comet assay
DNA damage was estimated by alkaline single-cell gel electrophoresis (comet assay) according to the protocol described by Singh et al. [25]. Homogenates of liver were dispersed and immobilized in an agarose gel on microscope slides. The slides were placed in a lysis solution to lyse and disperse cell components, leaving the DNA immobilized in the agarose. The DNA was denatured for a specified period of time by immersing the slides in an alkaline solution. Strand breaks in the denatured cellular DNA resulted in supercoil relaxation. Given a sufficient degree of relaxation the application of an electric field across the slides created a motive force by which the charged DNA may migrate through the surrounding agarose away from the immobilized main bulk of nuclear DNA. Following electrophoresis, the slides were rinsed in neutral buffer and the gel and its contents were fixed using ethanol. The DNA in the fixed slides was stained with a fluorescent DNA-specific stain such as ethidium bromide, propidium iodide, 4, 6 diamidino-2-phenylindole hydrochloride (DAPI), SYBER green, Gel Red and benxoxazolium-4-quinolinum oxazole yellow homodimer (YOYO-1). Stained slides are examined using a fluorescent microscope [26].
Statistical analysis
The results were expressed as mean and stander error ( ± SE) using SPSS (13.0 software, 2009) program. The data were analyzed using oneway ANOVA [27], followed by Duncan’s test. Values were considered statistically significant when p<0.05.
Results
Effect on NO and lipid peroxidation
A significant increase in serum NO and liver L-MDA in metalaxyl intoxicated rats all over the periods of the experiment as compared to control. Conversely, ginger treatment to metalaxyl intoxicated male rats showed a significant decrease in NO and L-MDA concentration when compared to metalaxyl exposed group (Table 1).
| Animal groups | NO (μmol/l) | L-MDA (nmol/ g tissue) | ||
|---|---|---|---|---|
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | |
| Group Ι: Normal control |
19.36 ± 0.69 c | 23.17 ± 1.28 b | 4.08 ± 0.01 d | 4.46 ± 0.05 c |
| Group Π : Metalaxyl |
28.88 ± 0.9 a | 34.31 ± 1.42 a | 7.64 ± 0.13 a | 8.37 ± 0.07 a |
| Group III: Metalaxyl+ginger |
20.47 ± 0.49 c | 22.82 ± 0.99 b | 4.99 ± 0.18 c | 5.84 ± 0.47 b |
Data are presented as (Mean ± S.E). S.E=Standard error. Mean values with different superscript letters in the same column are significantly different at (P = 0.05).
Table 1: Effect of ginger administration on serum NO and liver tissue L-MDA levels in metalaxyl intoxicated male rats.
Effect on NF-kB, TNF-α, PPAR-α and caspase-8 gene expression
A significant up-regulation of NF-κb, TNF-α and caspase-8 genes expression level while a significant down-regulation of PPAR-α in livers of metalaxyl-intoxicated rats all over the period of experiment compared to control. However, ginger treatment to metalaxyl intoxicated male rats showed a significant down-regulation of NF-κb, TNF-α and caspase-8. On the other hand, there was a significant upregulation in liver PPAR-α level compared to metalaxyl exposed group (Table 2).
| Animal groups | Fold change in NF-kB gene expression | Fold change in TNF-α gene expression | Fold change in PPAR-α gene expression | Fold change in Caspase-8 gene expression | ||||
|---|---|---|---|---|---|---|---|---|
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | |
| Group Ι: Normal control |
1.00 ± 0.09 d | 1.00 ± 0.08 d | 1.00 ± 0.08 e | 1.00 ± 0.10 e | 1.00 ± 0.07 a | 1.00 ± 0.08 a | 1.00 ± 0.08 d | 1.00 ± 0.07 d |
| Group Π : Metalaxyl |
13.00 ± 0.34 a | 17.75 ± 0.38 a | 10.13 ± 0.37 a | 12.82 ± 0.39 a | 0.42 ± 0.0 4c | 0.32 ± 0.03 c | 5.43 ± 0.17 a | 9.32 ± 0.23 a |
| Group III: Metalaxyl+ginger |
4.44 ± 0.19 c | 2.58 ± 0.14 c | 5.35 ± 0.24 c | 4.26 ± 0.25 c | 0.60 ± 0.05 b | 0.68 ± 0.05 b | 2.58 ± 0.13 c | 1.92 ± 0.12 c |
Data are presented as (Mean ± S.E). S.E=Standard error. Mean values with different superscript letters in the same column are significantly different at (P = 0.05).
Table 2: Effect of ginger administration on the relative expression of NF-kB, TNF-a, PPAR-a and caspase-8 gene in liver of metalaxyl-intoxicated rats.
Effect on DNA fragmentation
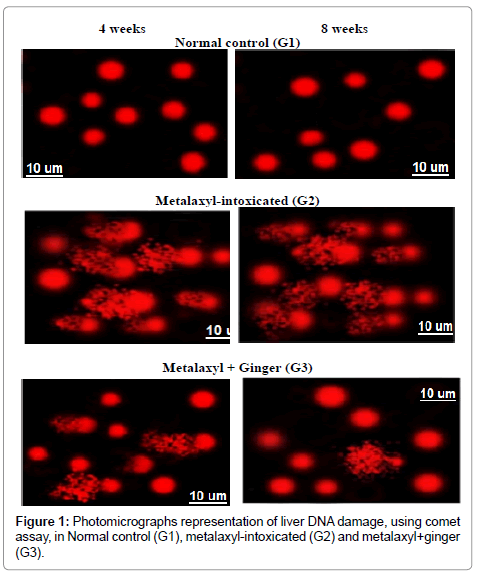
A significant increase in DNA damage that was indicated by an increase in tail length and tail DNA% in liver tissue was observed in metalaxyl-intoxicated rats all over the period of experiment compared to control. However, ginger treatment significantly reduced DNA damage that was indicated by comet assay in metalaxyl intoxicated male rats all over the periods of the experiment (Figure 1).
Histopathological and immunohistochemical alterations
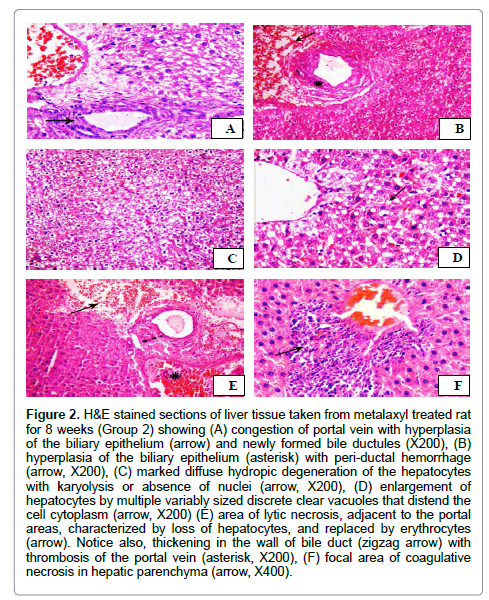
The microscopical examination of the hepatic tissues obtained from rats treated with metalaxyl after 8 weeks revealed various histopathological alterations in the hepatic parenchyma of the examined rats. Marked dilatation and congestion of central veins and hepatic blood sinusoids was observed. In addition, mild peri-vascular leukocytic cellular aggregations with activation of Von Kuepfer’s cells were also observed. The portal areas showed injury of the endothelial cell lining of the portal blood vessels with thrombus formation was seen. Marked congestion of portal vein with hyperplasia of the biliary epithelium in association with formation of newly formed bile ductules (Figure 2A) was detected. Additionally, the lumen of bile duct was expanded with eosinophilic debris. Occasionally, the portal areas were expanded by moderate numbers of leukocytes mainly lymphocytes and macrophage. Furthermore, peri-ductal edema admixed with erythrocytes was also observed (Figure 2B). Additionally, extensive thickening of the hepatic capsule in association with sub-capsular hemorrhage was also demonstrated. Extensive hydropic degeneration of the hepatocytes characterized by swollen, pale, vacuolated cytoplasm with occasional pyknosis, rarely karyolysis or absence of nuclei of degenerated hepatocytes was noticed (Figure 2C). Additionally, degenerative changes that characterized by enlargement of the cells by multiple variably sized discrete empty vacuoles that distend the cell cytoplasm and flattened, displaced nucleus to the periphery was demonstrated in the centri-lobular zones of hepatic lobules (Figure 2D). Focal areas of lytic necrosis characterized by disappearance of hepatocytes and replaced by erythrocytes (Figure 2E). Moreover, coagulative necrosis of small groups of hepatocytes that characterized by retention of hepatic cell outline, shrunken hepatocytes with hypereosinophilic cytoplasm and pyknotic nuclei in association with leukocytic cellular infiltrations mainly lymphocytes (Figure 2F). Degree 3 was observed in most treated animals. Accordingly, rats treated with metalaxyl had severe liver damage with a mean score of 3.
Figure 2: H&E stained sections of liver tissue taken from metalaxyl treated rat for 8 weeks (Group 2) showing (A) congestion of portal vein with hyperplasia of the biliary epithelium (arrow) and newly formed bile ductules (X200), (B) hyperplasia of the biliary epithelium (asterisk) with peri-ductal hemorrhage (arrow, X200), (C) marked diffuse hydropic degeneration of the hepatocytes with karyolysis or absence of nuclei (arrow, X200), (D) enlargement of hepatocytes by multiple variably sized discrete clear vacuoles that distend the cell cytoplasm (arrow, X200) (E) area of lytic necrosis, adjacent to the portal areas, characterized by loss of hepatocytes, and replaced by erythrocytes (arrow). Notice also, thickening in the wall of bile duct (zigzag arrow) with thrombosis of the portal vein (asterisk, X200), (F) focal area of coagulative necrosis in hepatic parenchyma (arrow, X400).
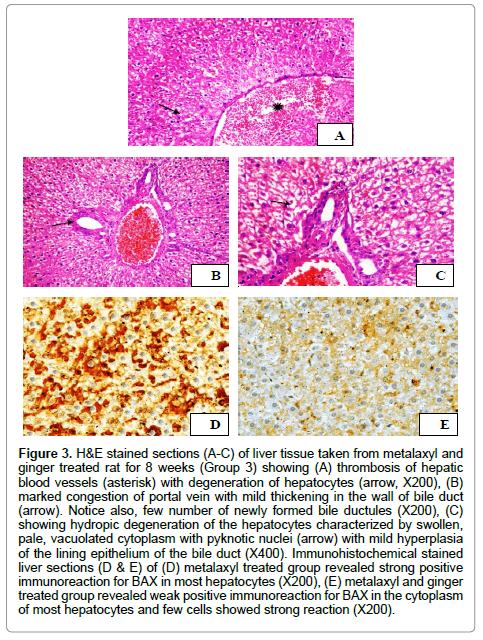
Histopathological changes in the livers of treated rats with metalaxyl and ginger for 8 weeks displayed mild improvement in hepatic tissue histology in comparable with that of the control group. The liver showed congestion of central veins and hepatic blood sinusoids with activation of Kuepfer’s cells. Moreover, thrombosis of hepatic blood vessels was also demonstrated (Figure 3A). Additionally, mild hyperplasia of the biliary epithelium with few number of newly formed bile ductules was observed in the liver tissues (Figure 3B). Furthermore, hydropic degeneration of hepatocytes that characterized by distention of the hepatocytes with variable size vacuoles was seen (Figure 3C). Moderate pathological changes (degree 2) were demonstrated during the microscopical examination of the liver that obtained from metalaxyl with ginger treated group. Rats of this group had liver scores with a mean 2. On the other side, normal histological structure of hepatic parenchyma, portal area and hepatic blood vessels was detected during the histopathological examination of the hepatic tissue taken from rats administrated distilled water (group 1).
Figure 3: H&E stained sections (A-C) of liver tissue taken from metalaxyl and ginger treated rat for 8 weeks (Group 3) showing (A) thrombosis of hepatic blood vessels (asterisk) with degeneration of hepatocytes (arrow, X200), (B) marked congestion of portal vein with mild thickening in the wall of bile duct (arrow). Notice also, few number of newly formed bile ductules (X200), (C) showing hydropic degeneration of the hepatocytes characterized by swollen, pale, vacuolated cytoplasm with pyknotic nuclei (arrow) with mild hyperplasia of the lining epithelium of the bile duct (X400). Immunohistochemical stained liver sections (D & E) of (D) metalaxyl treated group revealed strong positive immunoreaction for BAX in most hepatocytes (X200), (E) metalaxyl and ginger treated group revealed weak positive immunoreaction for BAX in the cytoplasm of most hepatocytes and few cells showed strong reaction (X200).
The microscopical evaluation of immunohistochemical stained hepatic sections for BAX revealed strong positive expression of BAX in most hepatocytes in metalaxyl treated rats (Figure 3D). In the meantime, moderate immunoreaction for BAX in the cytoplasm of most hepatocytes (Figure 3E) was demonstrated in metalaxyl plus ginger treated group.
Discussion
In the present study, there were significant increases in serum NO levels in metalaxyl intoxicated rats all over the periods of the experiment. Similarly, Kanbur et al. [28] reported that, propetamphos insecticide significantly increased the NO and other lipid peroxidation parameters in mice when given in the diet for 60 days. NO is highly reactive signaling molecule and it is an important regulator for cellular functions. Nitrosative stress also plays a critical role in inflammation. NO modifies DNA directly and inactivates the DNA repair enzymes [29]. The increase in NO level may be because of the up-regulation of TNF-α and other cytokines. TNF-α acts synergistically with other cytokines lead to increase of inducible NO synthase (iNOS) and increased NO production in cells [30]. After produced of NO, it can inhibit cytochrome c oxidase. NO found in the mitochondria increases the production of ROS and reactive nitrogen species which can change various processes activities such as mitochondrial biogenesis, respiration and oxidative stress [31]. Various studies have shown that ginger extracts, volatile oils and oleoresins the possess free radical scavenging effects, and to be effective in scavenge, superoxide, hydroxyl, nitric oxide in vitro [32,33]. The phytochemical zingerone is also recorded to be an effective scavenger of the free radicals such as peroxynitrite and block peroxynitrite-mediated tyrosine nitration formation [34]. Also, Kim et al. [35] and Aktan et al. [36] recorded that, gingerol suppress NO production in murine macrophages by partially blocking iNOS enzymatic activity and decreasing iNOS protein production, by mitigation of NF-kB mediated iNOS gene expression, providing a possible mechanism of action for the anti-inflammatory activity of ginger.
Malondialdehyde is the end point of lipid peroxidation process which may be defined as an oxidative deterioration of polyunsaturated lipids [37]. Treating rats with metalaxyl induced a significant increase in the oxidative stress, malondialdehyde which is lipid peroxidation marker. Also, Sultana et al. [38] recorded that, a significant increase of L-MDA level in erythrocyte lysate after 30th day of dermal treatment with metalaxyl compared to normal rat group. Lipid peroxidation and ROS are generated by electron leakage outside the electron transfer chains and these oxygen radicals can initiate lipid peroxidation [39]. According to Calviello et al. [40] fungicides-induced damage is closely associated with increase in the lipid peroxidation and decrease in the antioxidant enzymes. The mechanism of metalaxyl-induced toxicity may be due to metalaxyl metabolized to a reactive metabolite which may initiate a chain reaction with respect to lipid peroxidation and other tissue damaging effects Therefore, it is suggested that tissue injury induced by metalaxyl is mediated elevation of lipid peroxidation [41]. Ginger, which behaves as free radical scavenger and a potent antioxidant can decrease L-MDA level perturbed by metalaxyl in rat liver. The protective effect of ginger may be due to antioxidative properties. Shogaol and gingeriol and other chemicals in ginger inhibit lukotriene and prostaglandine biosynthesis through suppression of 5-lipooxygenase synthetase [42]. Also, ginger supplementation to rats modulates the antioxidant enzymes in a manner that favors the decreasing of lipid peroxidation and a possible adaptive mechanism to counteract oxidative stress situation [43], and this mechanism would have played a fundamental role in the observed hepatoprotective effects of ginger against varied class of toxicants [44].
As a signal protein, NF-kB is produced by inflammatory mediator including p65 and p50 subunits. It consists of inactivated NF-kB and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IkB-α), which are distributed in the cytoplasm. Following the activation of NF-kB by inflammatory cytokines, growth factors, or chemotactic factors, toll-like receptor 4 (TLR4) activates NF-kB to separate the p65 subunit from the IkB-α, and translocate into nucleus to initiate genetic transcription, which increases the expression of pro-inflammatory cytokines [45]. In present study, metalaxyl toxicity enhancement of L-MDA production in liver causing increases in NFKB. L-MDA contributes to inflammation through the activation of NF-kB [46]. A significant up-regulation of liver NF-kB was found in metalaxyl-treated group. This suggests that exposure to metalaxyl contributes to the activation of NF-kB in the liver, and stimulates the production of TNF-α. In the present study, administering metalaxyl for 4 and 8 weeks down regulation gene expression of NF-kB and TNF-α, consistent with these studies Habib et al. [14] suggested that, ginger extract can decrease the elevated expression of NF-kB and TNF-α in rats with liver cancer. Zingiber officinale contains active phenolic compounds such gingerol, shogoal and paradol that have antioxidant and anti-inflammatory effects [47]. These anti-inflammatory properties have been known and estimated for centuries, and several scientific studies have validated this beneficial effect of ginger and its phytochemicals in many study models [48]. Mechanistically, the pathogenesis of inflammatory diseases is proposed to be mediated by the reinforced expressions of NF-κB, TNF-α, COX-2, and increased generation of nitric oxide and proinflammatory eisonaoids [36,37].
Peroxisome proliferator activated receptor alpha in hepatocyte decreased in metalaxyl-exposed rats indicating that the fat burning machinery was compromised in these rats causing hepatic steatosis and steatohepatitis. The diminished effectiveness of oxidation systems is caused by genetic or toxic factors, and metabolic disturbances. In animal models, inefficient PPARα sensing enables the oxidation of the in fluxed fatty acids and cause severe hepatic steatosis development. Supplementation of PPARα agonists prevents these processes and even reverses hepatic fibrosis in animal models [49,50]. Similarly, Al-Eryani [51] reported that, intraperitoneal injections of dichlorodiphenyltrichloroethane (DDT) pesticide caused down-regulation of hepatic mRNA levels of PPAR-α target genes. Medicinal plants have been used to treat several diseases for thousands of years, and since the 19th century many bioactive pure compounds isolated from these plants became very effective drugs [52]. Zingerone is a pungent pyrolytic product of ginger that has been found to have potent antioxidant [34]. Chung et al. [53] observed that, oral administration of zingerone increased expression of PPAR-α in rat renal nuclear extracts, suggesting that zingerone may act as a peroxisome proliferator-activated receptor agonist. Previous studies have recorded that various other natural products exert their anti-inflammatory effect through activation of peroxisome proliferator-activated receptor. Who added that, the upregulation of expression of PPAR-α following ginger supplementation may be due to the elevated expression of hepatic nuclear factor-4 (HNF- 4).
Elevation of DNA damage in present study may be due to the increase in NF-kB, TNF-α, NO levels and caspase-8 activity. Many reports have indicated that high levels of NO induce apoptosis in many cell types. This effect appears to be mediated primarily by the effect of peroxynitrite on increases in mitochondrial permeability either directly [54,55]. Or through DNA damage with subsequent activation of the polyadenylate ribose synthase pathway [56,57]. This mitochondrial permeability transition results in the release of cytochrome c from the mitochondria, which constitutes a signal for apoptosis [58]. Also, some cytokines may activate specific intracellular pathways, i.e., proapoptotic signals via caspase cascade [59,60]. In some liver disorders, apoptosis eliminates a critical number of hepatocytes, leading to impaired liver function [59]. Injured hepatocytes may release apoptotic bodies and activate Kupffer cells, and these activated cells can promote inflammatory and fibrogenic responses, leading to a vicious cycle of hepatic injury [61]. During inflammation, immune system cells, among which mast cells and leukocytes, are recruited to the place of the injury, which leads to an exacerbation of cellular respiration because of increased oxygen consumption causing an increase in release and accumulation of ROS [62]. The generation of the inflammatory mediators, ROS and overexpression of pro-apoptotic proteins is associated with morphological and functional changes that induce an acute inflammatory response leading to several clinical complications [61,62]. NF-kB binding sites have been identified in the promoters of interleukin-1b converting enzyme protease, and TNF-α gene, which are commonly involved in signal-induced programmed cell death. Several studies have clearly demonstrated a pro-apoptotic role for NF-kB perhaps because it, along with activator protein 1, can induce Fas ligand (FasL) expression [63]. Ligation of FasL to Fas in the cell membrane triggers activation of caspase-8. Once activated, caspase-8 transduces a signal to effector caspases, including caspases 3, 6, and 7 and eventually caused the hydrolysis of cytosolic and nuclear substrates [64].
Ginger leads to suppression of apoptosis (DNA fragmentation) induced by metalaxyl. Khaki and khaki [65] reported that, ginger significantly reduced the opposite harmful effects of lead acetate exposure on the liver as well as lead acetate-induced apoptosis. Also, Sakr et al. [66] reported that, treatment with carbimazole induced significant damage in leucocyte DNA in rats as compared with control group meanwhile rats treated with ginger (24 mg/ml/rat) daily for six weeks showed an improvement in percentage of leucocyte DNA fragmentation. So the antioxidant characters of ginger could protect DNA and other important molecules from oxidation and damage, and can improve liver function. Ginger exhibited DNA protection, blocked lipid peroxidation, decrease apoptosis and free radical scavenging indicating strong antioxidant properties [67,68].
Histological findings showed various histopathological changes in the hepatic parenchyma of the examined rats. The livers of the rats treated with metalaxyl showed extensive dilatation and congestion of central veins and hepatic blood sinusoids. Also, peri-vascular leukocytic cellular aggregations with activation of Von Kuepfer’s cells were observed and marked congestion of portal vein with hyperplastic proliferation of the lining epithelium of the bile ducts in association with formation of newly formed bile ductules. Occasionally, coagulative necrosis of small groups of hepatocytes that characterized by retention of hepatic cell outline and shrunken hepatocytes with hypereosinophilic cytoplasm and pyknotic nuclei in association with leukocytic cellular infiltrations mainly lymphocytes was observed. Similarly, Lamfon [41] recorded that, metalaxyl induced many histopathological alterations in the hepatocytes of mice such tissue impairment, congestion of intrahepatic blood vessels and cytoplasmic vacuolization of the hepatocytes. Also, present study revealed strong positive BAX expression in most hepatocytes metalaxyl treated rats compared with control rats. Apoptosis caused by metalaxyl has been suggested in rats hepatocytes cells [69]. High Bax expression levels and formation of homo-or heterodimers with Bcl-2 may lead to cell death [70]. The Bax and Bak pro-apoptotic protein undergo changes in their structure after exposure to death signals and modify the mitochondrial membrane structure causing the release of cytochrome c and proapoptotic factors [71]. Histopathological changes in the livers of treated rats with metalaxyl and ginger for 8 weeks displayed mild improvement in hepatic tissue histology in comparable with that of the metalaxyl group. This finding was agreed with Lamfon [41] who reported that, treating rats with ginger and metalaxyl improved the biochemical and histopathological changes induced in hepatocytes by metalaxyl. Also, Badr, Sakr, and Abd-Eltawab, [72] observed that, normal liver architecture, histological structure with slight congested and enlarged blood vessels was observed in experimental Ehrlich ascites carcinoma treated with ginger.
Weak positive immunoreaction for BAX in the cytoplasm of most hepatocytes was demonstrated in metalaxyl plus ginger treated group. Similarly, EI-Ghonaimy [69] reported that, immune-detection of BAX protein in liver sections of metalaxyl plus ginger group showed weak positive reaction in most of hepatocytes compared to the strong positive reaction observed in metalaxyl-treated group and significant decrease in the mean area % of BAX when compared with metalaxyltreated group suggesting that ginger reduced apoptosis. These findings were supported by previously performed clinical and experimental investigations in liver of mouse [41] and kidney [73], which have shown that ginger has a protective effect against oxidative damage and apoptosis through its antioxidant properties. Also, Lee et al. [74] recorded that, (6)-gingerol protected against ß-amyloid-induced cytotoxicity and apoptotic cell death, such disruption of mitochondrial membrane potential, elevated Bax/Bcl-2 ratio, DNA fragmentation and activation of caspase-3, by inhibition of intracellular accumulation of reactive nitrogen species and/or ROS and subsequent oxidative and/or nitrosative damages.
In conclusion, the results of this study demonstrate that ginger was effective for inhibition of metalaxyl-induced oxidative stress and apoptosis in male rats. The results of this study show that the protective effects of ginger may be due to inhibition of both lipid peroxidation and pro-inflammatory cytokines.
References
- Ding F, Li X, Diao J, Sun Y, Zhang L, et al. (2012) Chiral recognition of metalaxyl enantiomers by human serum albumin: evidence from molecular modeling and photophysical approach. Chirality 24: 471-480.
- Sukul P, Spiteller M (2000) Metalaxyl: persistence, degradation, metabolism, and analytical methods. Rev Environ Contam Toxicol 164: 1-26.
- Reisinger K, Szigeti J, Várnagy L (2006) Determination of carbendazim residues in the eggs, liver and pectoral muscle of Japanese quail (Coturnix coturnix japonica). Acta Vet Hung 54: 127-133.
- Al-Attar A (2015) Effect of grapeseed oil on diazinon-induced physiological and histopathological alterations in rats. Saudi J Biol Sci 22: 284-292.
- Elzoghby R, Hamuoda A, Abdel-Fatah A, Farouk M (2014) Protective role of vitamin c and green tea extract on malathion-induced hepatotoxicity and nephrotoxicity in rats. Am J Pharmacol Toxicol 9: 177-188.
- Hrelia P, Maffei F, Fimognari C, Vigagni F, Cantelli-Forti G (1996) Cytogenetic effects of Metalaxyl on human and animal chromosomes. Mutat Res 369: 81-86.
- Al-Amoudi W (2012) Haematological and biochemical effects of metalaxyl fungicide on albino mice. Am J Biochem 2: 62-66.
- Hashem H (2012) Light and electron microscopic study of the possible protective effect of nigella sativa on metalaxyl induced hepatotoxicity in adult albino rats. J Cell Sci Ther 3: 1-6.
- Aoki K, Cortes A, Ramirez M, Gomez H, M Lopez (2007) Pharmacological study of antispasmodic activity of Mirabilis jalap Linn flowers. J Ethnopharmacol 28: 96-101.
- Lantz R, Chen G, Sarihan M, Solyom A, Jolad S, et al. (2007) The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine 14: 123-128.
- Gilani A, Rahman A (2005) Trends in ethnopharmocology. J Ethnopharmacol 100: 43-49.
- Grzanna R, Lindmark L, Frondoza CG (2005) Ginger--An herbal medicinal product with broad anti-inflammatory actions. J Med Food 8: 125-132.
- Aggarwal B, Shishodia S (2004) Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann N Y Acad Sci 1030: 434-441.
- Habib S, Makpol S, Abdul Hamid N, Das S, Ngah W, et al. (2008) Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 63: 807-813.
- Hinson J, Bucci T, Irwin L, Michael S, Mayeux P (2002) Effect of inhibitors of nitric oxide synthase on acetaminophen-induced hepatotoxicity in mice. Nitric Oxide 6: 160-167.
- Sakr S, Lamfon H (2005) Effect of green tea on metalaxyl fungicide induced liver injury in albino mice. Oxford Res Forum J 2: 65-69.
- Abdel-Azeem A, Hegaz A, Ibrahim K, Farrag A, El-Sayed E (2013) Hepatoprotective, antioxidant, and ameliorative effects of ginger (Zingiber officinale Roscoe) and vitamin E in acetaminophen treated rats. J Diet Suppl 10:195-209.
- Bancroft J, Stevens A (1996) Theory and Practice of Histological Techniques. 4nd ed. Edinburgh: Churchill Livingstone766 p.
- Kiernan J (2000) Histological and histochemical methods: Theory and practice. 3rd ed., Butterworth-Heinemann, Oxford, Boston and New Delhi, 129-139.
- Sakr S, Abdel-Samie H (2008) Apoptosis related protein Bax in liver of metalaxyl fungicide-treated mice. The effect of antox. Ozean J Appl Sci 1:17-27.
- Ortatatli M, OÄŸuz H (2001) Ameliorative effects of dietary clinoptilolite on pathological changes in broiler chickens during aflatoxicosis. Res Vet Sci 71: 59-66.
- Montgomery H, Dymock J (1961) The determination of nitrite in water. Analyst 86: 414-416.
- Mesbah L, Soraya B, Narimane S, Jean P (2004) Protective effect of flavonides against the toxicity of vinblastine cyclophosphamide and paracetamol by inhibition of lipid peroxidation and increase of liver glutathione. HAEMA 7: 59-67.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25: 402-408.
- Singh N, McCoy M, Tice R, Schneider E (1988) A simple technique for the quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175: 184-191.
- Lee R, Steinert S (2003) Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutat Res 544: 43-64.
- Steel R, Torrie J, Dickey D (1997) Principles and procedures of Statistics: A Biometrical Approach, 3rd ed, McGraw-Hill, New York, NY.
- Kanbur M, Liman B, Eraslan G, Altinordulu S (2008) Effects of cypermethrin, propetamphos, and combination involving cypermethrin and propetamphos on lipid peroxidation in mice. Environ Toxicol 23: 473-479.
- Salman K, Ashraf S (2013) Reactive oxygen species: A link between chronic inflammation and cancer. AsPac J Mol Biol Biotechnol 21: 42-49.
- Morris SM, Billiar TR (1994) New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol 266: E829- E839.
- Bolisetty S, Jaimes E (2013) Mitochondria and reactive oxygen species:physiology and pathophysiology. Int J Mol Sci 14: 6306-6344.
- Jagetia G, Baliga M, Venkatesh P (2004) Ginger (Zingiber officinale Rosc.), A dietary supplement, protects mice against radiation-induced lethality: mechanism of action. Cancer Biother Radiopharm 19: 422-435.
- Al-Tahtawy R, El-Bastawesy A, Monem M, Zekry Z,Al-Mehdar H, et al. (2011) “Antioxidant activity of the volatile oils of Zingiber officinale (ginger). Spatula DD 1: 1-8.
- Shin S, Kim J, Chung H, Jeong J (2005) Zingerone as an antioxidant against peroxynitrite. J Agric Food Chem 53: 7617-7622.
- Kim H, Murakami A, Abe M, Ozawa Y, Morimitsu Y, et al. (2005) Suppressive effects of mioga ginger and ginger constituents on reactive oxygen and nitrogen species generation, and the expression of inducible proinflammatory genes in macrophages. Antioxidants and Redox Signaling 7: 1621-1629.
- Aktan F, Henness S, Tran V, Duke C, Roufogalis B, et al. (2006) Gingerol metabolite and a synthetic analogue capsarol inhibit macrophage NF-kappaB-mediated iNOS gene expression and enzyme activity. Planta Med 72: 727-734.
- Dar M, Khan A, Raina R, Kumar P, Sultana M (2013) Effect of repeated oral administration of bifenthrin on lipid peroxidation and antioxidant parameters in wistar rats. Bull Environ Contam Toxicol 91: 125-8.
- Sultana M, Prawez S, Dar M, Ahmad M, Naseem S (2015) Sub acute dermal toxicity of metalaxyl with special reference to oxidative stress in wistar rats. J Sci Technol 6: 1316-1318.
- Hanukoglu I, Rapoport R, Weiner L, Sklan D (1993) Electron leakage from the mitochondrial NADPH-adrenodoxin reductase-adrenodoxin scc (cholesterol side chain cleavage) system. Arch Biochem Biophys 305: 489-498.
- Calviello, G, Piccioni E, Boninsegan A, Tedesco B, Maggiano N, et al. (2006) DNA damage and apoptosis induction by the pesticide mancozeb in rat cells: involvement of oxidative mechanism. Toxicol Appl Pharmacol 211: 1202-1209.
- Lamfon H (2011) Protective effect of ginger (Zingiber officinale) against metalaxyl induced hepatotoxicity in albino mice. American J Sci 7: 1093- 1100.
- Volmar B, Menger M (2009) The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev 89: 1269–1339.
- Wilkinson J (2000) Effect of ginger tea on the fetal development of Sprague–Dawley rats, Reprod Toxicol 14: 507-512.
- Haniadka R, Saxena A, Shivashankara A, Fayad R, Palatty P, et al. (2013) Ginger Protects the Liver against the Toxic Effects of Xenobiotic Compounds: Preclinical Observations, J Nutri food sci 3: 226.
- Crews F, Qin L, Sheedy D, Vetreno R, Zou J (2013) High mobility group box 1/ Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry 73: 602-612.
- Diesen D, Kuo P (2011) Nitric oxide and redox regulation in the liver: Part II. Redox biology in pathologic hepatocytes and implications for intervention. J Surg Res 167: 96-112.
- Hudson E, Fox L, Luckett J, Manson M (2006) Ex vivo cancer chemoprevention research possibilities. Environment Toxicol pharmacol 21: 204-214
- Grzanna R, Lindmark L, Frondoza C (2005) Ginger--An herbal medicinal product with broad anti-inflammatory actions, J Med Food: 125-132.
- Baliga M, Haniadka R, Pereira M, D’Souza J, Pallaty P, et al. (2011) Update on the chemopreventive effects of ginger and its phytochemicals, Crit Rev Food Sci Nutr 51: 499-523.
- Ip E, Farrell G, Robertson G, Hall P, Kirsch R, et al. (2003) Central role of PPAR alpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 38: 123-132.
- Al-Eryani L (2014) The role of pesticides in non-alcoholic fatty liver disease.
- Chung S, Kim M, Chung J, Kim D, Choi J, et al. (2009) Peroxisome proliferator-activated receptor activation by a short-term feeding of zingerone in aged rats. J Med Food 12: 345-350.
- Balakirev M, Khramtsov V, Zimmer G (1997) Modulation of the mitochondrial permeability transition by nitric oxide. Eur J Biochem 246: 710-718.
- Hortelano S, Dallaporta B, Zamzami N, Hirsch T, Susin S, et al. (1997) Nitric oxide induces apoptosis via triggering mitochondrial permeability transition. FEBS Lett 410: 373-377.
- Szabo C (1996) DNA strand breakage and activation of poly-ADP ribosyltransferase: a cytotoxic pathway triggered by peroxynitrite. Free Radic Biol Med 21: 855-869.
- Szabo C, Ohshima H (1997) DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide 1: 373-385.
- Costantini P, Petronilli V, Colonna R, Bernardi P (1995) On the effects of paraquat on isolated mitochondria. Evidence that paraquat causes opening of the cyclosporin A-sensitive permeability transition pore synergistically with nitric oxide. Toxicology 99: 77-88.
- Center S (2004) Metabolic, antioxidant, nutraceutical, probiotic, and herbal therapies relating to the management of hepatobiliary disorders. Vet Clin North Am Small Anim Pract 34: 67-172.
- Compare D, Coccoli P, Rocco A, Nardone O, Maria S, et al. (2012) Gut-Liver axis: The impact of gut microbiota on non-alcoholic fatty liver disease. Nutri Metab Cardiovasc Dis 22: 471-476.
- Czaja A (2014) Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol 20: 2515-2532.
- Reuter S, Gupta S, Chaturvedi M, Aggarwal B (2010) Oxidative stress, inflammation, and cancer: How are they linked. Free Radic Biol Med 49: 1603-1616.
- Wallach D, Varfolomeev E, Malinin N, Goltsev Y, Kovalenko A M, et al. (1999) Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol 17: 331-361.
- De Maria R, Lenti L, Malisan F, d’Agostino F, Tomassini B, et al. (1997) Requirement for GD3 ganglioside in CD95- and ceramide induced apoptosis. Science 277: 1652-1655.
- Khaki A, Khaki A (2010) Antioxidant effect of ginger to prevents lead-induced liver tissue apoptosis in rat. J Med Plants Res 4: 1492-1495.
- Sakr S, El-Nabi S, Okdah Y, El-Garawani I, AM El-Shabka (2016) Cytoprotective effects of aqueous ginger (zingiber officinale) extract against carbimazole-induced toxicity in albino rats. European J Pharm Med Res 3: 489-497.
- Siddaraju M, Dharmesh S (2007) Inhibition of gastric H+, k+, ATPase and Helicobacter pylori growth by phenolic antioxidants of Zingiber officinale. Mol Nutri Food Res 51: 324-332.
- Zahedi A, Fathiazad F, Khaki A, Ahmadnejad B (2012) Protective Effect of Ginger on Gentamicin-Induced Apoptosis in Testis of Rats. Adv Pharm Bull 2: 197-200.
- EI-Ghonaimy N (2015) Role of ginger (zingiber officinale) against metalaxyl induced hepatotoxicity in male albino rats: a histological and immunohistochemical study. J Histol Histopathol 2: 1-9.
- Liu G, Wang T, Song J, Zhou Z (2013) Effects of apoptosis-related proteins caspase 3, Bax and Bcl-2 on cerebral ischemia rats. Biomed Rep 1: 861e-867e.
- Lalier L, Cartron P, Juin P, Nedelkina S, Manon S, et al. (2007) Bax activation and mitochondrial insertion during apoptosis. Apoptosis 12: 887-896.
- Badr M, Sakr S, Abd-Eltawab H (2016) Ameliorative effect of ginger extract against pathological alterations induced in mice bearing solid tumors. J Biosci Applied Res 2: 185-196.
- Sakr S, Hawazen L, Amina E (2011) Ginger (Zingiber officinale) extract ameliorates metalaxyl fungicide induced nephrotoxicity in albino mice. African J Pharm Pharmacol 5: 104-112.
- Lee C, Park G, Kim C, Jang J (2011) Gingerol attenuates ß-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem Toxicol 49: 1261-1269.
Citation: Hassa MF, Hussein S, Senosi YEl, Mansour MK, Amin A (2018) Role of Ginger as Anti-inflammatory and Anti-apoptotic in Protection of Liver Damage Induced by Metalaxyl Fungicide in Male Albino Rats. J Clin Exp Pathol 8:346. DOI: 10.4172/2161-0681.1000346
Copyright: © 2018 Hassa MF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4589
- [From(publication date): 0-2018 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 3710
- PDF downloads: 879