Risk of Developing Sudden Sensorineural Hearing Loss in Patients with Acute Otitis Media: A Multicenter Retrospective Analysis
Received: 22-Jan-2014 / Accepted Date: 11-Feb-2014 / Published Date: 18-Feb-2014 DOI: 10.4172/2161-119X.1000157
Abstract
Objective: The aim of the study was to determine the etiology of Sudden Sensorineural Hearing Loss (SSHL) and to call attention to Acute Otitis Media (AOM) with SSHL.
Study Design and Setting: We conducted a retrospective, multicenter analysis of SSHL. We were used spearman correlation matrix test for correlations between all variables. One hundred twelve patients with SSHL were evaluated.
Results: A total of 112 patients (62 males, 50 females) ranging in age from 17 to 70 years (average male 40.21 ± 14.04, average female 40.26 ± 11.16) were included. Fourteen of these had AOM. The majority of patients had moderate hearing loss. Flat and down-sloping types of audiogram were also observed (P<0.05). There was a positive relationship between SSHL and AOM, SOM, cardiac pathology as hypertension. No significance was established in terms of age or sex (p>0.05). Otoscopic examination was consistent with AOM. SSHL occurred as mixed-type hearing loss. Tympanometry was observed as type A.
Conclusion: In the treatment and follow-up periods, AOM patients should be checked and treated for the presence (if any) of early hearing loss.
Keywords: Acute otitis media, Sudden sensorineural hearing loss, Hearing loss, Sensorineural hearing loss, Otitis media, Pure-tone audiogram, Tympanometry
257248Introduction
Acute Otitis Media (AOM) is one of the most common ear diseases. It is an inflammation within the middle ear cleft, located behind an intact tympanic membrane. Patients with AOM may exhibit symptoms and signs specific to ear disease, including pain, fever, bulging tympanic membrane, middle ear effusion, otorrhea and hearing loss [1]. Uncommon symptoms and signs of AOM include hearing loss, tinnitus, vertigo, and nystagmus. Although both diagnosis and treatment of AOM have improved enormously, serious complications are still common albeit less frequent now than in the past. However, non-lifethreatening complications, such as hearing loss, frequently trouble many patients and have led to controversy regarding the importance and management of such complications [2].
Hearing loss may occur in the form of Sudden Sensorineural Hearing Loss (SSHL). SSHL is defined as a decrease in hearing greater than 30 dB over at least three contiguous frequencies, occurring in a total of 72 h or less. The incidence is equal in men and women, while individuals of all ages can be affected; however, the peak incidence is in the fourth or fifth decades [3].
Features associated with disorders underlying hearing loss need to be check-listed. However, the etiopathogenesis of SSHL in AOM is still controversial. Temporary sensorineural hearing impairment is generally attributed to the effect of increased tension and stiffness of the Round Window (RW). Aggressive middle ear infections may result in the release of infection, involving the round or oval windows [4]. Today, widespread use of antimicrobial agents in the management of Otitis Media (OM) has significantly reduced the incidence of hearing loss or complications such as labyrinthitis [5,6]. SSHL may occur as labyrinthitis and may result from various diseases, but is mostly idiopathic.
The main problem still consists of understanding both the etiopathogenesis and etiology of SSHL. The diagnosis and treatment of SSHL is considered a medical emergency. Delay in the diagnosis and treatment of SSHL may result in temporary or permanent sensorial hearing loss. SSHL can be caused by AOM or one of its complications or sequelae.
This study focused, in particular, on AOM cases with SSHL, since the diagnosis of SSHL in AOM cases has been neglected.
Materials and Methods
The study was approved by the Dicle university medical ethics committee (11.06.2012/592) and was carried out in accordance with the Declaration of Helsinki as amended in 2008. We reviewed the medical records of 112 patients between these dates that 1996-1998 and 2010- 2012 with SSHL treated in the Department of Otorhinolaryngology- Head and Neck Surgery in the medical hospitals at Dicle and Cumhuriyet universities and the private Akademi ENT surgery center in Turkey. We were observed SSHL that will be appear after AOM frequently in first ten days most ofour medical records.
We excluded subjects with a history of acoustic trauma, head trauma, barotrauma, ototoxic drug use or otological surgery, and those with any other otological diseases such as otosclerosis, Meniere’s disease or suppurative labyrinthitis, since all of these may involve changes in the inner ear. After reviewing the medical records of these patients retrospectively, 112 cases were identified and medical records of patients who had undergone evaluation for AOM with SSHL were selected then they were reevaluated for their last visit during the study period (62 male; 55.4%, 50 females; 44.6%). Of these, 14 patients had AOM, 18 had secretory otitis media, 16 had upper respiratory diseases, 14 had systemic disease and 50 were idiopathic.
Following ear, nose and throat examinations, all patients underwent pure-tone audiogram, tympanometry, Speech Recognition Threshold (SRT), Word Recognition Score (WRS), biochemical and microbiological tests.
Pure tone audiometry was performed on all participants in sound-proofed booths for objective hearing assessment following the guidelines of the American Speech-Language-Hearing Association (ASHA) [7]. Pure-tone air-conduction thresholds were determined for each ear at 500, 1,000, 2,000, 3,000, 4,000, 6,000 and 8,000 Hz. Bone-conduction thresholds were measured at two frequencies at 500, 1000,2,000, and 4,000 Hz. The presence of hearing loss was defined as a Pure-Tone Average (PTA) of thresholds at 500, 1,000, 2,000 and 4,000 Hz greater than 15 dB HL (decibel hearing level). Pure-Tone Average (PTA) was calculated at the hearing thresholds 0.5, 1.0, 2.0, and 4.0 kHz (arithmetic mean). Pure-tone and speech audiometry were performed using a diagnostic audiometer (Madsen OB 822 Clinical Audiometer). A TDH-39 standard headset was used for air conduction thresholds and speech tests. Measurements were made using an ascendingdescending technique, at 5-dB steps at all frequencies. If a patient made two or more responses to a set of three stimuli, he/she was deemed to have heard the sound. We were differentiated between AOM and SOM by otoscopic examination and tympanometric test. Tympanometric measurements were performed using a TDH-39 headset and middle ear analyzer (Clinical Middle Analyzer AZ 26, Interacoustic). Severity of SSHL was classified as slight, 16-25; mild, 26-40; moderate, 41-55; moderately severe, 56-70; severe, 71-90; or profound, over 90 dB HL. The duration, side (unilateral or bilateral), severity, and type of auditory impairment were all recorded. Audiometry was performed at the initial clinic visit and was then repeated after the treatment regimen.
We selected temporal bone tomography or magnetic resonance imaging for cases with unilateral AOM or that remained resistant to treatment.
Statistical Analysis
Statistical analyses were carried out using SPSS 15.0 (SPSS. Inc. Chicago, IL, USA) for Windows. All of the data in this study was evaluated descriptive statistics analyses as mean ± Standard Deviation (SD). We were used spearman correlation matrix test. This test is symmetric and gives the correlations between all variables. We analyses between SSHL and acute otitis media, serous otitis media, cervical pathology, hypertension, diabetes mellitus, gentel, age and idiopatic.
Results
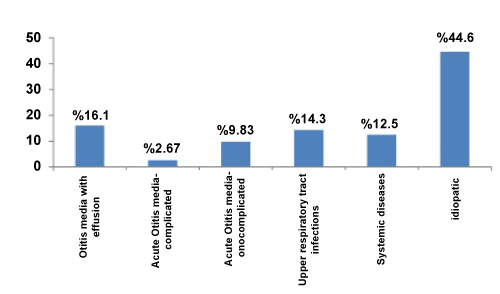
One hundred twelve patients, ranging in age from 17 to 70 years (male average 40.21 ± 14.04, female average 40.26 ± 11.16) were enrolled. Sixty-two (55.4%) right and 50 (44.6%) left ears were affected. SSHL distribution was statistically equal between the genders. Peak incidence was in the fourth decade. No significance was observed in terms of age or sex (p>0.05). Details obtained during the diagnosis of patients are shown in Figure 1.
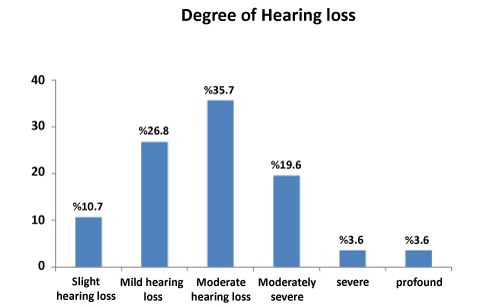
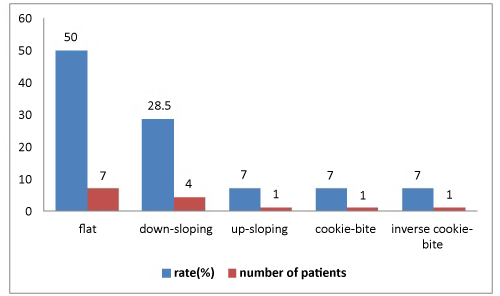
Patients with concurrent AOM and mixed hearing loss were selected. Fourteen patients with AOM were identified. Eight presented with hearing loss alone and six experienced dizziness or tinnitus together with hearing loss. Otoscopy revealed a thickened, hyperemic or bulging tympanic membrane in all the patients, while none exhibited nystagmus, signs of meningeal irritation or neurological deficits. All 14 patients had audiometrically confirmed, SNHL and decreased SRT and WRS at initial presentation, ranging from slight hearing impairment to profound hearing loss (Figure 2). Audiograms revealed mixed hearing loss in all patients. Hearing loss was at a minimum of three consecutive frequencies or more (the most common being 500- 1000- 2000 Hz) for SSHL. Audiometric tests were performed, and sensorineural hearing loss was identified as mixed-type hearing loss. In terms of the audiometric configuration of SSHL, the majority of audiogram shapes were flat (7 cases, 50%), followed by downsloping (4 cases, 28.5%), upsloping (1 case, 7.1%), cookie-bite (1 case, 7.1%) and inverse cookie-bite (1 case, 7.1%). PTA was 43.2 dB in flat shape, 41.0 dB in downsloping, 35.9 dB in upsloping, 33.5 dB in cookie-bite and 32.9 dB in inverse cookie-bite. Tympanometry was type A.
We were analyses between SSHL and acute otitis media, serous otitis media, cervical pathology, hypertension, diabetes mellitus, gentel, age and idiopatic. From this analyses; SSHL were positively correlated with acute otitis media, serous otitis media, cervical pathology, hypertension, and diabetes mellitus.
Tympanocentesis was performed in two patients due to a bulging tympanic membrane accompanied by severe pain and bullous myringitis.
Virological and microbiological tests for herpes virus, cytomegalovirus (CMV IgM and IgG), Epstein-Barr virus (EBV IgM and IgG), infection and syphilis (FTA-ABS) were negative in all patients. At complete blood count, five patients exhibited a slight elevation in white blood cell count. Other laboratory values did not reveal the specific cause of SSHL in any patient.
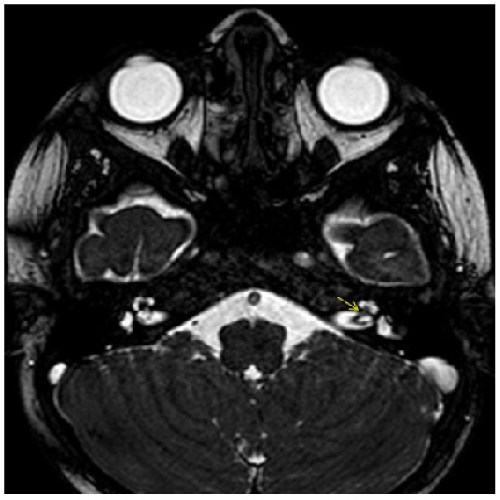
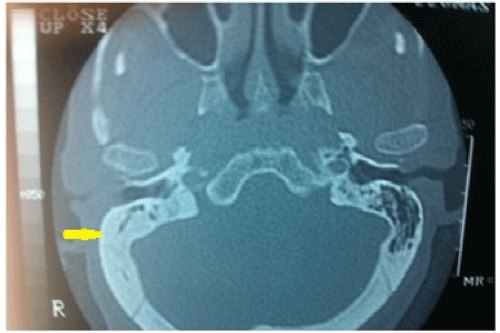
If the symptoms of tinnitus and hearing loss did not resolve after oral or parenteral antibiotic, antivirals, topical steroids, systemic steroid treatment or tympanocentesis then we performed CT or MRI. Temporal CT identified three patients with a minimal decrease in aeration in the mastoid and middle ear cavities (Figure 5). Temporal MRI identified two patients with a minimal enhancement in the inner ear cavities (Figure 4). However, no specific pathology was detected at CT or MRI for patients AOM with SSHL. Eleven of the 14 patients with AOM responded to medical treatment. However, hearing loss and tinnitus persisted in the other three, representing 12.5% of all patients; 9.82% of these were temporary, while 2.67% represented permanent hearing loss.
Apart from AOM, there are various other etiologies leading to SSHL (Figure 1). There was a positive relationship between SSHL and AOM, SOM, cardiac pathology as hypertension (Table 1). Eighteen patients had serous otitis media. Such patients are characterized by mixed-type hearing loss at audiometry and by type B at tympanometry. Fourteen patients had systemic etiologies. The foramina of the cervical vertebrae were narrow in six of these. Of the remaining eight patients, three had complications of diabetes and five had cardiovascular diseases. The characteristics of the cardiac population wasn’t different other patients. Another group of 16 patients presented with upper respiratory diseases. In this group, no hyperemia or other symptoms of AOM were observed in the tympanic membrane, and tympanometry showed type A. However, only the sensorineural type was identified in audiograms, compatible with upper respiratory diseases. The remaining 50 patients, who only had hearing loss and tinnitus with normal otoscopy and showed no evidence of any systemic disease, were deemed idiopathic.
| AOM | SOM | UR TI | DM | H pr | Servical path | Idiopatic | Gentel | Age | Control | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spearman rho | AOM | Correlation coefficient | 1,000 | -,165 | -,154 | -,063 | -,063 | -,090 | -,352 (**) | -,189 | -,053 | . |
| Sig. (2-tailed) | . | ,081 | ,104 | ,511 | ,511 | ,346 | ,000 | ,045 | ,581 | . | ||
| N | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | ||
| SOM | Correlation coefficient | -,165 | 1,000 | ,238 (*) | ,078 | ,073 | ,328 (**) | -,359 (**) | -,470 (**) | ,365 (**) | . | |
| Sig. (2-tailed) | ,081 | . | ,011 | ,414 | ,447 | ,000 | ,000 | ,000 | ,000 | . | ||
| N | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | ||
| UR TI | Correlation coefficient | -,154 | ,238 (*) | 1,000 | ,406 (**) | -,068 | ,243 (**) | -,380 (**) | -,336 (**) | ,099 | . | |
| Sig. (2-tailed) | ,104 | ,011 | . | ,000 | ,478 | ,010 | ,000 | ,000 | ,298 | . | ||
| N | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | ||
| DM | Correlation coefficient | -,063 | ,078 | ,406 (**) | 1,000 | -,028 | -,039 | -,154 | ,044 | ,037 | . | |
| Sig. (2-tailed) | ,511 | ,414 | ,000 | . | ,773 | ,679 | ,104 | ,648 | ,700 | . | ||
| N | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | ||
| H pr | Correlation coefficient | -,063 | -,073 | -,068 | -,028 | 1,000 | -,039 | -,154 | ,154 | -,233 (*) | . | |
| Sig. (2-tailed) | ,511 | ,447 | ,478 | ,773 | . | ,679 | ,104 | ,104 | ,013 | . | ||
| N | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | ||
| Servical path | Correlation coefficient | -,090 | ,328 (**) | ,243 (**) | -,039 | -,039 | 1,000 | -,221 (*) | -,256 (*) | ,083 | . | |
| Sig. (2-tailed) | ,346 | ,000 | ,010 | ,679 | ,679 | . | ,019 | ,007 | ,385 | . | ||
| N | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | ||
| Idiopatic | Correlation coefficient | -,352 (**) | -,359 (**) | -,380 (**) | -,154 | -,154 | -,221 (*) | 1,000 | ,328 (**) | -,031 | . | |
| Sig. (2-tailed) | ,000 | ,000 | ,000 | ,104 | ,104 | ,019 | . | ,000 | ,747 | . | ||
| N | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | ||
| Gentel | Correlation coefficient | -,189 (*) | -,470 (**) | -,336 (**) | ,044 | ,154 | -,256 (**) | ,328 (**) | 1,000 | -,321 (**) | . | |
| Sig. (2-tailed) | ,045 | ,000 | ,000 | ,648 | ,104 | ,007 | ,000 | . | ,001 | . | ||
| N | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | ||
| Age | Correlation coefficient | -,053 | ,365 (**) | ,009 | ,037 | -,233 (*) | ,083 | -,031 | -,321 (**) | 1,000 | . | |
| Sig. (2-tailed) | ,581 | ,000 | ,298 | ,700 | ,013 | ,385 | ,747 | ,001 | . | . | ||
| N | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | ||
| control | Correlation coefficient | . | . | . | . | . | . | . | . | . | . | |
| Sig. (2-tailed) | . | . | . | . | . | . | . | . | . | . | ||
| N | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 |
** Correlation is significant at the 0.01 level (2-tailed).
* Correlation is significant at the 0.05 level (2-tailed).
Table 1: The data of used in sperman correlation test.
Discussion
There are different theories have attempted to explain the pathogenesis of SSHL. These infectious, traumatic, neoplastic, autoimmune, toxic, circulatory, neurological and metabolic [8]. Endothelial function and cardiovascular factors is current cause of SSHL and speciality idiopathic sudden hearing loss [9].
This study concentrated on AOM and SSHL. AOM with accompanying SSHL has rarely been reported over the last few decades, and the literature on the subject is insufficient. AOM may be involved in the unknown etiologies of hearing loss and tinnitus. The aim of the study was to call physicians’ attention to AOM with SSHL. Audiograms have been compared and discussed on the basis of their shapes in many previous studies. Our statistical analysis revealed that the most common audiogram shape was flat, followed by downsloping (Figure 3). Cookie-bite, upsloping and inverse cookie-bite shapes was relatively uncommon. There was no improvement in subjects with tinnitus and a downsloping audiogram. Tinnitus at presentation with SHL has been identified as a negative prognostic indicator [10].
Additionally, imaging using CT and MRI was performed in the three patients resistant to therapy and those with unilateral AOM. We did not arrange imaging for all patients, since CT scanning has potential significant adverse events, including radiation exposure and side-effects of intravenous contrast, while offering no useful information that would improve initial management except in the event of a history of trauma, or chronic ear disease. CT can be used in situations where MRI is not possible, such as patients with pacemakers or severe claustrophobia, or even due to financial constraints [11]. CT or MRI revealed no anomalies in our patients’ middle or inner ear pathways. A decrease in mastoid aeration at CT may suggest labyrinthitis, from serous to purulent. There was association between lack of aeration and permanent sensorineural hearing loss [12]. However, this result is not statistically reliable due to the small sample sizes involved. MRI is not used in routine situations except in the case of retrocochlear pathology unresponsive to medical therapy. Thickening of the nerve localization was determined in only two patients. In addition, there was no apparent evidence in serological tests, except for systemic disease such as diabetes mellitus. In our study, the results of audiological-4 examinations other than diagnostic tests were consistent with the criteria set out in Robert et al. guidelines recently announced as a result of numerous studies [13].
Tympanogenic labyrinthitis or SHL is a rare intratemporal complication of otitis media. The decline in its incidence is partly due to earlier diagnosis, the development of better antibiotics, and greater awareness of the complications of otitis media among medical staff [14]. The etiopathogenesis of SSHL in AOM is still controversial. Viral infection is important in the pathogenesis of AOM, although it may be followed by bacterial colonization. AOM should therefore be primarily considered a bacterial infection. Many studies, using tympanocentesis, have identified Streptococcus pneumonia (up to 40%), Haemophilus influenzae (25-30%) and Moraxella catarrhalis (10-20%) as the organisms most commonly responsible for AOM [14]. Labyrinth irritation is induced by bacterial toxins or other mediators of inflammation [4]. Until recently, diagnosis of tympanogenic labyrentitis was made on clinical grounds. The presence of labyrinthitis may be suggested only if bone conduction loss co-exists with otitis media. In such a case, the toxins have presumably penetrated the RW to affect the cochlea, resulting in hearing loss. Acute purulent otitis media has been thought to cause temporary and permanent SSHL in the same way as chronic otitis media [15-18]. Engel et al. [17] investigated AOM in which streptolysin D damaged RW permeability, leading to SSHL. Morgolis and Nelson [19] published a case report of AOM with SSHL. The RW is probably more important than the oval window in this regard. The RW membrane is often thin and more susceptible to invasion in the nonchronically infected ear [19]. Additionally, the risk to hearing loss may be greater in acute than in chronic infection, since the RW membrane is demonstrably thicker in the latter condition, and pus may accumulate under pressure when the tympanic membrane is intact [20]. The RW is permeable to many biological substances and may function as a point of entry for harmful materials from the middle ear into the inner ear, leading to pathological changes in the latter [21,22]. The middle ears in the rat and humans exhibit numerous similar structural characteristics. It has recently been established that the reaction of the rat middle ear to S. pneumonia bears a close resemblance to that of the human middle ear [23]. On the basis of animal experiments, it may be suggested that RW response and penetrability to middle ear inflammatory conditions can vary according to the different stages of AOM [24].
Paparella et al. [25-27] indicated that proinflammatory molecules and mediators, along with bacteria and bacterial components, can pass through the RW into the inner ear and cause structural damage and hearing loss. Loss of outer hair cells at the base of the cochlea has been noted in otitis media. Accordingly, the infectious and inflammatory processes that occur in the middle ear, such as AOM, may result in cochlear or vestibular symptoms such as hearing loss or vertigo. Song et al. [28] described several patients with asymmetric SNHL and decreased SRS secondary to AOM.
In our study, we investigated the otoscopic and clinical characteristics of AOM in patients diagnosed with SSHL. The fact that the literature is compatible with our study may confirm the association between AOM and SSHL. We determined a 2.67% incidence of permanent SSHL in complicated AOM.Our scan of the literature revealed one study of the adult rate of SSHL with AOM. Swart [29] reported an incidence of SSHL with AOM of 8%. It was therefore impossible to perform a comparative discussion of the rate of SSHL with AOM, although, based on circumstantial evidence, both transient and permanent SHL can result from AOM. Margolis et al. [30] reported SHL that was more pronounced at higher frequencies (4,000 to 8,000 Hz) in two adults with documented purulent middle ear effusions. They also noted that children who have recovered from otitis media have significantly poorer hearing in the extended high-frequency range compared to children without significant histories of otitis media. Although our diagnostics from those of Morgolis, we identified four adult patients whose higher frequency hearing (down-sloping) loss was resistant to therapy in AOM. One developed permanent tinnitus. The remaining three patients recovered fully at all frequencies in the auditory pathway. Also Chul Ho et al. [4] also reported a case of tympanogenic labyrinthitis complicated by AOM.
The limitation of our study is that it is retrospective. There was few data and clinical trials in the world. In agreement with the literature, this study revealed that follow-up is essential for patients with AOM since they are likely to develop sudden hearing loss.
Conclusion
It is important to remember that SSHL can also develop in AOM cases. Following diagnosis, such patients should be examined audiometrically and treated promptly for the presence of early hearing loss, if identified.We think the further, more detailed studies on the subject are now required.
References
- Richard A (1993) Acute otitis media and otitis media with effusion. In: Cummings CW (Edr.) Otolaryngology-Head & Neck surgery. (2nd Edn.), Mosby-Year Book Missour, p: 2808-2886.
- Margaret A (1993) Otitis media with Effusion. In: Byron J. Bailey (Edr.) Head and Neck Surgery-Otolaryngology Kenna B. Lippincott company, Philadelphia,p: 1592.
- Shikowitz MJ (1991) Sudden sensorineural hearing loss. Med Clin North Am 75: 1239-1250.
- Jang CH, Park SY, Wang PC (2005) A case of tympanogenic labyrinthitis complicated by acute otitis media. Yonsei Med J 46: 161-165.
- Rappaport JM, Bhatt SM, Burkard RF, Merchant SN, Nadol JB Jr (1999) Prevention of hearing loss in experimental pneumococcal meningitis by administration of dexamethasone and ketorolac. J Infect Dis 179: 264-268.
- Morizono T, Giebink GS, Paparella MM, Sikora MA, Shea D (1985) Sensorineural hearing loss in experimental purulent otitis media due to Streptococcus pneumoniae. Arch Otolaryngol 111: 794-798.
- American Speech–Language–Hearing Association (2005) Guidelines for manual pure-tone threshold audiometry.
- Stew BT, Fishpool SJ, Williams H (2012) Sudden sensorineural hearing loss. Br J Hosp Med (Lond) 73: 86-89.
- Ciccone MM, Cortese F, Pinto M, Di Teo C, Fornarelli F, et al. (2012) Endothelial function and cardiovascular risk in patients with idiopathic sudden sensorineural hearing loss. Atherosclerosis 225: 511-516.
- Kuhn M, Heman-Ackah SE, Shaikh JA, Roehm PC (2011) Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif 15: 91-105.
- American College of Radiology (ACR). Expert Panel on Neurologic Imaging: Turski PA, Wippold FJ II, Cornelius RS, Brunberg JA, Davis PC et al. (1996) ACR appropriateness criteria, vertigo and hearing loss (last review date: 2008).
- Robert J Stachler, Sujana S Chandrasekhar, Sanford M Archer, Richard M Rosenfeld, Seth R Schwartz, et al. (2012) Otolaryngology- Head and Neck Surgery 146(1S) S1– S35.
- Tos M, Stangerup SE (1985) Secretory otitis and pneumatization of the mastoid process: sexual differences in the size of mastoid cell system. Am J Otolaryngol 6: 199-205.
- Schuknecht HF (1993) Infections: labyrinthitis. In: Pathology of the ear. Lea & Febiger, Pennsylvania, p: 211-216.
- Philip D Yates (2004) Otitis Media Current Diagnosis & Treatment in Otolaryngology. Head& Neck Surgery: 695.
- Cureoglu S, Schachern PA, Paparella MM, Lindgren BR (2004) Cochlear changes in chronic otitis media. Laryngoscope 114: 622-626.
- Engel F, Blatz R, Kellner J, Palmer M, Weller U, et al. (1995) Breakdown of the round window membrane permeability barrier evoked by streptolysin O: possible etiologic role in development of sensorineural hearing loss in acute otitis media. Infect Immun 63: 1305-1310.
- Mokhtar Bassiouni, Michael M Paparella (1984) Labyrinthitis. Volume II: Otology and Neuro-Otology Section 3: Diseases of the Ear Part 4: Inner Ear Chapter 42: 2.
- Margolis RH, Nelson DA (1993) Acute otitis media with transient sensorineural hearing loss. A case study. Arch Otolaryngol Head Neck Surg 119: 682-686.
- Ludman H (1987) Complications of suppurative otitis media In: Kerr AG(Edr.) Scott- Brown's Otolaryngology.(5th Edn.), Butterworths, London, P: 264-291.
- Carpenter AM, Muchow D, Goycoolea MV (1989) Ultrastructural studies of the human round window membrane. Arch Otolaryngol Head Neck Surg 115: 585-590.
- Nagahara K, Yoza T, Naito Y, Ogino F (1985) Oxygenation through the round window membrane and the inner ear function. Auris Nasus Larynx 12 Suppl 1: S120-122.
- Hermansson A, Emgård P, Prellner K, Hellström S (1988) A rat model for pneumococcal otitis media. Am J Otolaryngol 9: 97-101.
- Ikeda K, Sakagami M, Morizono T, Juhn SK (1990) Permeability of the round window membrane to middle-sized molecules in purulent otitis media. Arch Otolaryngol Head Neck Surg 116: 57-60.
- Huang M, Dulon D, Schacht J (1990) Outer hair cells as potential targets of inflammatory mediators. Ann Otol Rhinol Laryngol Suppl 148: 35-38.
- Schachern PA, Paparella MM, Hybertson R, Sano S, Duvall AJ 3rd (1992) Bacterial tympanogenic labyrinthitis, meningitis, and sensorineural damage. Arch Otolaryngol Head Neck Surg 118: 53-57.
- Schachern PA, Paparella MM, Goycoolea M, Goldberg B, Schlievert P (1981) The round window membrane following application of staphylococcal exotoxin: an electron microscopic study. Laryngoscope 91: 2007-2017.
- Song JE, Sapthavee A, Cager GR, Saadia-Redleaf MI (2012) Pseudo-sudden deafness. Ann Otol Rhinol Laryngol 121: 96-99.
- Swart SM, Lemmer R, Parbhoo JN, Prescott CA (1995) A survey of ear and hearing disorders amongst a representative sample of grade 1 schoolchildren in Swaziland. Int J Pediatr Otorhinolaryngol 32: 23-34.
- Margolis RH, Saly GL, Hunter LL (2000) High-frequency hearing loss and wideband middle ear impedance in children with otitis media histories. Ear Hear 21: 206-211.
Citation: Akdag M, Uysal IO, Bakir S, Ozkurt FE, Muderris S, et al. (2014) Risk of Developing Sudden Sensorineural Hearing Loss in Patients with Acute Otitis Media: A Multicenter Retrospective Analysis. Otolaryngology 4:157. DOI: 10.4172/2161-119X.1000157
Copyright: © 2014 Akdag M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16663
- [From(publication date): 5-2014 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 12012
- PDF downloads: 4651