Risks and Triggers of Psychosis in Parkinson Disease
Received: 25-Dec-2017 / Accepted Date: 03-Jan-2018 / Published Date: 10-Jan-2018 DOI: 10.4172/2161-0460.1000413
Abstract
Psychosis is a common non-motor complication in Parkinson disease, and affects the quality of life of patients and their care-givers. This psychosis is caused by intrinsic (pathological and genetic) and extrinsic factors. Pathological factors include the severity of Lewy body pathology, degeneration of cholinergic neurons and overstimulation of serotonin receptors. Genetic factors include apolipoprotein ε4, cholecystokinin genotyping, and glucocerebrosidase mutations. Extrinsic factors that trigger psychosis include systemic inflammation and medication of risky drugs. To prevent such psychosis, it is important to examine systemic infection, cease high-risk drugs, and then consider prescription of anti-psychotic drugs. This review is to discuss pathogenesis and therapeutic strategy of psychosis in Parkinson disease.
Keywords: C-reactive protein; Cognition; Psychosis; Hallucination; Delusion; Lewy body
Abbreviations
PD: Parkinson’s Disease; REM: Rapid Eye Movement; GBA: Glucocerebrosidase; 5-HT: 5-Hydroxytryptamine
Introduction
Parkinson disease (PD) is characterized by steadily progressive motor disturbance such as bradykinesia, muscular rigidity, and tremor. Pathologically, it is characterized by death of nigral dopaminergic neurons and the presence of Lewy bodies. Majority of motor symptoms and signs are caused by striatal dopamine deficiency due to nigral dopaminergic neuronal loss, and relieved by dopamine replacement therapy. In addition to motor symptoms and signs, cognitive decline [1], anxiety [2], depression, sleep disturbance, apathy [3], psychosis, orthostatic hypotension and sweating attacks [4] are recognized as non-motor problems. Among them, psychosis reduces the quality of life of patients and their care-givers [5]. Importantly, medications against psychosis can often worsen motor symptoms and signs. Serious psychosis often occurs in advanced stages of PD and requires use of dopaminergic antagonists. Dopamine antagonists exacerbate extrapyramidal signs, and as a result, elevate the risk of aspiration pneumonia by swallowing disturbance or bone fractures because of falling, which can increase psychosis [6]. Therefore, psychosis is a key problem in advanced stages of PD, and it is important to develop therapeutic strategies for psychosis in PD.
Prevalence
The prevalence of psychosis is reported as only 3% in initial untreated patients with PD [7]. However, with long-term pharmacological treatment, the prevalence of hallucinations is elevated to 40%-50% [8-10]. Simple visual hallucination with retained insight is common, occurring in approximately 50%. It can occur even in early stages of the disease [11] and often disappears spontaneously without any antipsychotic medications. However, hallucinations can sustain in some cases, and reduce the quality of life of patients especially without insight. Further, delusions usually sustain and require medical treatment. Comparing with hallucinations, delusions are rare, with a prevalence of approximately 7% [12,13]. The contents of the delusion are variable, delusions of poisoning, jealousy or infidelity; although rare but impressive is Capgras syndrome, whereby a patient believes that a familial person will be replaced by an identical imposter [14].
Sleep Disturbances and Hallucinations
Sleep disturbance is one of the most common non-motor symptoms of PD, and includes insomnia, fragmented sleep, daily excessive sleepiness, REM (rapid eye movement) sleep behavior disturbance, and sudden onset sleep. Daytime sleepiness is more common in patients taking dopamine agonists, although sleepiness is observed in patients without dopamine replacement therapy [15]. Sleepiness is thought to be caused by degeneration of neurons of the arousal system, locus coeruleus (noradrenergic), pendunculopontine nucleus and the basal forebrain (cholinergic), the raphe nucleus (serotoninergic), and the lateral hypothalamus (hypocretinergic) [15].
Sleep disturbances are associated with visual hallucinations [15,16]. However, pharmacological interventions against daytime sleepiness have low efficacy [17] and it remains unclear whether interventions against sleep disturbances also improve psychosis.
Cognition and Hallucinations
Cognitive function is often disturbed, especially in advanced stages of PD, and is characterized by reduced attention or difficulty in concentrating. Psychosis is associated with cognitive functional decline in language, memory, attention, executive function and visuospatial ability [18]. Although cognitive function is improved by inhibitors of brain cholinesterase, it is uncertain whether psychosis including hallucinations is improved or prevented [19].
Genetic Factors in Psychosis
Previous studies of genetic factors in psychosis have mainly focused on dopamine transporters and receptors, apolipoprotein E and cholecystokinin genotyping [20]. Monsell et al. investigated the association of apolipoprotein genotypes with development of clinical phenotype in 423 patients with PD, and found that apolipoprotein ε4 carriers were prone to developing dementia and that the risk of hallucinations was significantly higher (odds ratio of 5.29) [21]. By contrast, ε4 was not associated with motor function in PD in that study. An association of apolipoprotein ε4 with psychosis was also reported in non-demented PD patients [22], although this remains controversial [23].
Cholecystokinin is a gastrointestinal neuropeptide, and is found in dopaminergic neurons and regulates dopamine release. Polymorphisms in cholecystokinin were reported to be associated with development of psychosis [24,25]. Genetic mutations in glucocerebrosidase (GBA) are responsible for Gaucher disease and also represent a genetic risk factor for sporadic PD. Functional prognosis of PD patients with GBA mutations is poorer than of those without mutations. We also recently demonstrated that psychosis is more frequent and develops earlier in PD patients with GBA mutations than those without [26].
Pathological Background
Williams and colleagues investigated the pathological findings and clinical records of 787 patients presenting Parkinsonism, including PD and other symptomatic Parkinsonism and reported that vivid visual hallucinations were strongly associated with Lewy body pathology [10]. The concentration of β amyloid peptide in the cerebrospinal fluid is also associated with hallucinations [27]. As described above, there are several reports of associations of hallucinations with apolipoprotein ε4 that is a strong genetic risk for Alzheimer disease. Overall, these results suggest that Alzheimer pathology, including senile plaques and neurofibrillary tangles, is also among the pathological background of PD psychosis.
Acetylcholine and Hallucinations
Hallucinations can be a clinical hallmark of Lewy body pathology [10] and acetylcholine alterations are a biochemical feature of dementia with Lewy bodies [28,29]. As described, acetylcholine is a neurotransmitter involved in the arousal system and disruption of the arousal system is associated with psychosis. There are some reports of improvement in psychotic symptoms with use of donepezil and rivastigmine [30,31]. However, further studies examining the effect of cholinesterase inhibitors against psychotic symptoms in PD are required.
Serotonin and Hallucinations
Pathological studies of PD patients show degeneration of serotonergic raphe nuclei as well as nigral dopaminergic neurons, and as a result, serotonin 5-HT2A receptors are upregulated – upregulation of 5-HT2A receptors in the temporal cortex was also reported to be associated with visual hallucination [32,33]. The hypothesis of 5-HT2A receptor overactivation in the temporal cortex is supported by the fact that pimavanserin, an inverse serotoninergic receptor agonist, is efficacious against psychosis in PD [34,35]. Quetiapine, which has partial antagonistic activity against 5-HT2A, also provides beneficial effects against PD hallucinations [36,37].
Intrinsic and Extrinsic Factors
Psychosis often occurs suddenly, even in patients who have never experienced psychosis. In these patients psychosis is caused, not by intrinsic factors, but rather by extrinsic factors [38]. Extrinsic factors include systemic inflammation and use of trigger medications.
Trigger Medications
We previously examined potential trigger medications in a retrospective case-crossover study [38] by comparing drugs used in the period of psychosis development (hazard period) and those used in the periods without psychosis (control periods), and estimating the odds ratio (relative risk) of drugs for psychosis. Central anticholinergic drugs had the highest-risk (relative risk of 17.9) and the risk was dramatically increased in elderly patients. Dopaminergic agonists also had a high risk for psychosis, with an estimated odds ratio of 1.65 in patients aged ≥ 70 years.
Amantadine is well known to elicit psychosis, especially in patients with impaired renal function, and often causes encephalopathy presenting with myoclonus when the plasma concentration is elevated [39].
Systemic Inflammation
We previously reported that serious infections can exacerbate motor signs and symptoms of PD, with often irreversible effects [40,41]. Marked inflammation such as serious infection or surgical intervention can cause psychotic symptoms even in healthy people. In PD patients, even when infection is not identified clinically, a small elevation of C-reactive protein, a peripheral marker of systemic inflammation, is associated with development of psychosis [6]. Brain microglias are also activated in patients with PD and activated microglias are associated with the neurodegenerative process [42]. Using neuroimaging, Ouchi and colleagues reported that microglia is activated in the early stage of PD [43]. Microglia activation was also reported in early patients with dementia with Lewy bodies [44]. In Alzheimer disease, systemic infection can cause behavioral changes termed ‘sickness behavior’. In this context, a cross-talk between systemic inflammation and neuroinflammation may be an important clue to development of psychosis.
Concluding Remarks
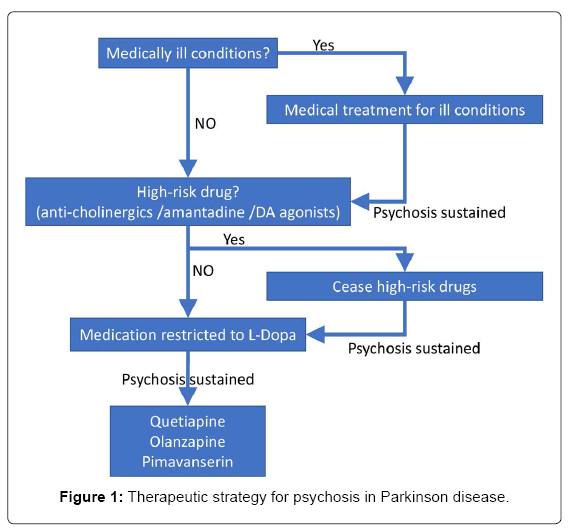
Psychosis is a multifactorial complication in PD. The causes of psychosis include intrinsic (or patient-related) factors and extrinsic trigger factors. Intrinsic factors include severity of Lewy body pathology, genetic risk factors (including apolipoprotein ε4, cholecystokinin, and glucocerebrosidase), older age, cognitive decline, and longer disease duration. Identifying patient at high risk of psychosis by these factors, trigger factors should be checked and fixed. Our recommendation is shown in Figure 1. If systemic ill conditions including elevated C-reactive protein are identified, the conditions should be treated. In cases psychosis sustains, risky drugs including anti-cholinergics, amantadine and dopamine agonists should be ceased and medication should be restricted to L-dopa if required. Finally, use of anti-psychotic drugs should be considered.
Acknowledgement
We thank Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- Lawson RA, Yarnall AJ, Duncan GW, Khoo TK, Breen DP, et al. (2014) Severity of mild cognitive impairment in early Parkinson’s disease contributes to poorer quality of life. Parkinsonism Relat Disord 20: 1071-1075.
- D'Iorio A, Vitale C, Piscopo F, Baiano C, Falanga AP, et al. (2017) Impact of anxiety, apathy and reduced functional autonomy on perceived quality of life in Parkinson’s disease. Parkinsonism Relat Disord 43: 114-117.
- den Brok MG, van Dalen JW, van Gool WA, Moll van Charante EP, de Bie RM, et al. (2015) Apathy in parkinson's disease: A systematic review and meta-analysis. Mov Disord 30: 759-769.
- Hirayama M (2006) Sweating dysfunctions in Parkinson’s disease. J Neurol 253: VII42-47.
- Gómez-Esteban JC, Tijero B, Somme J, Ciordia R, Berganzo K, et al. (2011) Impact of psychiatric symptoms and sleep disorders on the quality of life of patients with Parkinson’s disease. J Neurol 258: 494-499.
- Sawada H, Oeda T, Umemura A, Tomita S, Hayashi R, et al. (2014) Subclinical elevation of plasma C-reactive protein and illusions/hallucinations in subjects with Parkinson's disease: Case-control study. PLoS One 9: e85886.
- Weintraub D, Simuni T, Caspell-Garcia C, Coffey C, Lasch S, et al. (2015) Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. Mov Disord 30: 919-927.
- Fénelon G, Mahieux F, Huon R, Ziégler M (2000) Hallucinations in Parkinson's disease: Prevalence, phenomenology and risk factors. Brain 123: 733-745.
- Wood RA, Hopkins SA, Moodley KK, Chan D (2015) Fifty percent prevalence of extracampine hallucinations in Parkinson’s disease patients. Front Neurol 6: 263.
- Williams DR, Lees AJ (2005) Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: A retrospective autopsy study. Lancet Neurol 4: 605-610.
- Biglan KM, Holloway RG Jr, McDermott MP, Richard IH, Parkinson Study Group CALM-PD Investigators (2007) Risk factors for somnolence, edema and hallucinations in early Parkinson disease. Neurology 69: 187-195.
- Gallagher DA, Schrag A (2012) Psychosis, apathy, depression and anxiety in Parkinson's disease. Neurobiol Dis 46: 581-589.
- Fénelon G, Alves G (2010) Epidemiology of psychosis in Parkinson's disease. J Neurol Sci 289: 12-17.
- Hermanowicz N (2017) Delusional misidentification in Parkinson's disease: Report of two cases and a review. Postgrad Med 13: 1-4.
- Arnulf I, Leu S, Oudiette D (2008) Abnormal sleep and sleepiness in Parkinson’s disease. Curr Opin Neurol 21: 472-477.
- Gama RL, de Bruin VM, de Bruin PF, Távora DG, Lopes EM, et al. (2015) Risk factors for visual hallucinations in patients with Parkinson’s disease. Neurol Res 37: 112-116.
- Rodrigues TM, Castro Caldas A, Ferreira JJ (2016) Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson’s disease: Systematic review and meta-analysis. Parkinsonism Relat Disord 27: 25-34.
- Factor SA, Scullin MK, Sollinger AB, Land JO, Wood-Siverio C, et al. (2014) Cognitive correlates of hallucinations and delusions in Parkinson’s disease. J Neurol Sci 347: 316-321.
- Sawada H, Oeda T (2016) Protocol for a randomised controlled trial: Efficacy of donepezil against psychosis in Parkinson’s disease (EDAP). BMJ Open 3: e003533.
- Lenka A, Arumugham SS, Christopher R, Pal PK (2016) Genetic substrates of psychosis in patients with Parkinson’s disease: A critical review. J Neurol Sci 364: 33-41.
- Monsell SE, Besser LM, Heller KB, Checkoway H, Litvan I, et al. (2014) Clinical and pathologic presentation in Parkinson's disease by apolipoprotein e4 allele status. Parkinsonism Relat Disord 20: 503-507.
- de la Fuente-Fernández R, Núñez MA, López E (1999) The apolipoprotein E epsilon 4 allele increases the risk of drug-induced hallucinations in Parkinson’s disease. Clin Neuropharmacol 22: 226-230.
- Factor SA, Steenland NK, Higgins DS, Molho ES, Kay DM, et al. (2011) Disease-related and genetic correlates of psychotic symptoms in Parkinson’s disease. Mov Disord 26: 2190-2195.
- Fujii C, Harada S, Ohkoshi N, Hayashi A, Yoshizawa K, et al. (1999) Association between polymorphism of the cholecystokinin gene and idiopathic Parkinson’s disease. Clin Genet 56: 394-399.
- Wang J, Si YM, Liu ZL, Yu L (2003) Cholecystokinin, cholecystokinin-A receptor and cholecystokinin-B receptor gene polymorphisms in Parkinson’s disease. Pharmacogenetics 13: 365-369.
- Oeda T, Umemura A, Mori Y, Tomita S, Kohsaka M, et al. (2015) Impact of glucocerebrosidase mutations on motor and non-motor complications in Parkinson's disease. Neurobiol Aging 36: 3306-3313.
- Ffytche DH, Pereira JB, Ballard C, Chaudhuri KR, Weintraub D et al. (2017) Risk factors for early psychosis in PD: Insights from the Parkinson's progression markers initiative. J Neurol Neurosurg Psychiatry 88: 325-331.
- Colloby SJ, Pakrasi S, Firbank MJ, Perry EK, Piggott MA, et al. (2006) In vivo SPECT imaging of muscarinic acetylcholine receptors using (R,R) 123I-QNB in dementia with Lewy bodies and Parkinson's disease dementia. Neuroimage 33: 423-429.
- Shiozaki K, Iseki E, Uchiyama H, Watanabe Y, Haga T, et al. (1999) Alterations of muscarinic acetylcholine receptor subtypes in diffuse Lewy body disease: Relation to Alzheimer's disease. J Neurol Neurosurg Psychiatry 67: 209-213.
- Bergman J, Lerner V (2002) Successful use of donepezil for the treatment of psychotic symptoms in patients with Parkinson's disease. Clin Neuropharmacol 25: 107-110.
- Oh YS, Kim JS, Lee PH (2015) Effect of rivastigmine on behavioral and psychiatric symptoms of Parkinson's disease dementia. J Mov Disord 8: 98-102.
- Huot P, Johnston TH, Darr T, Hazrati LN, Visanji NP, et al. (2010) Increased 5-HT2A receptors in the temporal cortex of Parkinsonian patients with visual hallucinations. Mov Disord 25: 1399-1408.
- Ballanger B, Strafella AP, van Eimeren T, Zurowski M, Rusjan PM, et al. (2010) Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol 67: 416-421.
- Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, et al. (2014) Pimavanserin for patients with Parkinson's disease psychosis: A randomised, placebo-controlled phase 3 trial. Lancet 383: 533-540.
- Hermanowicz S, Hermanowicz N (2016) The safety, tolerability and efficacy of pimavanserin tartrate in the treatment of psychosis in Parkinson's disease. Expert Rev Neurother 16: 625-633.
- Fernandez HH, Friedman JH, Jacques C, Rosenfeld M (1999) Quetiapine for the treatment of drug-induced psychosis in Parkinson's disease. Mov Disord 14: 484-487.
- Fernandez HH, Okun MS, Rodriguez RL, Malaty IA, Romrell J, et al. (2009) Quetiapine improves visual hallucinations in Parkinson disease but not through normalization of sleep architecture: Results from a double-blind clinical-polysomnography study. Int J Neurosci 119: 2196-2205.
- Sawada H, Oeda T, Yamamoto K, Umemura A, Tomita S, et al. (2013) Trigger medications and patient-related risk factors for Parkinson disease psychosis requiring anti-psychotic drugs: A retrospective cohort study. BMC Neurol 13: 145.
- Nishikawa N, Nagai M, Moritoyo T, Yabe H, Nomoto M (2009) Plasma amantadine concentrations in patients with parkinson's disease. Parkinsonism Relat Disord 15: 351-353.
- Umemura A, Oeda T, Tomita S, Hayashi R, Kohsaka M, et al. (2014) Delirium and high fever are associated with subacute motor deterioration in Parkinson disease: A nested case-control study. PLoS One 9: e94944.
- Umemura A, Oeda T, Yamamoto K, Tomita S, Kohsaka M, et al. (2015) Baseline plasma c-reactive protein concentrations and motor prognosis in Parkinson disease. PLoS One 10: e0136722.
- McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38: 1285-1291.
- Ouchi Y, Yagi S, Yokokura M, Sakamoto M (2009) Neuroinflammation in the living brain of Parkinson's disease. Parkinsonism Relat Disord 15: S200-204.
- Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, et al. (2013) In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson's disease. Parkinsonism Relat Disord 19: 47-52.
Citation: Sawada H, Oeda T, Umemura A, Satoshi T, Kohsaka M, et al. (2018) Risks and Triggers of Psychosis in Parkinson Disease. J Alzheimers Dis Parkinsonism 8: 413. DOI: 10.4172/2161-0460.1000413
Copyright: ©2018 Sawada H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5999
- [From(publication date): 0-2018 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 5125
- PDF downloads: 874