Review Article Open Access
Role of Gastric Acid in Dyspepsia
Samal SC1*, Pratap Behera1 and George M Chandy2
1Department of GI and Liver Sciences, Amri Hospitals, Khandagiri, Bhubaneswara, Odisha, India
2MIOT Advanced Center for GI and liver diseases, Chennai, India
- *Corresponding Author:
- Subash Chandra Samal M.D, DM
Department of GI And Liver Sciences, Amri Hospitals
Khandagiri, Bhubaneswara, Odisha, India
Tel: 91-9938756409
E-mail: subashsamal@hotmail.com
Received date: March 01, 2015; Accepted date: April 01, 2015; Published date: April 08, 2015
Citation: Samal SC, Behera P, Chandy GM (2015) Role of Gastric Acid in Dyspepsia. J Gastrointest Dig Syst 5:280. doi:10.4172/2161-069X.1000280
Copyright: © 2015 Samal SC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Functional dyspepsia is a clinical syndrome in which there is an absence of underlying organic disease to explain the symptoms. Empiric acid suppression is one among the many options available in the therapeutic armamentarium to treat this common condition. However the response to acid suppression is not always a consistent one. It is because of the heterogeneous nature of the pathophysiologic mechanisms involving gastric acid in this scenario. This variation in the role of the gastric acids is determined possibly by the genetic constitution and it is influenced by the factors like H. pylori infection, irritants, allergens and diet. All these events primarily lead to visceral hypersensitivity. Finally, it is the degree of affection, whether gastric or duodenal, decides its clinical presentation. If it is a predominant gastric visceral hypersensitivity, presentation would be of Rome III-EPS type, whereas duodenal sensitisation would present as Rome III-PDS.
Keywords
Gastric acid; Functional dyspepsia; Rome-III criteria; H. pylori; Visceral hypersensitivity
Introduction and Back Ground
“Dyspepsia” is a Greek word, which means bad digestion. Functional dyspepsia is a clinical syndrome characterized by chronic or recurrent upper abdominal pain or discomfort, in the absence of underlying organic disease to explain the symptoms [1]. The symptom complex includes epigastric pain, bloating, early satiety, fullness, epigastric burning, belching, nausea and vomiting. The Rome II definition excludes patients with predominant reflux symptoms [2]. The annual prevalence of recurrent abdominal pain or discomfort is approximately 25% [3]. Functional dyspepsia tends to be a rather common, chronic condition, which, with the ever-increasing cost of drugs, poses a constant challenge to the treating physician [4,5]. The management of dyspepsia represents a major component of clinical practice; 2-5% of family practice consultations are for dyspepsia [6]. Empirical acid suppression is one of the main approaches for the treatment of functional dyspepsia [7].
Gastric Acid in Health
Gastric acid remains an important pathogenic factor for a variety of common upper gastrointestinal disorders. Further, gastric acid has played a significant role in shaping Gastroenterology as a specialty. It was in the acid era that our specialty became defined and flourished. More recently, the identification of hydrogen potassium stimulated adenosine triphosphatase (H+K+-ATPase) as the proton pump of the parietal cells and Helicobacter pylori (HP) infection as the main cause of peptic ulcer, heralded a new revolution in our understanding and treatment of acid peptic disorder. Warren and Marshall were awarded the Nobel Prize for their discovery of HP [8-12].
Parietal cells secrete hydrochloric acid at a concentration of approximately 160 mmol/L or pH 0.8. Acid is thought to gain access to the lumen via channels in the mucus layer created by the relatively high intraglandular hydrostatic pressures generated during secretion, approximately 17 mm Hg [13]. Most studies indicate that the rate of acid secretion by the human stomach changes little with aging, unless there is coexisting disease of the oxyntic mucosa such as infection with HP or atrophic gastritis [14,15]. Acid facilitates the digestion of protein, absorption of iron, calcium, and Vit B12 as well as prevents bacterial overgrowth and enteric infection [16]. Gastric acid secretion is controlled by a highly coordinated interaction of neural, hormonal and paracrine pathways. These pathways can be activated directly by stimuli originating in the brain or reflexively by stimuli originating in the stomach such as distension, protein and acid.
The principal stimulants of acid secretion are histamine, released from ECL (enterochromaffin like) cells (paracrine); Gastrin, released from G cells (hormonal); and Ach (acetyl choline), released from postganglionic enteric neurons (neurocrine). These agents interact with receptors coupled to 2 major signal transductions pathways: The adenylate cyclase in the case of histamine and intracellular calcium in the case of gastrin and acetyl choline [17]. The main inhibitor of acid secretion is somatostatin, released from oxyntic and pyloric D cells (paracrine). Each of these agents acts directly on the parietal cell as well as indirectly by modulating the secretion of neuro endocrine cells [18].
H. pylori and Gastric Acid
Most of the therapeutic trials in functional dyspepsia include acid suppression and/ or anti H. pylori medication. Hence their inter-relationship needs a brief review in this review. Acute infection with H. pylori results in hypochlorhydria, whereas chronic infection results in either hypo or hyperchlorohydria. This decrease in acid secretion is thought to facilitate survival of the organism and colonization of the stomach during acute infection [19].
Chronic infection with HP may be associated with either decreased or increased acid secretion, depending on the severity and distribution of gastritis [20]. Approximately 10% to 15% of patients chronically infected with HP have antral predominant inflammation. These patients produce increased amounts of acid as a result of reduced antral Somatostatin content and elevated basal and stimulated gastric secretion [21-23]. Gastrin, in turn, stimulates histamine secretion from ECL cells leading to enhanced acid secretion.
Functional Dyspepsia is a Heterogenous Entity
Systematic reviews of large trials show that suppressing acid secretion, eradicating H. pylori, prokinetics and antidepressants have inconsistent effects on the treatment of functional dyspepsia [24,25]. This inconsistent therapeutic efficacy has been attributed to the heterogeneity of patients, and the contribution of multiple mechanisms to the development of symptoms. To provide more homogenous patients for inclusion in clinical trials, the Rome II consensus criteria recommended distinguishing between patients with epigastric pain and those with discomfort as a means of identifying pathophysiologically distinct subgroups [1]. The Rome III consensus criteria [26] proposed differentiating two subcategories of functional dyspepsia- post prandial distress syndrome (PDS) (early satiation or post-prandial fullness) and epigastric pain syndrome (EPS) (pain or burning in the epigastrium). It has been showed that delayed gastric emptying is associated with nausea, vomiting and fullness; [27,28] impaired accommodation to a meal is associated with early satiety and weight loss; [29] hypersensitivity to gastric distension is associated with epigastric pain, belching and weight loss [30]. We do need to examine whether gastric acid has anything to do with these pathophysiologic mechanisms.
Gastric Acid and Visceral Hyperalgesia
Gastric acid secretion and the perceived sensation associated with it are among the potentially important players in the genesis of dyspeptic symptoms. In patients with functional dyspepsia (FD), especially those with EPS, suppression of gastric acid secretion by anti-secretory agents such as proton-pump inhibitors (PPIs) or histamine type 2-receptor antagonists (H2 blocker) seems to ameliorate the epigastric pain or burning. Furthermore, even in PDS, as the initial gastric acid emptying may play a pathogenic role in symptom generation through the early outset of duodenal brake, acid suppression might be effective, at least in part, against the bothersome post-prandial fullness [31]. Basal and peak acid outputs do not differ in patients with functional dyspepsia compared with controls [32,33]. The threshold of acidity for damage and pain is probably not the same. Greater acidity is probably needed to damage epithelial cells compared to acidity required to stimulate pain fibres. This is because the epithelial cells have acid recovery mechanisms which are not present in pain fibres. Depending on the location of the pain fibres and access thereto of H+, individuals will have different sensitivity to luminal acidity [34].
Conceptually, the pain of dyspeptic patients may reflect pathological alterations in gut function and/ or signify that events in the gastrointestinal tract are represented in the brain in an exaggerated fashion, because the sensory gain of afferent neurons or the central gain of afferent input from the gut is set abnormally high [35,36]. Acid can sensitize mechanosensitive afferents in the stomach [37]. These observations and the ability of anti-secretory therapy to alleviate symptoms in some dyspeptic patients suggest that acid hypersensitivity, but not acid hyper secretion, is a factor in functional dyspepsia [38,39].
The observation that the vagus nerve plays a major role in the communication between the gut and the brain is beyond doubt. It is the largest visceral sensory nerve in the body [40]. These vagal sensory neurons are responsible for carrying chemonociception generated by the presence of gastric acid [41]. This can happen under pathophysiological circumstances where vagal afferents are stimulated by back diffusion of luminal acid through a leaky gastric mucosal barrier whose permeability may increase, e.g., following the intake of irritating food or liquid, infection, inflammation, stress and nonsteroidal anti-inflammatory drug use [42]. It is very likely that the summation of vagal afferent input elicited by physiological and noxious stimuli may have an important bearing on dyspeptic pain. Unperceived electrical stimulation of mechano-insensitive jejunal afferents can increase the perception of background distension to an uncomfortable level [43]. The fact that under the circumstances vagal afferent pathways are sensitized for a prolonged period of time makes a strong case for chemo-sensitive vagal nerve fibre being involved in the upper abdominal hyperalgesia associated with acid related disorders including functional dyspepsia [42].
Gastric Acid and Duodenal Brake
Duodenal hypersensitivity to acid, increased duodenal acid exposure and abnormal responses to duodenal acid have been observed in patients with functional dyspepsia [44]. Isolated duodenal acidity can be measured by using catheterless radio telemetry pH monitoring system to examine the relationship between duodenal acidity and upper gastro intestinal symptoms [45]. Lee et al. reported that acid infusion into the duodenal bulb induced dyspepsia in healthy volunteers, and symptoms of dyspepsia are more readily observed in patients with [46,47] FD than in healthy subjects. A recent study has indicated that acid infusion into the stomach predominantly induced dysmotility- like dyspeptic symptoms in healthy Japanese control subjects [48].
It has been documented that duodenal acidification suppresses antral contractions in healthy volunteers [49]. It has also been observed that the greater the concentration of acid in the duodenum, the greater is the inhibition of gastric emptying [50]. The occurrence of phase III migrating motor complex is also delayed by low duodenal pH [51]. To further compound the problem, spontaneous duodenal exposure to endogenous acid was increased in a subset of FD patients who displayed reduced clearance of exogenous acid in the duodenum [52].
Apart from the above mentioned effects, duodenal acidification induced proximal gastric relaxation, increases sensitivity to gastric distension, and inhibits gastric accommodation to a meal [53].
Chemical irritation with acid appears to increase sensory input into inter neurons and/ or projection neurons in the dorsal horn of the spinal cord, resulting in secondary hyperalgesia in adjacent, undamaged visceral tissue and central hyper excitability [54]. Through these mechanisms, duodenal acidification may increase gastric mechanosensitivity. Thus, increased duodenal acid exposure present in a subset of FD patients might potentiate perception of a different concurrent stimulus, leading to dyspeptic symptoms.
Acid Suppression
Proton pump inhibitors (PPI) therapy is more effective than placebo or histamine 2 receptor antagonists (H2RAs) in relieving symptoms in patients with un-investigated dyspepsia [55-65]. Eleven trials compared H2RA therapy with placebo in 2164 patients with functional dyspepsia.
The proportion of patients that continued to have dyspeptic symptoms was statistically significantly reduced in patients allocated to H2RA therapy (RR,0.78; 95% CI, 0.65-0.93). There was significant heterogeneity between studies, and the methodological quality of the trials influenced the results.
Eight trials [64,66-70] reported in 6 papers compared PPI therapy with placebo in 3293 patients with functional dyspepsia. PPI therapy was given for 2-8 weeks and was statistically significantly superior to placebo (RR, 0.86; 95% CI, 0.78-0.95). There was significant heterogeneity between the results, but publication bias or the quality of the trials did not explain this.
Safety of Acid Suppression
Inhibition of gastric acid by different therapeutic agents like antacids, H2RA and PPI continues to be the major medical strategy to treat dyspepsia. PPIs are safe drugs but concerns have been voiced regarding potential adverse effects related to hyper-gastrinemia rebound acid hypersecretion, malabsorption, infection, and drug interaction.
Humans respond to a decrease in luminal acid with a lesser increase in serum gastrin than rats and do not develop gastric carcinoids unless in the setting of severe atrophic gastritis or Zollinger-Ellison syndrome (ZES) associated multiple endocrine neoplasia (MEN-I) [71,72]. Although there is no convincing evidence that hypergastrinemia per se induces neoplasia, the possibility exists that it might accelerate the growth and invasiveness of cancer harboring its receptors [73-77].
Rebound hyper secretion is an issue, which can occur after as little as a 2-month course of therapy and lasts at least 2 months after the PPI is stopped [78]. One way to prevent this from happening would be to, taper the PPI and switch to tapering doses of H2RA over a period of 2 months instead of discontinuing the drug abruptly [79].
Chronic hypochlor- hydria induced by PPIs could interfere with absorption of nutrient such as Vit B12, iron and calcium [80-82].
Conclusions
Gastric acid plays an important role in the pathophysiology of functional dyspepsia. We hypothesize that initial contact of gastric acid with the vagal nerve terminal occurs in the background of a leaky mucosa.
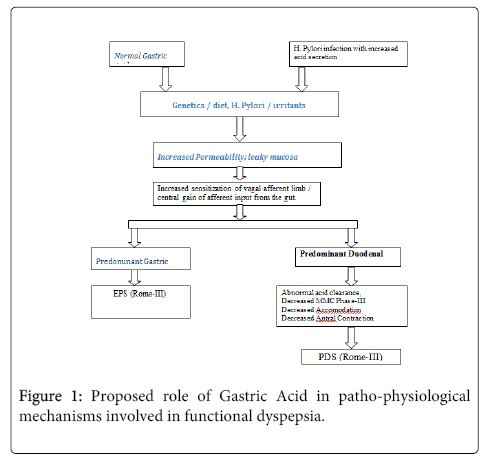
This permeability is altered in a genetically susceptible individual in response to H. pylori infection, irritants, allergens or diet. Either an abnormal central nervous system processing of afferent information or/and sensitization of vagal afferent nerve ending during gastric stimuli is a mechanism of visceral hypersensitivity (Figure 1).
If it is a predominant gastric visceral hypersensitivity, presentation would be of Rome-III EPS. In case of duodenal sensitization, it predominantly presents as PDS-Rome-III. At the level of duodenum, abnormalities may exist in stimulus intensity, mucosal mRNA expression, biosynthesis, release, or inactivation of mucosal mediators; or receptor expression of afferent nerve endings. Elucidation of the abnormalities involved will provide a basis for rational treatment of functional dyspepsia.
References
- Tally NJ, StanghelliniV, Heading RC, Koch KL, Malagelada JR, et al. (1999) Functionalgastrodudenol disorders.Gut 45: 37-42.
- Tally NJ, StanghelliniV, Heading RC, Koch KL, Malagelada JR, et al. (2000) The functional gastrointestinal disorders.pp: 299-350.
- Talley NJ, Silverstein MD, Agreus L, Nyren D, Sonnenbers A, et al. (1998) AGA technical review: Evaluation of dyspepsia. Gastroenterology 114: 582-595.
- Henke CJ, Levin TR, Henning JM, Potter LP (2000) Work loss costs due to peptic ulcerdisease and gastro esophageal reflux disease in a health maintenance organization. Am J Gastroenterol 95:788-792.
- Kurata JH, Nogawa AN, Everhart JE (2002) A prospective study of dyspepsia in primaryCare Dig Dis Sci47: 797-803.
- Manjumdar SR, Soumerai SB, Farraye FA, LeeM, Kemp JA, et al. (2003) Chronic acid related dis-orders arecommon and under investigated. Am J Gastroenterol 98: 2409-2414.
- Talley NJ, Vakil NB, Moayyedi P (2005) American Gastro-entrological association technical review on evaluation of dyspepsia. Gastroentrology 129: 1756-1780.
- Konturek SJ,Konturek PC, Brzozowski T, Konturek JW,Pawlik WW (2005) From nerves and harmones to bacteria in the stomach. Nobel prize for achievements in gastroentrology during last century. J Physiolpharmacol 56: 507-530
- McColl KEL, El-Omar E, Gillen D(1998) Interactions between H pylori. infection, gastric acid secretion and anti secretory therapy. Br Med Bull54: 121-138.
- Fellenius E, Berglinda, Sachs G, Olbe L, Elander B, et al. (1981) Substituted benz-imidazoles inhibit gastric acidSecretion by blocking (H+K+) ATPase290:159-161.
- Marshall BJ, Warren JR (1984)Un-identified curved bacilli in the stomach of patients with gastritis and peptic ulcer. Lancet 1: 1311-1315.
- Wolosin JM, Forte JG (1984) Stimulation of oxyntic cell triggers K+ CI- conductancesin H+K+ ATPase membrane. Am J physiol cell physiol 246: C537-C545.
- Johansson M, Synnerstad I, Holm L (2000) Acid transport through channels in themucus layer ofrat stomach. Gastroenterology119: 1297-1304.
- Hurwitz A, Brady DA, Schaal SE, Samloff IM, Dedon J,et al.(1997) Gastric acidity in older adults. JAMA278: 659-662.
- Trey G, Marks IN, Louw JA, Jaskiewicz K, Sipponen P, et al. (1997) Changes in acid secretion over the years. A 30 years longitudinal study. J clinGastroenterol 25: 499-502.
- Schubert ML, Shamburek R (1990) Control of acid secretion. Gastroenterolclin northAm 19: 1-25.
- Soll AH (1982) Potentiating interactions of gastric stimulants on (14C) aminopyrineaccumulation by isolated canine parietal cells. Gastroenterology 83: 216-223.
- Mitchell L, Schubert, David A Peura (2008) Control of gastric acid secretion in health anddisease. Gastroenterology134: 1842-1860.
- Meyer-Rosberg K, Scott DR, Rex D, Melchers K, Sachs G (1996) The effect of environment pH on theproton motive force of Helicobacter pylori. Gastroenterology 111: 886-900
- El-Omar EM (2006) Mechanisms of increased acid secretion after eradication of helicobacter pylori infections. Gut55: 144-146.
- Gillen D, El-Omar EM, Wirz AA,Ardill JES, McColl KEL(1998) The acid response to gastrin distinguishes duodenal ulcer patients from helicobacter pylori infected healthy subjects.Gastroenterology 114: 50-57.
- Moss SF, Legon S, Bishop AE, Polak JM (1992) Effect of helicobacter pylori on gastricsomatostatin in duodenal ulcer disease. Lancet 340: 930-932.
- El- Omar EM, Penman ID, Ardill JES,Chittajallu RS, Howie C,et al. (1995) Helicobacter pylori infection andabnormalities of acid secretion in patients with duodenal ulcer disease.Gastroenterology 109: 681-691.
- Moayyedi P, Soo S, Deeks J, Delaney B, Harris A, et al. (2005) Eradication of Helicobacter pylori for non ulcer dyspepsia. Cochrane database syst rev 1: CD002096.
- Moayyedi P, Soo S, Deeks J, Delaney B, Innes M, et al. (2004)Pharmacological interventions for non ulcer dyspepsia. Cochrane database syst rev4: CD001960.
- Tack J, Talley NJ, Camilleri M,Holtmann G, Hu P, et al. (2006)Functional gastroduodenal dis-orders.Gastroenterology 130: 1466-1479.
- Stanghellini V, Tosseti C, Paternico A, Barbara G, Morselli-Labate AM, et al. (1996) Risk indicators of delayed gastric emptying ofsolids in patients with functional dyspepsia. Gastroenterology 110: 1036-1042.
- Sarnelli G, Caenepul P, Geypens B, Janssens J, Tack J (2003) Symptoms associatedwith impaired gastric emptying of solids and liquids in functional dyspepsia. Am JGastroenterol 98: 783-788.
- Tack J, Piessevaux H, Couli B, Caenepeel P, Janssens J(1998) Role of impaired gastricaccommodation to a meal in functional dyspepsia. Gaastroenterology115:1346-1352.
- Tack J, Caenepeel P, Fischter B, Piessevaux H, Janssens J (2001) Symptoms associated with hyper sensitivity to gastric distention in functional dyspepsia. Gastroenterology121: 526-535.
- Grudell ABM, Camilleri M, Burton DD, Stephens DA (2006) Effect of proton pump inhibitor onpost prandial gastric volume, emptying and symptoms in healthy human subjects. Apilot study. Aliment pharmacolther24: 1037-1043.
- El-Omer E, Pennman I, Ardil JES, McColl KEL(1995) A substantial proportion of non-ulcerdyspepsia patients have the same abnormality of acid secretion as duodenal ulcer patient. Gut 36: 534-538.
- Collen MJ, Loeben berg MJ (1989) Basal gastric secretion in non-ulcer dyspepsia with orwithout duodenitis. Dig Dis Sci 34: 246-250.
- Sachs G, Shin JM, Munson K, Vagin O, Lambrecut M, et al. (2000) Review article: Thecontrol of gastric acid and helicobacter pylori eradication. Alimentary pharmacolther 14: 1383-1401.
- Holzer P (2002) Sensory neurone responses to mucosal noxae in the upper gut: relevanceto mucosal integrity and gastrointestinal pain. Neurogastroenterol Mot 14:459-475.
- Malagelada JR (2001) The continuing dilemma of dyspepsia. Alimentary pharmacolther15(suppl): 6-9.
- Coffin B, Chollet R, Flourie B, Lémann M, Franchisseur C, et al. (2001) Intraluminal modulation of gastric sensitivity todistension: effect of hydrochloric acid and meal. Am J Physiol 280: G904-G909.
- Talley NJ, Lauristen K (2002) The potential role of acid suppression in functionaldyspepsia: The BOND, OPERA, PILOT, and ENCORE studies. Gut50 (suppl 4):iv36-iv41.
- Read NW (1999) Functional dyspepsia: A case of indecision. Gastroenterology 116:761-762.
- Zagon A (2001) Does the vagus nerve mediate the sixth sense? Trends Neurosci 24:671-673.
- Lamb K, Kang YM, Gebhart GF, Bielefeldt K (2003) Gastric inflammation triggershypersensitivity to acid in awake rats. Gastroenterology 125: 1410-1418.
- Holzer P (2003) Afferent signaling of gastric acid challenge. Journal of physiology and pharmacology 54 (supp-4): 43-53.
- Accarino AM, Azpiroz F, Malagelada JR (2002) Gut perceptions in humans is modulated byinteracting gut stimuli. AmJphysiol 282: G220-G225.
- Lee KJ, Tack J (2010)Duodenal implications in the patho-physiology of functionaldyspepsia. J NeurogastroenterolMotil16(3): 251-257.
- Tanimura T, Adachi K, Furuta K, Ohara S, Morita J, et al. (2011) Usefulness of catheterless radiometry pH monitoring system to examine therelationship between duodenal acidity and upper gastrointestinal symptoms. J.GastroenterolHepatol26(1): 98-103.
- Lee KJ, Demarchi B, Demedts I, Sifrin D, Raeymaekers P, et al. (2004) A pilot studyon duodenal acid exposure and its relationship to symptoms in functional dyspepsiawith prominent nausea. Am. J. Gastroenterol99: 1765-1773.
- Samsom M, Verhagen MA, Van Berge Hengouwen GP, Smout AJ (1999) Abnormalclearance of exogenous acid and increased acid sensitivity of proximal duodenum indyspeptic patients. Gastroenterology 116: 515-520.
- Miwa H, Nakajimma K, Yamaguchi K, Fujimoto K, Veldhuyzen VAN, et al. (2007) Generation of dyspeptic symptomsby direct acid infusion into the stomach of healthy Japanese subjects. Alimentarypharmacolther26: 257-264.
- Simren P, Vos R, Janssens J, Tack J (2003) Acid infusion enhances duodenal mechano-sensitivity in healthy subjects. Am J physiol285: G309-G315.
- Hunt JN, Knox MT (1972) The slowing of gastric emptying by four strong acids and threeweak acids. J. Physiol222: 187-208.
- Woodtli W, Owyang C (1995) Duodenal PH governs interdigestive motility in humans.Am J Physiol268: G146-G152.
- Lee KJ, Demarchi B, Vos R, Demedts I, Janssens J, et al. (2002) Comparison ofduodenal acid exposure in functional dyspepsia patients and healthy controls using24 hour abulatory duodenal PH monitoring (Abstract). Gastroenterology 1223(suppl): 788.
- Lee KJ, Rita V, Janssens J, Tack J (2004) Influence of duodenal acidification on thesensorimotor functions of the proximal stomach of the humans. Am J PhysiolGastrointest Liver physiol 286: G278-G284.
- Mayer EA,Gebhart GF (1994) Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 107: 271-293.
- Hansen JM, Bytzer P, Schaffalitzky de Muckadell OB (1998) Placebo controlled trial ofcisapride and nizatidine in unselected patient with functional dyspepsia. Am J.Gastroenterol 93: 368-374
- Delattre M, Malesky M, Prinzie A (1985) Symptomatic treatment of non-ulcer dyspepsia with cimetidine. CurrTher Res 37: 980-991
- Gothard R, Bodemar G, Brodin U, Jonson KA (1988) Treatment with cimetidine, antacids,or placebo in patients with dyspepsia of unknown origin. Scand J Gastroenterol23: 7-18.
- Hadi S (1989) Clinical investigation of ranitidine in patients with gastritis. ClinTher11: 590-594.
- Kelbaek H, Linde J, Eriksen J, Mungaard S, Moesgaard F, et al. (1985) Controlledclinical trial of treatment with cimetidine for non ulcer dyspepsia. Acta Med Scand217:281-287.
- Nesland AA, Berstand A (1985) Effect of cimetidine in patients with non-ulcer dyspepsiaand erosive pre-pyloric changes. Scand J Gastroenterol 20: 629-635.
- Saunders JH, Oliver Rj, Higson DL (1986) Dyspepsia: incidence of a non-ulcer disease in acontrolled trial of ranitidine in general practice. Br. Med J (Clin Res Ed) 292:665-668.
- Singal AK, Kumar A, Broor SL(1989) Cimetidine in the treatment of non-ulcer dyspepsia:results of a randomized double-blind, placebo-controlled study. Curr Med Res Opin11: 390-397.
- Muller P, Hotz J, Franz E, Simon B (1994) Ranitidine in the treatment of non-ulcerdyspepsia. A placebo controlled study in the federal Republic of Germany.Arzneimittel for schung44: 1130-1132.
- Blum AL, Arnold R, Stolte M, Fisher M, Koelz HR (2000) Short course acid suppressivetreatment for patients with functional dyspepsia: results depend on helicobacterpylori status. Gut47: 473-480.
- Olubuyide IO, Atoba MA (1986) Non-ulcer dyspepsia in nigerians clinical and therapeuticresults. ScandGastroenterol124 (suppl): 83-87.
- Talley NJ, MeinecheSchmidt V, Pare P, Duckworth M, Raisanen P, et al. (1998) Efficacy of omeprazole in functional dyspepsia: double-blindrandomized placebo–controlled trial (TheBond and Opera studies). AlimentpharmacolTher 12: 1055-1065.
- Wong WM, Wong BC, Hung WK, Yee YK, Yip AW, et al. (2002) Double blind, randomised, placebo controlledstudy of four weeks of lansporazole for the treatment of functional dyspepsia inchinese patients. Gut51: 502-506.
- Bolling-Sternevald E, Lauritsen K, Aalykke C, Havelund T, Knudsen T, et al. (2002) Effect of profound acid suppression in functional dyspepsia: adouble-blind, randomised, placebo controlled trial. Scand J Gastroenterol 37:1395-1402
- Farup PG, Havde O, Torp R, Wetterhus S (1999) Patients with functional dyspepsiaresponding to omeprazole have a characteristic gastro-oesophageal reflux patternScand J Gastroenterol34: 575-579.
- Peura DA, Kovacs TOG, Metz DC, Siepman N, Pilmer BL, et al. (2004)Lansoparazolein the treatment of functional dyspepsia: two double blind, randomized, placebo–controlled trials.Am J Med 116: 740-748.
- Carlesson E, Larsson H, Mattsson H,Ryberg B, Sundell G (1986) Pharmacology and toxicology ofomeprazole with special reference to the effects on the gastric mucosa. Scand JGastroenterol 118: 31-38.
- Freston JW, Borch K, Brand SJ, Carlsson E, Creutzfeldt W, et al. (1995) Effects of hypochlorhydria andhypergastrinemia on structure and function of gastrointestinal cells. A review andanalysis. Dig Dis Sci 40: s50-s62.
- Dufresne M, Seva C, Fourmy D (2006)Cholecytokinin and gastrin receptors. Physiol Rev86: 805-847.
- Hoosein NM, Kiener PA. Curry RC, Rovati LC, McGilbra DK, et al. (1988)Antiproliferative effects of gastrin receptor antagonists and antibodies to gastrin on human color carcinoma cell lines. CancerRes48: 7179-7183.
- Yang YX, Hennessy S, Propert K, Hwang WT, Sedarat A, et al. (2007) Chronic proton pump inhibitor therapy andthe risk of colorectal cancer. Gastroenterology 133: 748-754.
- Robertson DJ, Larsson H, Friis S, Pedersen L, Baron JA, et al. (2007) Proton pump inhibitor use and risk ofcolorectal cancer: a population based, case control study. Gastroenterology 133: 755-760.
- Clerc P, Dufresne M, Saillan C,Chastre E, André T, et al. (1997) Differential expression of the CCK-A andCCK-B/gastrin receptor genes in human cancers of the esophagus, stomach andcolons. Int J cancer72: 931-936.
- Larsson H, Carlsson E, Hakanson R,Mattsson H, Nilsson G, et al. (1988) Time course of development andreversal of gastric endocrine cell hyperplasia after inhibition of acid secretion. Gastroenterology 95: 1477-1486.
- Schubert ML, Peura DA (2008) Control of Gastric acid secretion in health anddisease. Gastroenterology 134: 1842-1860.
- Miner P, Katz PO, Chen Y, Sostek M (2003) Gastric acid control with esomeprazol, lansoprazol,omeprazole, pantoprazole, and rabeprazole: a five way cross over study. Am J Gastroenterol 8: 2616-2620.
- Hutchinson C, Geissler CA, Powell JJ,Bomford A (2007) Proton pump inhibitors suppressabsorption of dietary non-haem iron in hereditary hemochromatosis. Gut56:1291-1295.
- Yang YX, Lewis JD, Epstein S, Epstein S, Metz DC (2007) Long-term protonpump inhibitor therapy andrisk of hip fracture. JAMA 297: 470.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 17454
- [From(publication date):
June-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 12804
- PDF downloads : 4650