Role of CT and MRI in the Follow-Up of Operated Middle Ear Cholesteatoma
Received: 08-Oct-2016 / Accepted Date: 24-Oct-2016 / Published Date: 31-Oct-2016 DOI: 10.4172/2161-119X.1000275
Abstract
Background: Recurrence is the main risk that may occur during the follow-up of operated middle ear cholesteatoma. Imaging plays an important role in its diagnosis, leading to avoid surgical second look when it is not mandatory. The aim of our study was to evaluate postoperative CT and MRI in patients who had undergone middle ear cholesteatoma surgery. Methods: Retrospective study from June 2010 to June 2015 including operated patients for middle ear cholesteatoma whom follow-up was made in the ENT department of Rabta hospital and who had postoperative CT and/or MRI in the imaging department. Comparison of radiological and second look surgical findings was made with analysis of sensitivity, specificity, PNV, PPV for each type of imaging exam. Results: Forty ears included (36 patients, median age=38. 5, sex-ratio=1.1). Thirty four ears had CT showing well aerated middle ear cleft (n=1), total opacification (n=7), partial soft-tissue opacity with convex margins (n=11), pearl-shaped lesion (n=7) and concave margins opacity (n=8). CT was not able to further characterize these opacities (specificity 20%) but it was efficient in the evaluation of ossicular and bony walls lysis. Twenty five ears had MRI showing recurrent cholesteatoma (n=15), scar tissue (n=8) and aerated postoperative cavity with alteration of the labyrinth T2 signal (n=2). MRI specificity was about 25%. 100% PNV allowed excluding recurrence when MRI was showing no soft tissue mass. PPV of diffusion weighted imaging (DWI) and delayed post contrast T1 weighted imaging was respectively 83.3% and 71.4%. A hypersignal on DWI and no contrast uptake were highly in favor of cholesteatoma. Conclusion: CT is insufficient for the diagnosis of recurrent cholesteatoma. MRI contribution is hindered by false negatives due to too small lesions to be detected.
Keywords: CT scan; MRI; Cholesteatoma; Middle ear; Tympanoplasty
253607Abbreviations
CT scan: Computed Tomography Scan, MRI: Magnetic Resonance Imaging
Introduction
Middle ear cholesteatoma is defined by the presence, in the tympanic cavity, of keratizing squamous epithelium [1]. It isn’t a neoplasic tumor despite its destructive properties [2]. It can be removed using two techniques depending on the size of the cholesteatoma and the damage done to the ossicular bones, the eardrum and the posterior wall of the ear canal [3]. The closed technique, opposed to the open technique, is usually preferred. Postoperative surveillance is based on the CT scan, which used to be the gold standard in the detection of recurrent cholesteatoma [4] and the MRI showing better results due to different imaging sequences.
The purpose of our study is to illustrate the imaging findings in the follow-up of operated middle ear cholesteatoma and to evaluate their performance is detecting recurrent cholesteatoma.
Material and Methods
We conducted a retrospective study of the charts of 36 patients (40 ears) who had undergone a surgery for middle ear cholesteatoma in the Departement of Otolaryngology of Rabta hospital, between June 2010 and 2015 and who had a postoperative CT and/or MRI in the Imaging department.
CT scan of the temporal bone was performed for almost all patients. The protocol consisted on a spiral acquisition with a slice plan parallel to the lateral semicircular canal with multiplanar reconstruction in coronal and sagital plans and a slice thickness of 0.6 mm. The injection of X-ray contrast medium was used only in cases when infectious or thrombo-embolic complications were suspected. The MRI was conducted with axial and coronal views on T1, T2 weighted, echoplanar diffusion-weighted (when available on the machine) and contrast enhanced images. We compared the radiological findings with those of the second look surgery to evaluate the performance of each type of imaging exam.
Results
The number of patients reviewed in this study was 36 including 19 males (53%) and 17 females (47%), aged from 16 to 70 years (mean 38.5).
Clinical findings
Four patients (11%) had cholesteatoma in both ears, while 22 (61%) had their left ear operated and 10 (28%) had the right ear operated. For the initial surgery, 17 ears had a closed technique, 10 had an open technique and for the rest of the patients the surgical findings were missing. These were patients who didn’t undergo surgery in our hospital.
The post-operative symptoms were otorrhea in 20 ears, worsening of hearing loss in 5 ears, vertigo in 5 years and otalgia in 5 ears. Twenty patients only noted the fact that they didn’t get any better.The recurrence of cholesteatoma was certain for seven ears after the otoscopic examination.
Radiologic findings
CT scan
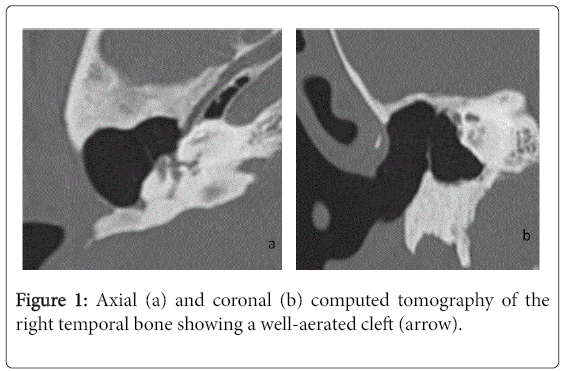
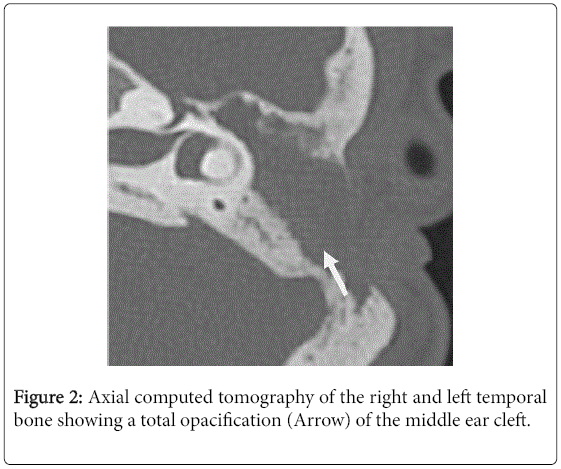
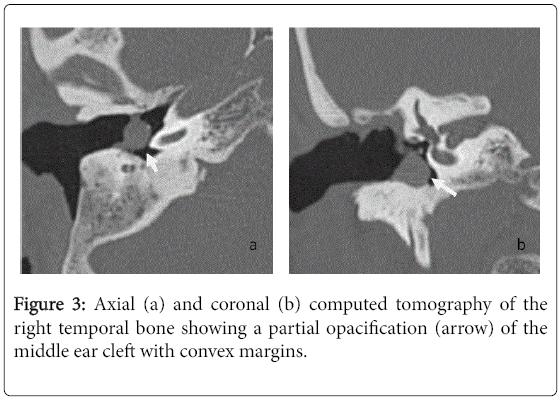
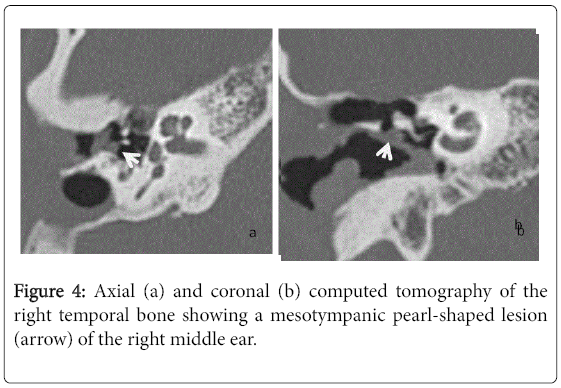
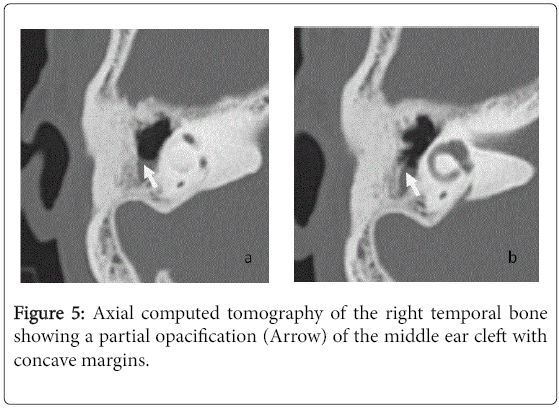
For the 40 operated ears, 34 had a post-operative CT scan showing a well-aerated cleft (n=1) (Figure 1), total opacification (n=7) (Figure 2), partial soft-tissue opacity with convex margins (n=11) (Figure 3), pearl-shaped lesion (n=7) (Figure 4) and concave margins opacity (n=8) (Figure 5). Newly appeared ossicular erosion, when comparing to the initial surgical findings concerned the incus bone (n=10), the malleus (n=6) and the stapes (n=5). In most case the erosion consisted in a total loss of the bone. Erosion of the middle ear cleft walls was observed most frequently in the outer attic wall (n=30), this erosion didn’t match the initial surgery findings for 4 ears. The other walls were damaged in 5 cases concerning the tegmen tympani and also in 5 cases concerning the other walls of the middle ear cleft.CT scan showed also destruction of facial nerve canal (n=7), erosion of the lateral SCC (n=2), erosion of the upper SCC (n=1), labyrinthitis (n=1) and mastoiditis (n=1).
MRI
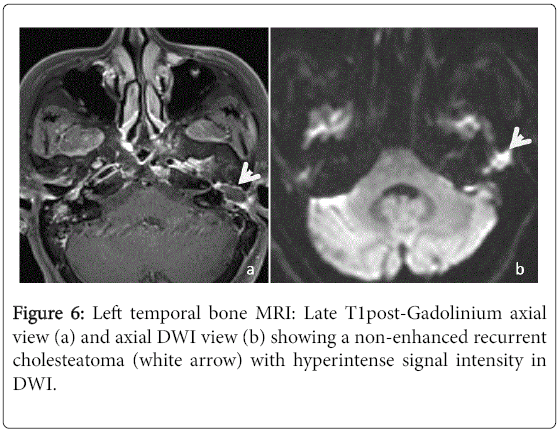
Among the 40 ears included in this study, 25 had an MRI of the temporal bone. Nineteen of those had already a post-operative CT scan. In 15 cases, the MRI concluded to a recurrent cholesteatoma (Figure 6). On standard T1-weighted sequence, 12 (80%) had an intermediate signal intensity when compared with the brain gray matter, two (13.3%) had hypointense signal intensity and one (6.7%) had hyperintense signal intensity.
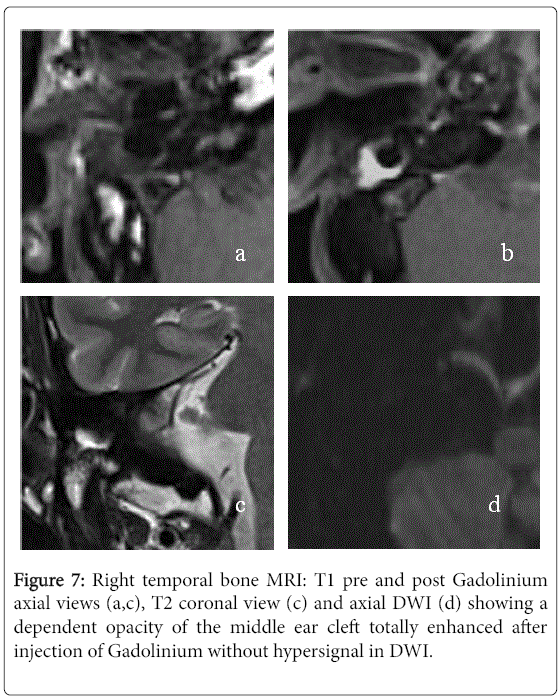
On standard T2-weighted sequence, 12 (80%) had a hyperintense signal intensity and three (20%) had intermediate signal intensity. Late post-gadolinium T1-weighted sequence was performed for 14 ears and there were no enhancement in all cases. The DWI was performed for eleven ears showing a marked hyperintense signal in 7 cases and it was not specific in the others cases. In 8 cases, the MRI concluded to fibrous tissue (Figure 7). It was described as intermediate signal intensity in T1-weighted imaging in 7 cases and all cases had hyperintense signal in T2-weighted sequence as well as an enhancement in late post-gadolinium T1-weighted sequence. The DWI didn’t show marked hyperintense signal in all cases. The two left MRI exams were performed to confirm a labyrinthine abnormality.
Second-look surgery findings
Among the 27 ears who had second-look surgery, 13 were evaluated by CT scan only, three by MRI only and 11 by both CT scan and MRI. The indication of the second-look surgery was determined by the imaging findings as resumed in Table 1. Recurrent cholesteatoma was noted in 22 cases (81.5%). In the others cases, three ears had fibrous tissue associated with cholesterol granulomas and two ears had only fibrous tissue. According to these findings, CT scan was judged as a poor exam in the diagnosis of recurrent cholesteatoma as it has low specificity (20%) and negative predictive value (NPV) (14.3%) and average sensitivity (68.4%) and positive predictive value (PPV) (76.4%). MRI, on the other hand, proved to be an excellent exam in detecting recurrent cholesteatoma when associating late postgadolinium T1-weighted and DWI sequences as it reached 100% in sensitivity and NPV. However we noted a significant number of false positives dropping its specificity to 25%. Taken separately, DWI and late post-gadolinium T1 sequences showed both high PPV (83.3% and 71.4%). Nonetheless, with a low NPV (25%), the DWI sequence can’t rule out the diagnosis of recurrent cholesteatoma when showing no abnormality.
| Indication | Number of cases | Percentage (%) |
|---|---|---|
| Recurrent cholesteatoma on MRI | 3 | 11.1 |
| Partial soft-tissue opacity with convex margins or Pearl-shaped lesion on CT scan+Recurrentcholesteatoma on MRI | 6 | 22.2 |
| Total opacification on CT scan+Recurrentcholesteatoma on MRI | 5 | 18.5 |
| Partial soft-tissue opacity with convex margins or Pearl-shaped lesion on CT scan | 11 | 40.8 |
| Total opacification on CT scan | 2 | 7.4 |
| Total | 27 | 100 |
Table 1: The indication of the second-look surgery depending on imaging results.
Discussion
The limits of our study consist in the fact that the 40 ears initially included in the study is a number comparable to those found in other studies when reviewing the literature. However, the number of ears who had both CT scan and MRI (19 ears) was quite low. The fact our study was retrospective made it impossible to have a unique MRI protocol containing all necessary sequences explaining the fact that the DWI sequence wasn’t done in all cases due to different MRI machines. The major issues in cholesteatoma surgery are residual and recurrent cholesteatoma. Residual cholesteatoma develops from a vestige of keratinized epithelium left in the middle ear cleft at the first surgery. Its rate varies from 10% to 40% [5] depending on the type and the extent of the cholesteatoma as well as of the operative technique and the surgeon’s experience. Recurrent cholesteatoma, on the other hand, is newly appeared cholesteatoma resulting from the recurrence of tympanic membrane retraction or perforation favored by impaired middle ear ventilation. It is rarer than residual cholesteatoma and its rate varies from 10% to 20%b [6]. The known risk factors of recurrent cholesteatoma are the age of patients with a higher risk for children [7], ossicular erosion, the initial extent of cholesteatoma, the lack of the surgeon’s experience and the operative technique with higher rates of recurrence with closed techniques [8,9]. Post operative follow-up is recommended at least for 3 years [10] or even for life according to some studies [11]. Patients with recurrent cholesteatoma usually show no symptoms particularly for the residual type. In our study, the most frequent symptom was otorrhea (50% of ears). CT scan is an essential complementary exam in both pre operative and post operative assessment of middle ear cholesteatoma.
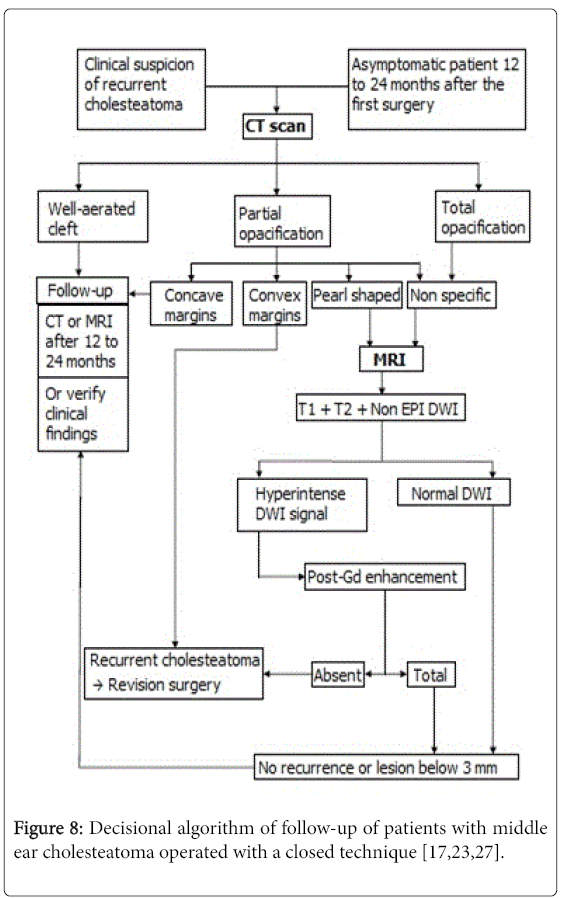
It is recommended to diagnose residual cholesteatoma in asymptomatic patients, in case of confirmed recurrence in the otoscopic exam and in case of unexplained non improvement of hearing loss.CT scan can show a well-aerated cleft almost ruling out recurrent cholesteatoma with an excellent NPV that reached 100% according to Thomassin and al. and Gaillardin et al. [5,12] with no false negatives in both studies. In our study, a low NPV (14.3%) was under estimated by the fact that, as said earlier, a well-aerated cleft is considered safe from recurrent or residual cholesteatoma and thus no second-look surgery is conducted to confirm it. CT scan can also reveal pearl-shaped, highly suggestive of recurrent cholesteatoma particularly when located in the atticus or the retrotympanum [13-15]. CT scan is very effective in detecting these cholesteatoma pearls up to a size of 2 mm. When this type of lesion is shown, the second-look surgery is recommended straight away [6,16]. In our study, pearlshaped opacification was found in 7 ears with recurrent cholesteatoma confirmed in six cases after second-look surgery. When showing a partial opacification with convex margins, CT scan suspects the recurrence of the cholesteatoma especially when associated with newly appeared bone erosion. This emphasizes the importance of comparing the findings of post operative and pre operative CT scans [14]. In our study, this type of opacification was found in 11 ears, the majority of which (n=8) had an MRI confirming the diagnosis of recurrence in 5 cases which were operated and ruling it out in 3 cases who didn’t get to surgery. The second-look surgery revealed recurrent cholesteatoma in 3 ears only. Three patients had surgery based of CT findings only showing recurrence in 2 cases. The principal flaw of CT scan consists on showing either a total or a non specific opacification of the post operative cleft. The reason is that CT scan can’t tell the difference between hyperplasic inflammatory mucous, cholesterol granuloma, fibrous tissue and recurrent cholesteatoma all having almost the same density [6,16] .This situation occurs in 20% of cases [14] in CT scans with poor specificity and PPV. In our study, they reached 20% and 76.4% respectively correlating with the results of Geoffray et al. [17] demonstrating that, when showing this kind of opacification, CT scan in unable to confirm of rule out the diagnosis of recurrent cholesteatoma.MRI has emerged as the complementary exam needed to characterize the non specific opacifications shown in CT scans [18]. There is no codified MRI protocol when looking for a recurrent cholesteatoma. However, it should contain T1-weighted sequences pre and post-contrast (the acquisition is made 30 to 45 min after the injection of Gadolinium), T2-weighted sequence and diffusionweighted imaging with a b-value varying between 800 and 1000 [17,19].
The T1-weighted pre contrast sequence is useful for two reasons. The first is that it is necessary to affirm if there is an enhancement is late contrast sequence [19] and the second is that it can show hyperintense lesions as cholesterol granuloma which can be imperceptible within the enhancement after injection of Gadolinium [15,20]. The principal purpose of the late post-contrast T1-weighted sequence is to differentiate between the absence of enhancement of cholesteatoma and the constant enhancement of fibrous tissues [21]. This enhancement is centripetal [22] and becomes more intense past 45 min [23]. This time limit must be at least 45 min for Lehman et al. [24] and averaged 60 min for Blanco Cabellos et al. [19] and in our study. The major inconveniences of this sequence are the fact that it lengthens the duration of exams [17,21] and depends strongly on the radiologist’s experience. It also has frequent false positives lowering its specificity and PPV. These false positives can be due to some material (silastic) used in surgery having the same pearl-shaped and indifference to Gadolinium as cholesteatoma [2]. In 2002, Fitzek and al. were the firsts to use the DWI in temporal bone MRI to detect cholesteatoma and proved that it has diffusion restriction.
Cholesteatoma is hyperintense in DWI not only because of this restriction phenomenon but also due to a T2 shine-through effect. In our study we used the EPI (echo planar imaging) DWI and our results showed lower specificity (50%), PPV (83%) and NPV (25%) than found in literature. The explanation would be the fact that small cholesteatoma pearls can’t be diagnosed with this sequence. The minimum size estimated for EPI DWI is 5 mm. Other diffusion imaging techniques were developed in order to have better performances. The TSE DWI technique has a longer duration but lesser susceptibility artifacts and better spatial resolution [17,25,26]. TSE DWI sequence made it possible to detect smaller lesions up to 3 mm equalizing T1 post-contrast sequence though the latter has a better spatial resolution. Some studies showed that TSE DWI has better sensitivity and specificity than T1 post-contrast sequence. De Foer et al. [26] went even further recommending using solely the DWI TSE denying the need for Gadolinium injection by demonstrating that associating both sequences didn’t provide significant difference in PPV and NPV in comparison with using TSE DWI only. The newest diffusion technique is the PROPELLER sequence (acronym used by GE heathcare®) manufactured to maximize the signal-to-noise ratio and to minimize artifacts. It has higher performances than the other DWI techniques but has a longer duration (4 to 5 min) with more frequent kinetic artifacts [25]. It can only be acquired in axial plane which limits the exploration of the tegmental area [24]. Some teams decided to give up on late T1 post-contrast sequence and rely only on the DWI technique [26]. Others, including ours, continue with associating both sequences as they give MRI specificity and PPV close to 100% and a NPV averaging 90% [17,23]. This close to perfect NPV is due to the barrier of 3 mm size below which the MRI can’t detect cholesteatoma lesions. However, many studies agree that those small lesions grow slowly and consequently they can be diagnosed by another MRI one or two after the first one [17,23,27].The comparison of the results of our study with those of literature enabled us to make a decisional algorithm for the diagnosis of recurrent cholesteatoma for adult patients (Figure 8).
Consent Statement
As our article is a retrospective study and did not publish any personal photo or information regarding any of the patient in our manuscript thus the consent is not required for the article publication. However, we had the approval of the Rabta hospital of Tunis to conduct the study in the departments of Radiology and of Otolaryngology before the article.
References
- Bordure P, Bailleul S, Malard O, Wagner R (2009) Otitechroniquecholestéatomateuse: Aspects cliniques et thérapeutiques. Encycl Med Chir. Oto-Rhino-Laryngologie 4: 1-16.
- Khemani S, Singh A, Lingam RK, Kalan A (2011) Imaging of postoperative middle ear cholesteatoma.ClinRadiol 66: 760-767.
- Migirov L, Tal S, Eyal A, Kronenberg J (2009) MRI, not CT, to rule out recurrent cholesteatoma and avoid unnecessary second-look mastoidectomy.Isr Med Assoc J 11: 144-146.
- Plouin-Gaudon I, Bossard D, Ayari-Khalfallah S, Froehlich P (2010) Fusion of MRIs and CT scans for surgical treatment of cholesteatoma of the middle ear in children.Arch Otolaryngol Head Neck Surg 136: 878-883.
- Gaillardin L, Lescanne E, Morinière S, Cottier JP, Robier A (2012) Residual cholesteatoma: prevalence and location. Follow-up strategy in adults.Eur Ann Otorhinolaryngol Head Neck Dis 129: 136-140.
- Ayache D, Schmerber S, LavieilleJP, Roger G, Gratacap B (2006) Middle ear cholesteatoma.Ann OtolaryngolChirCervicofac 123: 120-137.
- Preciado DA (2012) Biology of cholesteatoma: Special considerations in pediatric patients.Int J PediatrOtorhinolaryngol 76: 319-321.
- Park KT, Song JJ, Moon SJ, Lee JH, Chang SO, et al. (2011) Choice of approach for revision surgery in cases with recurring chronic otitis media with cholesteatoma after the canal wall up procedure.AurisNasus Larynx 38: 190-195.
- Tomlin J, Chang D, McCutcheon B, Harris J (2013) Surgical technique and recurrence in cholesteatoma: A meta-analysis.AudiolNeurootol 18: 135-142.
- Roux A, Bakhos D, Lescanne E, Cottier JP, Robier A (2015) Canal wall reconstruction in cholesteatoma surgeries: Rate of residual.Eur Arch Otorhinolaryngol 272: 2791-2797.
- Beltaief N, Sellami M, Tababi S, Zainine R, Charfeddine A, et al. (2012) Le cholestéatome de l’oreillemoyenne. J Tun ORL 28: 1-6.
- Thomassin JM, Braccini F (1999) Role of imaging and endoscopy in the follow up and management of cholesteatomas operated by closed technique.Rev LaryngolOtolRhinol (Bord) 120: 75-81.
- Ayache D, Williams MT, Lejeune D, Corré A (2005) Usefulness of delayed postcontrast magnetic resonance imaging in the detection of residual cholesteatoma after canal wall-up tympanoplasty. Laryngoscope 115: 607-610.
- Williams MT, Ayache D (2004) Imaging of the postoperative middle ear.EurRadiol 14: 482-495.
- Veillon F, Riehm S, Roedlich MN, Meriot P, Blonde E, et al. (2000) Imaging of middle ear pathology.SeminRoentgenol 35: 2-11.
- Williams MT, Ayache D (2006) Imaging in adult chronic otitis.J Radiol 87: 1743-1755.
- Geoffray A, Guesmi M, Nebbia JF, Leloutre B, Bailleux S, et al. (2013) MRI for the diagnosis of recurrent middle ear cholesteatoma in children-can we optimize the technique? Preliminary study.PediatrRadiol 43: 464-473.
- Kösling S, Bootz F (2001) CT and MR imaging after middle ear surgery.Eur J Radiol 40: 113-118.
- Cabellos JAB, Vélez SO, Cáceres IA, Lluch ES, Galobardes J (2011) CT and MRI correlations in patients with suspected cholesteatoma after surgery. Neuroradiol J 24: 367-378.
- Baráth K, Huber AM, Stämpfli P, Varga Z, Kollias S (2011) Neuroradiology of cholesteatomas.AJNR Am J Neuroradiol 32: 221-229.
- Venail F, Bonafe A, Poirrier V, Mondain M, Uziel A (2008) Comparison of echo-planar diffusion-weighted imaging and delayed postcontrast T1-weighted MR imaging for the detection of residual cholesteatoma. Am J Neuroradiol 29: 1363-1368.
- Williams MT, Ayache D, Alberti C, Héran F, Lafitte F, et al. (2003) Detection of postoperative residual cholesteatoma with delayed contrast-enhanced MR imaging: Initial findings. EurRadiol 13: 169-174.
- Emonot G, Richard C, Dumollard JM, Veyret C, Martin C (2008) Apport de l’imagerie au diagnostic de cholestéatomeresiduel. J FrOto Rhino Laryngol 94: 366-374.
- Lehmann P, Saliou G, Brochart C, Page C, Deschepper B, et al. (2008) 3T MR imaging of postoperative recurrent middle ear cholesteatomas: Value of periodically rotated overlapping parallel lines with enhanced reconstruction diffusion-weighted MR imaging. Am J Neuroradiol 30: 423-427.
- Schwartz KM, Lane JI, Bolster BD Jr, Neff BA (2011) The utility of diffusion-weighted imaging for cholesteatoma evaluation.AJNR Am J Neuroradiol 32: 430-436.
- De Foer B, Vercruysse JP, Bernaerts A, Meersschaert J, Kenis C, Pouillon M et al. (2010) Middle ear cholesteatoma: Non–echo-planar diffusion-weighted MR imaging versus delayed gadolinium-enhanced T1-weighted MR imaging. Radiology 255: 866-872.
- Lincot J, Veillon F, Riehm S, Babay N, Matern JF, et al (2015) Middle ear cholesteatoma: Compared diagnostic performances of two incremental MRI protocols including non-echo planar diffusion-weighted imaging acquired on 3T and 1.5T scanners. J Neuroradiol42: 193-201.
Citation: Jrad M, Graiess F, Behi S, Bachraoui R, Besbes G, et al. (2016) Role of CT and MRI in the Follow-Up of Operated Middle Ear Cholesteatoma. Otolaryngol (Sunnyvale) 6:275. DOI: 10.4172/2161-119X.1000275
Copyright: © 2016 Jrad M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16491
- [From(publication date): 10-2016 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 15446
- PDF downloads: 1045