Research Article Open Access
Role of Dermatophagoides Pteronyssinus in the Skin Lesions of Atopic Dermatitis in Children
Cantani A*Division of Pediatric Allergy and Immunology, Roma University "La Sapienza", Italy
- *Corresponding Author:
- Cantani A
Division of Pediatric Allergy and Immunology
Roma University "La Sapienza", Italy
Tel: 0644230256
E-mail:acantani13@gmail.com
Received date: December 09, 2014; Accepted date: December 28, 2015; Published date: December 30, 2015
Citation: Cantani A (2014) Role of Dermatophagoides Pteronyssinus in the Skin Lesions of Atopic Dermatitis in Children. Interdiscip J Microinflammation 1:126. doi: 10.4172/2381-8727.1000126
Copyright: © 2014, Cantani A This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at International Journal of Inflammation, Cancer and Integrative Therapy
Abstract
Atopic dermatitis (AD) is a common, complex, multifactorial skin disorder of infancy and childhood. Many affected children show positive SPT and IgE antibodies towards a large variety of foods and some aeroallergens. The relationship between AD and food allergy has been established by double blind placebo controlled food challenge tests and by significant improvement after appropriate elimination diet. Although it has been suggested for many years that inhalant allergens may induce AD only in the last decade the role of house dust mite in AD has been investigated.
Keywords
Allergy; Clinical; Immunology division; Dermatophagoides pteronyssinus
Introduction
It has been reported that adult patients with AD and without asthma have high levels of IgE [1-7] and IgG antibodies towards the Der P1 allergen of Der p [8-12]. In addition, eczematous skin lesions occurred applying mite allergen by PT to the cutis of patients with AD, thus suggesting a link between AD and Der p exposure [12-18]. However the methodologies used by different authors widely varied, namely the procedure and the site of the PT application, the allergen preparation and the time between PT application and reading.
The aim of the present study was to evaluate the PT response to Der p in children with AD and in their parents and to evaluate the cutaneous xerosis in the parents of the atopic children [19].
The data of the present study show that a significantly higher proportion of children with AD and their parents have positive PT to Der P than the controls (p<0.001). In addition we have shown that xerosis was more common in the parents of the children with AD in comparison to the parents of the atopic children without AD (p<0.0005).
Patients, Material and Methods
Seventy-nine atopic children with AD who consecutively attended our clinic from October 1990 until January 1991 were enrolled into the study.
They were 45 males and 34 females with median age of 4 years and 8 months (range 5 months to 13 years) and, as a control group, 20 healthy children (11 males and 9 females) with median age of 4 years and 6 month (range 6 months to 11 years and 6 months) and 11 healthy adults (5 males and 6 females aged 29-36 years) with negative personal and familial history of atopic diseases.
The 79 children were divided into 3 groups: group 1 comprised 31 children who had AD at the enrollment (median age: 4 years and 8 months, range: 5 months to 13 years); group 2 comprised 23 children who had suffered from AD, but the disease was cured (median age: 3 years, range: 1 year and 6 months to 9 years and 7 months); group 3 comprised 25 atopic children with asthma and/or rhinitis who never had suffered from AD (median age: 5 years and 5 months, range: 1 years and 4 months to 12 years).
The diagnosis of AD was made according to the criteria of Hanifin and Rajka [20]. The severity of AD lesions was assessed using a semiquantitative score as previously described [6].
In addition to AD, 5/54 (9%) children of the group 1 and 2 suffered from asthma, 10/54 (19%) had asthma and rhinitis, 6/54 (11%) had rhinitis, 1/54 (2%) had rhinitis and conjunctivitis, 1/54 had rhinitis and urticaria, 2/54 (4%) had urticaria.
149 parents of the atopic children (78 mothers and 71 fathers aged from 21 to 46 years) were also studied.
The following tests were performed in all the subjects: SPT with Der p, Lolium perenne, Parietaria officinalis, Alternaria tenuis, lactalbumin, casein, egg-white and wheat (Lofarma - Milano, Italy) on the volar surface of the forearm and the reaction was read at 20 minutes. In some cases additional allergens were used. A negative control with glycerosaline solution and a positive one with histamine (1:1000) were done. A wheal 3 mm larger than the negative control was considered positive.
PTs with a fecal extract of Der p in vaseline (60 biological units of Der p/cc of vaseline) (SARM - Guidonia, Italy) were done. For each test 0.1 ml of the ointment was used. PTs were done on the healthy skin of the interscapular region as follows: the ointment was applied with and without a previous scratching of the skin, the scratching was performed with a plastic needle without bleeding; the region was occluded with a patch-test plaster (Curatest - Lohmann, Germany). PTs with vaseline and without any substance (with and without previous skin scratching) were also performed. PT reactions were read after 48-72h evaluating the onset of pruritus, papules, erythema and vesicles through an arbitrary score (0 to 5 for each parameter). The highest total score was 20 and a PT with a score higher than 6 was considered positive. Application and reading of PTs were done by two different people. Who read PTs (P.M.) was not aware of the substances' disposition. Antihistaminic drugs and topical steroids were stopped at least 2 weeks before the application of the PT. No child enrolled in this study needed systemic steroids. Total serum IgE levels were determined using Phadebas IgE PRIST test (Pharmacia - Uppsala, Sweden). Specific IgE to the same allergens checked with SPT were measured by the Phadebas RAST method (Pharmacia - Uppsala, Sweden). First class RAST was considered positive.
Skin xerosis in parents was evaluated on clinical basis by an arbitrary score considering skin chap, scales, keratosis pilaris and hyper-linearity. A score from 0 to 5 could be assigned to each parameter. We considered a parent to have "dry skin" if score was higher than 10. Informed consent was obtained from the parent of each child.
Statistical analysis
The analyses were performed using the X2 test and the Wilcoxon test.
Results
Allergic features
Twenty-three out of the 54 children of the group 1 and 2 (43%) and 11/25 children of the group 3 (44%) had a positive familial history of atopy (n.s.).
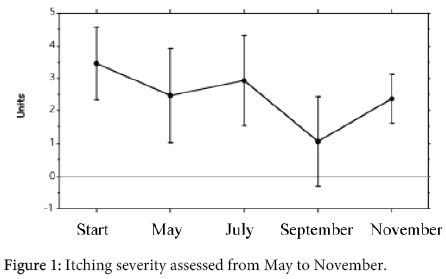
Twenty-eight out of 107 parents of the children of the group 1 and 2 (26%) and 13/50 parents of the children of the group 3 (26%) had a positive personal history of atopic diseases (n.s.). In particular, among the parents of the children of the group 1 and 2, 4/107 (3.7%) had asthma and 13/107 (12%) had allergic rhinitis; among the parents of the children of the group 3, 2/50 (4%) had either asthma and rhinitis (n.s.). The skin was xerosic in 47/107 (44%) parents of the children of the group 1 and 2 and only in 6 out of 50 parents of the children of the group 3 and in no parents of the children of the control group. This difference was highly significative (p<0.0005). Seventy-eight out of 107 (73%) parents of the children of the groups 1+2 and 37/50 (74%) parents of the children of the group 3 had no clinical allergy (n.s.) (Figure 1). No parent referred to have suffered from AD and no sign and symptom due to AD were present at a careful clinical investigation. The children with in progress or previous AD (groups 1 and 2) and the atopic children without AD of the group 3 had positive SPT to CM respectively in 19/54 cases (35%) and in 1/23 cases (4%) (p<0.005), to egg in 19/54 cases (35%) and in 3/23 cases (13%) (p<0.05), to wheat in 7/51 cases (14%) and in 2/23 cases (9%) (n.s.) and in no control group.
Skin prick test response to Der p
SPT to Der p was positive in 17/31 children (55%) of the group 1, in 12/23 children (52%) of the group 2, in 12/25 children (52%) of the group 3 and in no control subject. RAST to Der p was positive in 14/30 children (47%) of the group 1, in 12/21 children (57%) of the group 2, in 16/24 children (67%) of the group 3 and in no control subject. All the differences among the groups 1 and 2 and 3 were not significative.
Patch test response to Der p
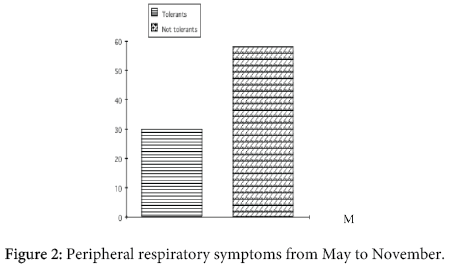
Figure 2 shows the results of the PTs in children. The PT was positive in 27/31 children (87%) with AD (group 1), in 19/23 children (83%) with previous AD (group 2), in 11/25 atopic children (44%) without AD (group 3) and in no control subject. The difference between group 1 and group 2 was not significative, the difference between group 1 and 2 versus group 3 was highly significative (p<0.001).
Among the children of the groups 1 and 2, 27/54 (50%) had positive SPT and positive PT, 19/54 (35%) had negative SPT and positive PT (n.s.), 8/54 (15%) had both negative SPT and PT. Among the children of the group 3, 7/25 (28%) had positive SPT and positive PT, 4/25 (16%) had negative SPT and positive PT (n.s.) and 14/25 had both negative SPT and PT.
The median severity score of the skin lesions induced by the PT in the children of all groups was 11 (range 7-20) with previous scratching and 9 (range 7-19) without previous scratching. This difference was highly significative (p<0.0001, with the Wilcoxon matched-pairs signed-ranks test).
In addition the median severity score of the skin lesions induced by the PT was 11 (range 7-20) in children with positive SPT and 10 (range 7-15) in children with negative SPT. This difference was significative (p<0.05).
The SPT to Der p was positive in at least 1 parent in 9/31 (29%) children of group 1, in 8/23 (36%) children of the group 2, and in 8/24 (33%) children of the group 3. All these differences were not significative. 28/54 (52%) parents of the children of the group 1 and 2 had positive PT. Only 17/54 (31%) had positive SPT to Der p. This difference was significative (p<0.05).
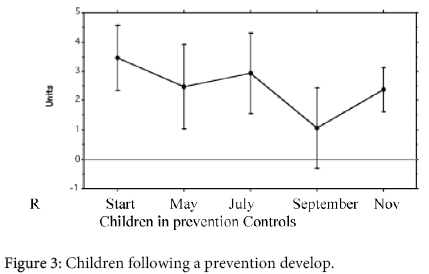
Figure 3 outlines the PT results in the parents. The PT was positive in at least 1 parent in 15/31 (48%) of the children of group 1, in 13/23 (57%) of the group 2, and in 5/25 (20%) of the children of the group 3. The difference between the group 1 and the group 2 was not significative; the difference between the group 1 + 2, and the group 3 was significative (p<0.01).
In the parents of the children of the group 1, 2 and 3 the median severity score of the skin lesions induced by PT was 9 (range 7-18) with previous scratching and 8 (range 7-17) without previous scratching. This difference was highly significative (p<0.0005, with the Wilcoxon matched-pairs signed-ranks test).
In addition the median severity score of the skin lesions induced by PT in the parents of the children of the group 1, 2 and 3 was 9 (range 7-15) in parents with negative SPT and 13 (range 7-18) in parents with positive SPT. This difference was highly significative (p<0.005).
Discussion
We have studied the PT response to Der p in 79 atopic children and in their parents. The atopic children were divided into 3 groups: children with AD (group 1), children who had suffered from AD but the disease was cured (group 2), atopic children with asthma who have never suffered from AD (group 3). We performed PTs on the healthy skin of the interscapular region, with and without a previous skin scratching.
The data of the present study show that a significantly higher proportion of children with being or previous AD (groups 1 and 2) have positive PT to Der P than the atopic children who have never suffered from AD (group 3) (p<0.001), and the controls.
As previously pointed out, studies on PT performed in patients with AD are not fully comparable due to the different methodologies employed .
Mitchell et al. [21] carried out PT with aqueous Der p1 extract on mildly abraded skin in 10 adult patients with AD and the PT was positive only in AD patients with positive SPT to Der p. In addition, 4/6 atopic adults not suffering from AD had PT positive response.
Reitamo et al. [19] induced eczematous lesions on normal not manipulated skin in 3/17 patients with AD employing Der p lyophilized commercial preparation.
Gondo et al. [18] induced a typical AD lesion on healthy skin in 4/13 adults with AD by applying twice a day for 2-5 days an ointment containing Der f. These authors also demonstrated the penetration of Der f (which was linked with ferritin) into the stratum corneum, the epidermis and the dermis. However, the lesions were present only in typical areas and only with previous skin scratch.
Adinoff et al. and Clark et al. [13, 22-23] elicited delayed cutaneous response in 18 patients with AD applying various aeroallergens (included Der f) extracts (20w/v in 50% glycerin) on clinically uninvolved and not manipulated skin. Only patients with positive SPT response to Der p had positive PT response to Der. Atopic patients not suffering from AD did not show positive PT responses.
Norris et al. [24] applied for 5 days, 1 ml of a SPT solution containing Der p on the unmanipulated antecubital or popliteal skin of atopic adults with or without AD. Worsening of the skin lesions occurred in 1/3 patients with AD and positive SPT response to Der p. All patients with AD and negative SPT to Der p had negative PT response.
Finally Bruynzeel-Koomen et al. [12] showed 70% positive PT response, applying house dust mite (and pollen allergens) on the back of AD adult patients, previously removing the superficial stratum corneum by 15 consecutive applications of adhesive tape. No positive responses were found in atopic patients without AD or in controls.
As for us, we obtained a multiform result since subpopulations of the 54 children with AD of groups 1 and 2 with different biological characteristics there were. The larger subpopulation was of 27/54 (50%) children with positive PT and positive SPT; 19/54 children (35%) had positive PT and negative SPT; 5/54 children (9%) had negative PT and positive SPT and 3/54 children (6%) had either negative PT and SPT.
We may speculate that in children with AD and either positive PT and SPT the allergen makes contact with IgE+-LCs which, in turn, presents allergen to skin T-cells so inducing the late response. It has been shown that epidermal LCs from patients with AD and high serum IgE levels express an FcR for IgE [25]; these IgE+-LCs are able to present allergen to autologous T cells from peripheral blood [26,27]. Moreover the important role of skin T-cell in inducing the PT test reaction has been emphasized by a recent work of van Reijsen et al. [28] who indicated that activated skin T-cell of a PT-biopsy was of Th2 phenotype and allergen specific.
Positive PT reaction in children with positive PT but negative SPT may be explained by means of antigen presentation to T-cell by dermal macrophages. These may act as antigen presenting cell thus inducing a true late response not depending from IgE. Several lines of evidence suggest that in mice differentiation of Th1 cells might be inhibited during a strong Th2 response (and vice versa) [29,30]. Cytokine synthesis inhibitory factor, produced by Th2 clones inhibits the synthesis of several Th1 cytokines [31]. We may hypothesize, as suggested in mice [32], that in the beginning a ThO-type T-cells stimulation may produce both IL-4 and INF-gamma. After further and more intense allergenic stimulation the ThO type of T-cell response may differentiate in Th2-type response, with the inhibition of the Th1-type cells.
A different explanation of the reaction in children with positive PT but negative SPT may be due to the intrinsic characteristics of the allergen of Der p. As we know the fecal allergens of the house dust mites are synthesized and secreted into the alimentary canal [33] and it has been shown that a great part of these allergens have an enzymatic activity [34]. In particular Takahashi et al. [35] have shown that an aqueous extract from a mite culture of Dermatophagoides farinae activated prekallikrein to kallikrein in normal plasma. As a consequence, in our opinion, the proteasic activity of these substance may trigger in a non specific and non allergologic manner the inflammatory response.
Overall, these data indicate that the positive PT response to Der p is not only due to the delayed phase of the IgE-mediated immune reaction, because it may occur even without a positive SPT. As suggested by Zachary et al. [36] we may speculate that delayed type immune reaction to Der p may play a role in at least a subpopulation of children with AD who have positive PT to this allergen.
Moreover, we found that PT score in all groups was significantly higher after previous skin scratching (p<0.0001) and in children with positive SPT to Der p compared to children with negative SPT (p<0.05). We obtained the same significant results in the parents of all allergic children (groups 1, 2 and 3). In our opinion the skin scratching increases PT response because a higher quantity of allergen may penetrate and reach the deeper layer of the skin so inducing a more vigorous response. We may hypothesize that pruritus, whatever it is induced, in addition to mechanical damage to the skin, worsens the response to allergen which can easier reach and stimulate dermal mast-cells and/or Langherans cells. Moreover, it is reasonable to speculate that children with positive SPT to Der p have a higher quantity of specific IgE on the surface of the cutaneous mast-cells and Langherans' cells. This may explain the more intense response of the PT to Der p in children with positive SPT.
Consequentially, PT to Der p appears to be more useful than SPT and it should be performed in children with AD when house dust mite exposure is suspected to be clinically relevant.
In order to correlate the "atopic status" of the children and the PT response in their parents we performed PT in them, as well. Interestingly, we found that 28/53 (53%) children of the group 1 and 2 and only 5/25 (20%) of the group 3 had at least 1 parent with PT positive response (p<0.01) (Figure 2). Therefore positive PT response to Der p seems to be significantly associated not only to the children with AD, but also to their parents. Moreover, as in children, the PT score in the parents was significantly higher both after previous skin scratching (p<0.005) and in parents with positive SPT to Der p compared to parents with negative SPT (p<0.0005).
Several hypothesis can be done in order to explain these data. First of all, if positive PT to Der p is a peculiar feature of AD, the parents of the children of group 1 and 2 might have suffered from AD, early in life, but they might have forgotten to have had AD. Another explanation may be that the parents of the children of group 1 and 2 may have a trivial form of AD. But this is not the case, because AD was carefully investigated and this possibility was eventually ruled out.
It is noteworthy that the parents of the children of the group 1, 2 and 3 did not show any significant difference about the atopic state, however, xerosis was significantly more common (p<0.0005) in the parents of the children of the group 1 and 2 (Figure 3). Xerosis is a rough clinical sign and it is a frequent secondary sign of AD [20]. However, it has been suggested that it may be due to an increased trans-epidermal water loss [37,38] or to a deficiency of intercellular lipid (namely ceramides) of the stratum corneum [39-41]. Moreover Tupker et al. [42] have demonstrated that dehydrated skin of AD patients is more susceptible to non specific irritant agents. We speculate that xerosis may get the skin more prone to environmental allergens attack. Children with AD may have inherited (independently each other) not only the propensity to produce IgE antibodies ("atopic status"), but even xerosis which increases the skin susceptibility to environmental irritants and allergens. This is an attempt of understanding why hypersensitivity to the same allergen provokes AD in someone and asthma in others.
In conclusion: a) AD clinical course, at least in a subpopulation of children, may be influenced by environmental factors and Der p may play an important role; b) sensitivity to Der p can be demonstrated either by an immediate (SPT) or late (PT) skin positive response. These 2 reactions may be not necessarily correlated. PT to Der p may be positive while SPT is negative, therefore PT should be done in all children with AD; c) we hypothesize that AD outcome may be influenced independently by both the "atopic status" and "skin condition". The latter may be inherited beside the atopic condition.
References
- Sampson HA (1983) Role of immediate food hypersensitivity in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol 71: 473-480.
- Sampson HA, McCaskill CC (1985) Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatr 107: 669-675.
- Sampson HA, Albergo R (1984) Comparison of results of skin tests, RAST, and double-blind, placebo-controlled food challenges in children with atopic dermatitis. J Allergy Clin Immunol 74: 26-33.
- Atherton DJ (1983) The role of foods in atopic eczema. Clin Exp Dermatol 8: 227-232.
- Atherton DJ, Sewell M, Soothill JF, Wells RS, Chilvers CE (1978) A double-blind controlled crossover trial of an antigen-avoidance diet in atopic eczema. Lancet 1: 401-403.
- Meglio P, Farinella F, Trogolo E, Giampietro PG, Cantani A, et al. (1988) Immediate reactions following challenge-tests in children with atopic dermatitis. Allerg Immunol (Paris) 20: 57-62.
- Meglio P, Giampietro PG, Farinella F, Cantani A, Businco L (1989) Personal experience in the diagnostic procedures in children with atopic dermatitis and food allergy. Allergy 44 Suppl 9: 165-173.
- Peck SM, Salomon G (1933) Eczema of infancy and childhood: contacts as etiologic agents, with particular reference to feathers. AM J DIS CHILD 46: 1308-1328.
- Hill LW (1937) Sensitivity of house dust and goose feathers in infantile eczema: the role of specific allergens. J ALLERGY 9: 37-47.
- Cazort AH (1936) The house dust antigen in allergy. SOUTH MJ 29: 1022-1026.
- TUFT L (1949) Importance of inhalant allergens in atopic dermatitis. J Invest Dermatol 12: 211-219.
- Bruynzeel-Koomen CA, Van Wichen DF, Spry CJ, Venge P, Bruynzeel PL (1988) Active participation of eosinophils in patch test reactions to inhalant allergens in patients with atopic dermatitis. Br J Dermatol 118: 229-238.
- Adinoff AD, Tellez P, Clark RA (1988) Atopic dermatitis and aeroallergen contact sensitivity. J Allergy Clin Immunol 81: 736-742.
- Platts-Mills TA, Mitchell EB, Rowntree S, Chapman MD, Wilkins SR (1983) The role of dust mite allergens in atopic dermatitis. Clin Exp Dermatol 8: 233-247.
- Chapman MD, Rowntree S, Mitchell EB, Di Prisco de Fuenmajor MC, Platts-Mills TA (1983) Quantitative assessments of IgG and IgE antibodies to inhalant allergens in patients with atopic dermatitis. J Allergy Clin Immunol 72: 27-33.
- de Groot AC, Young E (1989) The role of contact allergy to aeroallergens in atopic dermatitis. Contact Dermatitis 21: 209-214.
- Mitchell EB, Crow J, Rowtree S, Webster ADB, Platts-Mills TAE, et al. (1984) Cutaneous basophil hypersensitivity to inhalant allergens in atopic dermatitis patients: elicitation of delayed responses containing basophils following local transfer of immune serum but not IgE antibody. J INVEST DERMATOL 83: 290-295.
- Gondo A, Saeki N, Tokuda Y (1986) Challenge reactions in atopic dermatitis after percutaneous entry of mite antigen. Br J Dermatol 115: 485-493.
- Reitamo S, Visa K, Kähönen K, Käyhkö K, Stubb S, et al. (1986) Eczematous reactions in atopic patients caused by epicutaneous testing with inhalant allergens. Br J Dermatol 114: 303-309.
- Hanifin JM, Rajka G (1980) Diagnostic features of atopic dermatitis. ACTA DERM VENEREOL 92: 44-47.
- Mitchell EB, Crow J, Chapman MD, Jouhal SS, Pope FM, et al. (1982) Basophils in allergen-induced patch test sites in atopic dermatitis. Lancet 1: 127-130.
- Clark RA, Adinoff AD (1989) Aeroallergen contact can exacerbate atopic dermatitis: patch tests as a diagnostic tool. J Am Acad Dermatol 21: 863-869.
- Clark RA, Adinoff AD (1989) The relationship between positive aeroallergen patch test reactions and aeroallergen exacerbations of atopic dermatitis. Clin Immunol Immunopathol 53: S132-140.
- Norris PG, Schofield O, Camp RD (1988) A study of the role of house dust mite in atopic dermatitis. Br J Dermatol 118: 435-440.
- Bruynzeel-Koomen C, van der Donk EM, Bruynzeel PL, Capron M, de Gast GC, et al. (1988) Associated expression of CD1 antigen and Fc receptor for IgE on epidermal Langerhans cells from patients with atopic dermatitis. Clin Exp Immunol 74: 137-142.
- Bruijnzeel-Koomen CAFM, Boland GJ, Mudde GC. IgE-mediated antigen presentation by epidermal Langherans cells from patients with atopic dermatitis. In: Thivolet J, Schmitt D, eds. The Langherans cell. Montrouge, France: Colloque INSERM/John Libbey Eurotext Ltd, 1988:335-341.
- Mudde GC, Van Reijsen FC, Boland GJ, de Gast GC, Bruijnzeel PL, et al. (1990) Allergen presentation by epidermal Langerhans' cells from patients with atopic dermatitis is mediated by IgE. Immunology 69: 335-341.
- van Reijsen FC, Bruijnzeel-Koomen CA, Kalthoff FS, Maggi E, Romagnani S, et al. (1992) Skin-derived aeroallergen-specific T-cell clones of Th2 phenotype in patients with atopic dermatitis. J Allergy Clin Immunol 90: 184-193.
- Katsura Y (1977) Cell-mediated and humoral immune responses in mice. III. Dynamic balance between delayed-type hypersensitivity and antibody response. Immunology 32: 227-235.
- Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, et al. (1989) Reciprocal expression of interferon gamma or IL-4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T-cell subsets. J EXP MED 169: 59-72.
- Fiorentino DF, Bond MW, Mosmann TR (1989) Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med 170: 2081-2095.
- Street NE, Schumacher JH, Fong TA, Bass H, Fiorentino DF, et al. (1990) Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol 144: 1629-1639.
- Thompson SJ, Carswell F (1988) The major allergen of the house dust mite, Dermatophagoides pteronyssinus, is synthesized and secreted into its alimentary canal. Int Arch Allergy Appl Immunol 85: 312-315.
- Stewart GA, Ward LD, Simpson RJ, Thompson PJ (1992) The group III allergen from the house dust mite Dermatophagoides pteronyssinus is a trypsin-like enzyme. Immunology 75: 29-35.
- Takahashi K, Aoki T, Kohmoto S, Nishimura H, Kodera Y, et al. (1990) Activation of kallikrein-kinin system in human plasma with purified serine protease from Dermatophagoides farinae. Int Arch Allergy Appl Immunol 91: 80-85.
- Zachary CB, Allen MH, MacDonald DM (1985) In situ quantification of T-lymphocyte subsets and Langerhans cells in the inflammatory infiltrate of atopic eczema. Br J Dermatol 112: 149-156.
- Werner Y, Lindberg M (1985) Transepidermal water loss in dry and clinically normal skin in patients with atopic dermatitis. Acta Derm Venereol 65: 102-105.
- Werner Y (1986) The water content of the stratum corneum in patients with atopic dermatitis. Measurement with the Corneometer CM 420. Acta Derm Venereol 66: 281-284.
- Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, et al. (1991) Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol 96: 523-526.
- Imokawa G, Hattori M (1985) A possible function of structural lipids in the water-holding properties of the stratum corneum. J Invest Dermatol 84: 282-284.
- Schäfer L, Kragballe K (1991) Abnormalities in epidermal lipid metabolism in patients with atopic dermatitis. J Invest Dermatol 96: 10-15.
- Tupker RA, Pinnagoda J, Coenraads PJ, Nater JP (1990) Susceptibility to irritants: role of barrier function, skin dryness and history of atopic dermatitis. Br J Dermatol 123: 199-205.
Relevant Topics
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery
- Immunology: Current Research
- Current Trend in Gynecologic Oncology
- Journal of Cancer Diagnosis
- Journal of Gastrointestinal Cancer and Stromal Tumors
- Cervical Cancer: Open Access
- Journal of Mucosal Immunology Research
- Journal of Oncology Research and Treatment
- Journal of Orthopedic Oncology
- Journal of Prostate Cancer
- Research and Reviews on Pathogens
Article Tools
Article Usage
- Total views: 20325
- [From(publication date):
December-2014 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 15744
- PDF downloads : 4581