Role of Education and Counseling for the Prevention of Japanese Encephalitis in the Eastern U.P, India
Received: 15-Mar-2014 / Accepted Date: 27-May-2014 / Published Date: 30-May-2014 DOI: 10.4172/2161-1165.1000161
Abstract
Japanese encephalitis is numerically one of the most important causes of mosquito borne encephalitis not only in asia but over the world. The major vector of JEV is a culicine mosquito, Culex tritaeniorhynchus. Pigs act as important amplifiers of the virus, and birds can also be involved in its amplification and spread in the environment .An epidemic of viral encephalitis was reported from July through November 2005 in Gorakhpur, Uttar Pradesh, India. It was the longest and most severe epidemic in 3 decades; 5,737 persons were affected in 7 districts of eastern Uttar Pradesh, and 1,344 persons died. The geographic features of this region are conducive for the spread of JEV; an abundance of rice fields and a bowl-shaped landscape allow water to collect in pools. In this present study attempts will be made to find out the role of education and counseling in prevention of occurrence and reducing number of morbidity due to Japanese encephalitis. The illiteracy is most important factor of spreading of Japanese encephalitis. By the educational programs and counseling we can make a barrier for Japanese Encephalitis and this is very important for society.
Keywords: Counselling; Prevention; Japanese Encephalitis
162442Introduction
The JE virus is a member of the genus Flaviviridae, together with the Yellow Fever virus and Dengue Virus. The JE virus belongs to the same serological group as the West Nile virus (WNV) and the St. Louis Encephalitis Virus (SLEV). With the help of genome sequencing studies, it has been possible to determine the various genotypes of JEV in circulation in different geographic areas. The two Indian isolates [GP78 and Vellore P20778] show genetic similarity to the Chinese SA14 and Beijing genotypes.
Fast Facts of Japanese Encephalitis
• 30,000-50,000 cases reported annually, resulting in 10,000-15,000 deaths annually;
•3 billion people, including 700 million children, are at risk of contraction; Children are the most vulnerable (highest mortality rates) Over 10 million children have been infected, 3 million dead and 4 million permanently disabled from JE;
• JE affects the brain and is commonly mistaken for Meningitis and other brain related diseases; Occurs primarily in rural areas; Mosquitoes, pigs, birds, humans and horses are involved in the transmission and infection process [1].
Methodology to Prevent Japanese Encephalitis
Identification of geographical location where JE is Para endemic
Identification of the patients under medication
Patient counseling
Health education
Collection of data in specified format
Evaluation of data
Occurrence
Japanese encephalitis was recognized in southern India from 1955, but was confined to the south until the 1970s. Since then, large outbreaks (2000–7000 cases a year) have been reported from eastern and northeastern states. The fact that adults and children were equally affected in these Indian states strongly supports the idea that the virus was introduced here for the first time. The late 1970s also saw the first cases in Burma and Bangladesh, and large epidemics (up to 500 cases a year) in southwestern Nepal. In 1985 Sri Lanka experienced its first epidemic with 410 cases and 75 deaths. Japanese encephalitis virus continues to spread west with cases occurring in Pakistan [2] and new epidemics in the Kathmandu valley of Nepal [3].
Vectors
In India, the virus has been isolated from more than 15 species of mosquitoes belonging to genera Culex, Aedes and anopheles. Cx tritaneniorhynchus and Cx Vishnui. however, are considered as the main vectors.
Chances of infection: During the rainy summer months of June to August, the incidence of the disease reaches its peak [4] (Table 1).
| Table 1 | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assam | Peak | |||||||||||
| Manipur | Peak | |||||||||||
| Nagaland | Peak | |||||||||||
| Arunanchal Pradesh | Peak | |||||||||||
| West Bengal | Peak | Peak | ||||||||||
| U.P | Peak | Peak | ||||||||||
| Uttarakhand | Peak | Peak | ||||||||||
| Bihar | Peak | Peak | ||||||||||
| Haryana | Peak | Peak | ||||||||||
| Maharashtra | Peak | |||||||||||
| Goa | Peak | |||||||||||
| Tamil Nadu | 1st Peak | 2nd Peak | ||||||||||
| Kerala | Peak | |||||||||||
| Karnataka | Peak | |||||||||||
| Andhra Pradesh | Peak |
Table 1: Transmission season for the 15 states undertaken/ to be undertaken in the JE campaigns – Source NVBDCP- 8th March 2010 [5].
Symptoms
Most people who are infected show only mild symptoms or no symptoms at all. However, at advanced stages, the disease may be fatal. It has an incubation period of 1-2 weeks. The disease begins like flu with headache, fever, chills, anorexia, vomiting, dizziness and drowsiness which in children is often accompanied by abdominal pain and diarrhea [6].
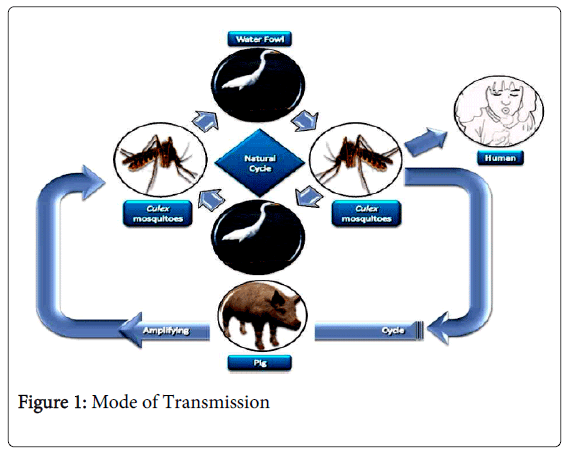
Mode of Transmission
The transmission of JE and highlights contextual determinants because infected pigs act as amplifying hosts, domestic pig rearing is an important risk factor in the transmission to humans [7]. Two distinct epidemiologic patterns of JE have been described. In temperate zones, such as the northern part of the Korean peninsula, Japan, China, Nepal, and northern India, large epidemics occur in the summer months; in tropical areas of southern Vietnam, southern Thailand, Indonesia, Malaysia, the Philippines, and Sri Lanka, cases occur more sporadically and peaks are usually observed during the rainy season [8,9]. Thus far, the reasons for the spread of JE are not fully understood. The Transportation of vectors, human migration, and international travel seem to be of little importance because viremia in humans is usually low and of short duration and because humans are dead-end hosts JE was likely introduced into northern Australia by wind-blown mosquitoes from Papua New Guinea (Figure 1).
Districts Affected
The deaths, mostly of children, took place in various districts, including Gorakhpur, Kushinagar, Siddharthnagar, Basti and Sant Kabir Nagar in eastern UP and Gopalganj, Siwan, Chappra and Muzafferpur in Bihar. Gorakhpur is the worst affected area with maximum cases (Table 2).
| S.N. | District affected | Patient admitted | No. of death |
|---|---|---|---|
| 1. | Gorakhpur | 595 | 95 |
| 2. | Kushinagar | 340 | 56 |
| 3. | Maharajganj | 176 | 33 |
| 4. | Deoria | 269 | 62 |
| 5. | Basti | 67 | 12 |
| 6. | Santkabir Nagar | 138 | 19 |
| 7. | Siddharthnagar | 84 | 24 |
| 8. | Gonda | 05 | 02 |
| 9. | Azamgarh | 06 | 02 |
| 10. | Balrampur | 03 | 01 |
| 11. | Mau | 12 | 01 |
| 12. | Balia | 08 | 01 |
| 13 | Gazipur | 01 | 00 |
Table 2: District wise position of Japanese Encephalitis*; * District wise position of Japanese Encephalitis collected from Hindustan Hindi daily news at date 03 Oct 2012.
Origin of the Research Problem
Japanese Encephalitis (JE) virus is the most important cause of viral encephalitis in the Asia–Pacific region, accounting for more than 16000 reported cases and 5000 deaths annually. Although all age group are susceptible to the disease, children between the age of 1 to 15 years are the most commonly affected [10]. JE virus is a mosquito- borne flaviviral infection. In the last 25 years, JE virus transmission has intensified in certain countries and the disease has extended its geo-graphical range to previously unaffected areas of Asia and to northern Australia. The high fatality rate and frequent residual neuropsychiatric sequelae in survivors make JE a considerable health problem. For example, in a in Thailand in which patients with JE received a high standard of supportive care that included treatment with dexamethasone, 25% of the patients died and 45% demonstrated neurological sequelae 3 months after diagnosis. Furthermore, in the best-documented study of long-term disability due to JE, which was conducted 10 years after the 1947 outbreak of the disease in Guam, neurological sequelae were reported in 40% of surviving patients, 11% of which were considered severe. Neither of these studies evaluated the proportion of cases with psychomotor retardation, fine motor deficits or behavioural disorders. JE virus is amplified in nature in a cycle involving Culex mosquitoes and vertebrate animals, especially pigs. Humans of all ages are susceptible unless immunized by natural infection or vaccination. Evidence shows that effective vaccines will protect animals and humans against illness and will remove the vaccinated animals from the pool of potential amplifying hosts of the virus. Although the control of mosquitoes and the vaccination of pigs are effective in certain circumstances, these measures are not practical as a means of preventing human illness. It is also important to recognize that humans are incidental hosts, and that, for vaccination to be effective, coverage must be maintained indefinitely in all persons who may be exposed to the virus. The virus replicates in a variety of cultured cells of vertebrate and arthropod origin. Since the 1960s, both live and inactivated vaccines have been developed that provide active immunity against JE virus. The development of these vaccines represents a major advance in the ability to control JE virus infection and reduce the burden of disease. Viruses isolated from human patients in Japan in 1935 and in China in 1949 provided the prototype Nakayama and Beijing and P3 strains, respectively, that are in principal use in the production of inactivated JE vaccine today [11]. National vaccination programmes in China (Province of Taiwan), Japan and the Republic of Korea, using an inactivated mouse-brain-derived vaccine that meets international requirements have controlled the disease to the point of elimination, but in other countries, the expense and complexity of producing the vaccine and the need for repeated doses have limited vaccine use. In addition to the problems posed by multiple doses, use of the vaccine to protect travellers has led to hypersensitivity events, including demyelination sequelae, such events have been reported in North America, Australia and Europe. As an alternative to inactivated vaccines, a live-attenuated JE vaccine (the SA 14-14-2-PHK strain) was developed in China. Since its licensure in China in 1988, more than 300 million doses of JE vaccine (live) have been produced for administration to children in annual vaccination programmes. The vaccine is of considerable interest to countries where JE virus is endemic but, as of the year 2000, is not yet licensed elsewhere [12,13]. Due to lack of information, lack of availability of the facilities to the patients and lack of awareness to the community creates the problem in prevention of this disease. Educational programs and other counseling can affect or prevent the spreading of JE in district Gorakhpur and nibour districts.
International Status of JE
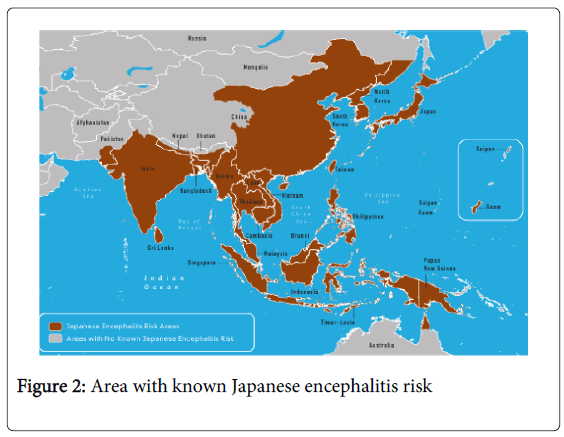
There are so many educational and counseling programs are usually performed for different diseases which are very harmful to community like diabetes, hypertension, asthma, cancer malaria etc. But Japanese encephalitis related programs are underestimated. Although all age group are susceptible to the disease, children between the age of 1 to 15 years are the most commonly affected. The impact of Japanese encephalitis not only results from the actual illness and death that occurs, but from the longer consequences of illness among survivors. Until many other diseases where the outcome is either complete recovery or death, permanent squealed after Japanese encephalitis are common with disability. This can result in the significant burden for the family and the community, as well as the health system. The physical and economic burden can be removed possibly by removing the problem of the community through educational programs. The problem of increasing concern to countries, including Cambodia, China, Indonesia, Japan, Laos, Malaysia, Myanmar, Philippines, Korea, Thailand, Vietnam, South-eastern Russian Federation and the Indian subcontinent in Southeast Asia and the Western Pacific because of the occurrence of thousands of cases over the past decade, with high case-fatality rates and expansion into new areas (Figure 2).
National Status
In India, JE was first recorded in Vellore & Pondicherry in mid 1950s. JE has been reported from 24 states/ Union territories so far. Frequently affected states include Andhra Pradesh, Assam, Bihar, Goa, Haryana, Karnataka, Manipur, Tamil Nadu, Uttar Pradesh & West Bengal. The Directorate of National Anti-Malaria Program (NAMP) is monitoring JE in India since 1978. An estimated 378 million population is living at the risk of JE in 12 states/ Union Territories that are frequently affected. An endemic of viral encephalitis was reported from July through November 2005 in Gorakhpur, U.P, India [14]. The spread of JE to new areas is probably due to agricultural development and intensive rice cultivation supported by irrigation schemes. It has high mortality and morbidity rates. In UP, there are 20 districts including Gorakhpur, Kushinagar, Siddharthnagar, Basti and Sant Kabir Nagar in eastern UP is affected by Japanese encephalitis. Gorakhpur is the worst affected area with maximum cases. Some recent data are shown the seviourity of Japanese Encephalitis (Table 3).
| Month | Admitted patients |
|---|---|
| Jan | 51 |
| Feb | 51 |
| March | 58 |
| April | 85 |
| May | 119 |
| June | 97 |
| July | 135 |
| Aug | 304 |
Table 3: Admitted patient of Japanese Encephalitis in BRD medical college, District Gorakhpur from Hindustan daily news at date 29 Aug 2012
Medical Facilities
In some rural areas, where medical care is not easily available, case fatality rates as high as 70% have been reported. In contrast, high quality medical care may result in rates less than 10%, CDC (1993) [6,15,16]. In a zoonotic cycle, JEV is transmitted by vector mosquitoes (Culex sp.) [17] between wild/domestic birds and pigs; where birds act as reservoir host [18] and pigs act as amplifying host [19] Pig-mosquito-pig and bird-mosquito- bird cycle is responsible for the maintenance of the virus in nature. Man is the “dead end” host [20,21] (Table 4).
| Date | Admitted Patient | No. of Dead |
|---|---|---|
| 28 Aug 2012 | 35 | 05 |
| 29 Aug 2012 | 36 | 00 |
| 30 Aug 2012 | 29 | 06 |
| 01 Sep 2012 | 28 | 05 |
Table 4: Patient Growth of Japanese encephalitis in BRD medical college [22]
Efforts from Government
The health department have released Rs 18 crore for a 100-bed ward at the BRD Medical College in Gorakhpur. Rs 2500 crore have been sanctioned under the National Rural Mission this year for UP out of which Rs 5 crore is specifically for encephalitis [23]. The health department have released Rs 1.5 crore for a BRD Medical College in Gorakhpur to improve the situation of Japanese encephalitis [5].
Preventive Activities for the Japanese Encephalitis Campaign
At the National level an orientation planning & consultation workshop should be conducted to plan for the JE campaigns. At the State level – State orientation and JE campaign planning meetings should be held for the districts undertaking the JE campaign [24,25].
Simple Information on JE - cause, transmission and prevention of mosquito bites.
Community action in reducing mosquito breeding places by filling pools, weekly drainage of accumulated water, lowering of water levels in rice fields etc.
National guidelines for diagnosis, management and prevention of JE- for programme managers and health professionals.
Inter- sectoral coordination (Health, Education, ICDS etc).
To review broad district plans and to conduct district microplanning meeting for both rural and urban areas to review preparedness, microplanning and progress in IEC/social mobilization, trainings, etc and to review the progress and solve last minute problems.
To meet before and after and as and when required to take corrective actions.
During the JE campaign - to review the activity and make further plans for the introduction of the JE vaccine in the routine immunization program.
Orientation of district and block level trainers/medical officers.
Identify one media spokesperson at the State and the District level.
Initiate preparation of block-wise microplans, procurement of logistic materials and printing of stationary like immunization cards, supervisory and vaccinators instructions, checklists and tally sheets etc.
Review microplans prepared at Blocks/PHCs/urban areas.
Identify ice factories/cold storages for procurement of ice or freezing of ice packs.
Verify functioning and availability of cold chain equipments, like deep freezers, ILRs, vaccine carriers, adequate icepacks and cold boxes.
Blocks/PHCs/urban areas to submit micro plans to the district.
Organize orientation meeting of community/religious leaders at district headquarters.
Finalize and release funds to blocks/urban areas.
Start orientation of supervisors, vaccinators and cold chain handlers.
Make supervisory visits to identified high risk pockets both in rural and urban areas before campaign to check preparedness and during campaign to monitor activities
M O to organize meetings/panch sammelans with community and religious leaders.
Continue orientation of supervisors and vaccinators and cold chain handlers.
Distribute vaccines and logistics to PHCs.
Intensify social mobilization; begin with display of IEC materials, rallies, prabhat pheris.
Start miking and public announcements from fixed sites like temples and markets three days prior to activity.
Daily evening meetings at block/PHC to get feedback from supervisors and plan for mid round corrective actions.
Send Block reports to district headquarters in the evening or the next day.
Consolidate immunization figures for the district and report to SEPIO & AC (Imm.), MOH&FW, GOI.s
Conclusion
Present review focusing on to short out the reasons for noncompliance and improving the drug therapy, it also provide role of education and counseling in prevention of occurrence and reducing the morbidity due to Japanese encephalitis.
References
- Igarashi A, Tanaka M, Morita K, Takasu T, Ahmed A, et al. (1994) Detection of West Nile and Japanese encephalitis viral genome sequences in cerebrospinal fluid from acute encephalitis cases in Karachi, Pakistan. MicrobiolImmunol38: 827-830.
- Zimmerman MD1, Scott RM, Vaughn DW, Rajbhandari S, Nisalak A, et al. (1997) Short report: an outbreak of Japanese encephalitis in Kathmandu, Nepal. Am J Trop Med Hyg 57: 283-284.
- Tsai TF (1999)Arboviruses. In: Murray PR, Baron EJ, Pfaller MA, et al. Manual of clinical microbiology. (edn), ASM Press, Washington, USA.
- Operational Guide for Japanese Encephalitis Vaccination in India (2010)MoHFW, September 2010.
- Gould EA (2002) Flavivirus Infections in Humans. Encyclopedia of Life Sciences, Macmillan Publishers Ltd., Nature Publishing group 220-244.
- Solomon T (2006) Control of Japanese encephalitis--within our grasp? N Engl J Med 355: 869-871.
- Vaughn DW, Hoke CH Jr (1992) The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol Rev 14: 197-221.
- Mackenzie JS, Gubler DJ, Petersen LR (2004) Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 10: S98-109.
- Report of Japanese encephalitis disability assessment kingdom of combodia (2008) ministry of health department of communicable disease control.
- Parts of the text of this section are abstracted from the minutes of a WHO/(Children’s Vaccine Initiative (CVI)) meeting on new initiatives for the control of Japanese encephalitis by vaccination, held in Bangkok, Thailand.
- Tsai TF (2000) New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13-15 October 1998. Vaccine 18 Suppl 2: 1-25.
- Requirements for Japanese encephalitis vaccine (inactivated) for human use (1988) In: WHO Expert Committee on Biological Standardization. Thirty-eighth report. Geneva, World Health Organization.
- Parida M,Dash PK, Tripathi NK, Ambuj, Sannarangaiah S, et al. (2006) Japanese encephalitis outbreak, India, 2005, Emerging infectious disease 12: 1427-1430.
- Centers for Disease Control and Prevention (1993) Inactivated Japanese Encephalitis virus vaccine recommendations of the advisory committee on immunization practices (ACIP) MMWR, 42.
- Easmon C (2005) Japanese Encephalitis and other forms of Viral Encephalitis transmitted by Mosquito.
- Geevarghese G, Kanogia PC, Mishra AC (2004) Japanese encephalitis-Vector Biology. NIV Pune Year Book. Orient Longman Publication, Himayatnagar.
- Reuben R, Gajanana A (1997) Japanese encephalitis in India. Indian J Pediatr 64: 243-251.
- Diagana M, Preux PM, Dumas M (2007) Japanese encephalitis revisited. J NeurolSci 262: 165-170.
- SCHERER WF, KITAOKA M, OKUNO T, OGATA T (1959) Ecologic studies of Japanese encephalitis virus in Japan. VII. Human infection. Am J Trop Med Hyg 8: 707-715.
- Sphere India Unified Response Strategy: Situation Report (2011) (Japanese Encephalitis).
- Prevention and control of dengue, Japanese Encephalitis and Kala-Azar in Sea Level (2002) Regional committee.
Citation: Srivastava P, Singh A, Srivastava AK, Singh AP, Prakash D (2014) Role of Education and Counseling for the Prevention of Japanese Encephalitis in the Eastern U.P, India. Epidemiol 4:161. DOI: 10.4172/2161-1165.1000161
Copyright: © 2014 Srivastava P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 20563
- [From(publication date): 6-2014 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 15858
- PDF downloads: 4705