Risk Factors for Metachronous Vertebral Osteomyelitis after Periprosthetic Joint Infections: A Minimum Five Years Retrospective Analysis
Received: 18-Mar-2022 / Manuscript No. JCIDP-22-57750 / Editor assigned: 21-Mar-2022 / PreQC No. JCIDP-22-57750 / Reviewed: 04-Apr-2022 / QC No. JCIDP-22-57750 / Revised: 17-May-2022 / Manuscript No. JCIDP-22-57750 / Published Date: 24-May-2022
Abstract
Background: Joint arthroplasty failure is primarily caused by Periprosthetic Joint Infection (PJI). PJI treatment may result in serious complications, such as Vertebral Osteomyelitis (VO). Risk factors for the development of metachronous VO after PJI, and the outcomes of these patients, are unknown. We aimed to 1) Identify the risk factors for developing meta-chronous VO following PJI and 2) Investigate the clinical outcomes of meta-chronous VO following PJI.
Methods: We included patients with PJI who underwent surgical intervention at our institute between January 2006 and December 2020. Patients with synchronous VO following PJI were excluded from the study. Patients with meta-chronous VO after PJI were identified and monitored during a minimum follow-up period of 5 years and we analyzed their comorbidities, procedures, causative pathogens, and clinical outcomes.
Results: We identified 567 patients with PJI, of whom seven developed VO (1.2%). We identified the following risk factors for metachronous VO after PJI: Systemic Inflammatory Response Syndrome (SIRS), drug abuse, polymicrobial infection, and 3-stage resection arthroplasty or more (Odds Ratios (ORs): 1.43, 52.98, 55.83, and 29.78, respectively). All patients who developed VO had poor clinical outcomes: two out of seven patients suffered from recurrent VO and six out of seven patients suffered from recurrent PJIs.
Conclusion: Patients with PJI who have risk factors that we identified may be predisposed to metachronous VO and likely to experience poor outcomes. We suspect that bacteremia may play a role in the pathogenesis of VO following PJI, but further research is required.
Keywords: Periprosthetic joint infection; Meta-chronous vertebral osteomyelitis; Systemic inflammatory response syndrome; Staged resection arthroplasty
Introduction
Background
Recent advances in orthopedic implants have led to more surgical options and better patient outcomes. The demand for joint replacement surgery is increasing in geriatric patients, with a decline in the average age of patients [1]. Moreover, the number of patients who undergo multiple joint replacement surgeries is increasing [2]. With increasing numbers of orthopedic surgeries, complications following arthroplasty have gained attention. The major perioperative complication is Periprosthetic Joint Infection (PJI), which may burden medical resources and is associated with significant medical expenses and poor clinical outcomes [3]. Patients with PJI often suffer from complications during treatment [4].
Some studies have reported an incidence of meta-chronous or synchronous PJI of approximately 20% [5-9]. In addition, patients may also suffer from meta-chronous Vertebral Osteomyelitis (VO) following PJI treatment. VO accounts for 2%–7% of all musculoskeletal infections, and its incidence has increased in the last few decades [10]. Spinal surgery is often necessary for patients who are unresponsive to antibiotic treatment.
Rationale
To the best of our knowledge, the incidence of meta-chronous VO following PJI is unknown. In addition, the risk factors for developing meta-chronous VO following PJI is also unknown, although hematogenous spread has been suggested to play a role in the pathogenesis of this condition. Therefore, the aim of this study was to identify potential risk factors-including comorbid conditions, methods of PJI management, and causative pathogens-and clinical outcomes in patients who develop VO following PJI.
Materials and Methods
Study design, setting, and participant selection
This was a retrospective cohort study of all patients with PJI of the hip or knee who were managed at our hospital, a referral medical center, between January 2006 and December 2020. With the approval of the Institutional Review Board, the electronic hospital database was searched retrospectively for patients with an ICD-9 (International Classification of Diseases, Ninth Revision, and Clinical Modification) code of 996.66. The extracted data were reviewed by two independent researchers. Patients who underwent arthroplasty with documented episodes of PJI and a minimum 5 years follow-up period were included in the study and followed up until the endpoint of the development of metachronous VO. The exclusion criteria included infection after open reduction and internal fixation or primary septic arthritis or the presence of synchronous VO and PJI or previous VO before PJI.
Definitions
In our study, we defined PJI as the presence of any one of the following three criteria:
- A sinus tract communicating with the prosthesis.
- The isolation of a pathogen from two or more samples obtained from the infected prosthetic joint.
- The existence of at least four of the following six criteria:
- Elevated serum Erythrocyte Sedimentation Rate (ESR) and serum C-Reactive Protein (CRP) concentrations.
- Elevated synovial leukocyte count.
- Elevated synovial neutrophil percentage (PMN %);
- Pus in the affected joint.
- A positive periprosthetic tissue or fluid culture.
- Histologic analysis of periprosthetic tissue at × 400 magnification demonstrating >5 neutrophils per high-power field in five high-power fields [11].
Outcome measures
We documented patient demographics, comorbidities, the site and date of arthroplasty, methods used to manage PJI, the time between PJI diagnosis and VO onset, duration of antibiotic treatment, and causative pathogens identified in cases of PJI and VO. To identify the risk factors contributing to the development of VO following PJI, we compared PJI patients with and without VO. These risk factors were analyzed, and the outcomes of these patients were recorded.
Treatment
Patients with PJI were treated according to the guidelines provided by Tsukayama [12]. Debridement and Irrigation (DAIR) was performed for acute hematogenous infections, in addition to modular component exchange. For chronic infections (infections persisting beyond 4 weeks after symptom onset), the gold standard treatment is second stage resection arthroplasty with interim Antibiotic Loaded Bone Cement (ALBC) mobile spacer implantation, which offers a high success rate. The second stages resection arthroplasty protocol used at our institute is as follows: before the first stage surgery, if the patient develops Systemic Inflammatory Response Syndrome (SIRS), two sets of blood cultures are obtained. The first stage surgery consists of radical debridement with intra-operative synovial fluid sampling, the collection of three sets of deep tissue specimens, prosthesis removal, and antibiotic loaded bone cement implantation. This is followed by the administration of 4 weeks of systemic Intra Venous (IV) antimicrobial therapy, guided by cultures yielded during the first stage surgery. In cases with negative cultures, broad spectrum dual-antibiotic regimens are prescribed by an infectious disease specialist and bridged by additional 2 weeks oral antibiotic therapy. To create the ALBC used in second stage resection arthroplasty, we hand-mix 4 g vancomycin and 4 g ceftazidime per 40 g cement. At our hospital, we generally use 3 months interim periods between each stage of resection arthroplasty, including 6 weeks drug holidays. During the interim period, ESR and CRP levels are checked monthly. Joint aspiration for microscopy and culture is routinely performed prior to the second stage of resection arthroplasty to ensure infection eradication. Moreover, second stage surgeries with prosthesis reimplantation is only performed once CRP levels have returned to normal or are on a downward trend, without symptoms and signs of infection such as localized erythema or swelling in the soft tissues.
However, in the case of treatment failure (positive joint aspirate culture prior to reimplantation, continued CRP elevation, erythema, soft tissue swelling, or any symptoms and signs suggestive of infection) after the first stage surgery, repeat resection arthroplasty with culture guided ALBC mobile spacer implantation is indicated. The second-stage surgery protocol with an ALBC mobile spacer is the same as previously mentioned, except that we utilize a culture guided ALBC mobile spacer. Following the second stage surgery, another 4 weeks of systemic IV antibiotic and 2 weeks of oral antibiotic treatment is prescribed. We define repeat resection arthroplasty by the number of surgeries performed before reimplantation (3rd stage or 4th stage resection arthroplasty).
In addition, if recurrent PJI is diagnosed after reimplantation following resection arthroplasty, we followed the previously mentioned principles. Another second stage resection arthroplasty might be indicated, and we define these procedures as “repeat second stage resection arthroplasties”.
Complications following recurrent PJI may include scar formation and contraction of the soft tissue, with development of resistant bacterial infections. In addition, some patients become severely ill and may develop septic shock, making them unsuitable candidates for complicated debridement surgery. In these situations, amputation or permanent resection arthroplasty can be performed through life saving salvage procedures [13].
Polymicrobial PJI is defined as the isolation of more than one microorganism from at least one (or more) cultures of periprosthetic tissue or synovial fluid. In the present study, we analyzed the microbiological profile of all episodes of infection.
In the present study, we diagnosed VO based on the clinical presentation of back pain, combined with elevated ESR and CRP levels. MRI imaging was the gold standard for the final diagnosis of VO, which was confirmed by spine surgeons in our institution. Depending on the clinical conditions of each case, CT guided biopsy or debridement surgery was performed to obtain additional tissue samples.
The treatment protocol for VO at our hospital is as follows: after the diagnosis of VO, the first line treatment is nonoperative management with long-term antibiotics. However, surgical intervention is indicated in patients with poor response to antibiotics, those with neurological compromise, mechanical instability, intractable back pain, or epidural abscess.
Statistical analysis
We evaluated the association between qualitative variables using the Chi-Square or Fisher’s exact test. Continuous variables with a normal distribution were compared using Analysis of Variance (ANOVA). We evaluated risk factors using univariate and multivariate logistic regression models. For all tests, statistical significance was set at p<0.05 (5%). Processing and data analysis were performed using SPSS (version 20.0; IBM Corp., Armonk, NY, USA).
Results
Risk factors for the development of meta-chronous VO following PJI
Of the 567 patients with PJI who had undergone a minimum clinical follow-up period of five years, seven patients (7/56,1.2%) developed VO. Among these, 57.1% were male (n=4) and 42.9% were female (n=3), with an average age of 70.3 years (standard deviation: 11.0; range: 55-83).
Demographic data were compared between patients with and without metachronous VO (Table 1).
| Variables | PJI with metachronous VO (n=7) | PJI (n=567) | p |
|---|---|---|---|
| Male/Female | 4 (57.1%)/3 (42.9%) | 331 (58.4%)/236 (41.6%) | 0.941 |
| Age (Medium) | Medium=70.50568 | Medium=72.015 | 0.961 |
| (range)(IQR) | Range=10.20304 | Range=34.373 | |
| (mean)(SD) | 70.286 (11.011) | 70.816 (10.203) | |
| Body mass index (Medium) | Medium=26.7 | MediuM=26.23 | 0.549 |
| (range)(IQR) | Range=9.2 | Range=21.92 | |
| (mean)(SD) | 26.029 (3.291) | 26.871 (4.239) | |
| Albumin level (SD)* | 3.0314 (0.676) | 3.836 (0.578) | 0.061 |
| eGFR(SD) | 72.633 (33.182) | 80.72 (35.451) | 0.597 |
| CRP(SD) | 71.03 (67.018) | 91.823 (87.653) | 0.469 |
| Hospital course | |||
| Admission from ER | 5 (71.43) | 73 (12.87) | <0.001* |
| SIRS | 6 (85.71) | 81 (14.29) | <0.001* |
| Blood culture positive | 5 (71.43) | 24 (4.23) | <0.001* |
| Underlying disease | |||
| Charlson comorbidity index | 3.571 (2.070) | 2.414 (2.302) | 0.163 |
| Cancer (%) | 2 (28.57) | 57 (10.05) | 0.132 |
| Solid tumor (%) | 1 (14.29) | 29 (5.11) | 0.123 |
| Hypertension (%) | 5 (71.43) | 364 (64.20) | 0.705 |
| Diabetes (%) | 3 (42.86) | 97 (17.11) | 0.101 |
| Liver disease (%) | 3 (42.86) | 153 (26.98) | 0.123 |
| HCV carrier (%) | 3 (42.86) | 36 (6.35) | 0.012* |
| HBV carrier (%) | 2 (28.57) | 49 (8.64) | 0.516 |
| Alcoholism (%) | 1 (14.29) | 25 (4.40) | 0.123 |
| Drug user (%) | 2 (28.57) | 15 (2.64) | <0.001* |
| COPD (%) | 1 (14.29) | 53 (9.43) | 0.141 |
| Renal insufficiency (%) | 1 (14.29) | 32 (5.64) | 0.38 |
| CV disease (%) | 3 (42.86) | 97 (17.10) | 0.123 |
| Af (%) | 3 (42.86) | 16 (2.82) | <0.001* |
| CAD (%) | 1 (14.29) | 24 (4.23) | 0.256 |
| Surgery related variables | |||
| Operation time(min)(SD) | 155 (39.233) | 125.08 (48.037) | 0.129 |
| Joint presentation | |||
| Hip (%) | 1 (14.29) | 164 (28.92) | 0.123 |
| Knee (%) | 6 (85.71) | 403 (71.08) | 0.113 |
| Procedures | |||
| 2nd stage resection arthroplasty(mobile spacer) | 1 (14.29) | 469 (82.72) | 0.092 |
| 2nd stage resection arthroplasty(static spacer) | 2 (28.57) | 40 (7.05) | 0.012* |
| 3rd stage resection arthroplasty or more (%) | 3 (42.86) | 16 (2.82) | 0.003* |
| DAIR | 1 (14.29) | 57 (10.05) | 0.136 |
| Amputation (%) | 1 (14.29) | 18 (3.17) | 0.576 |
| Repeat 2nd stage resection arthroplasty (%) | 4 (57.14) | 31 (5.47) | 0.023* |
| Bacteria of PJI | |||
| Culture-negative (%) | 3 (42.86) | 129 (22.75) | 0.242 |
| Gram positive (%) | 4 (57.14) | 397 (70.02) | 0.089 |
| Gram negative (%) | 0 (0) | 40 (7.05) | 0.465 |
| Fungus (%) | 2 (28.57) | 16 (2.82) | 0.003* |
| Tuberculosis (%) | 0 (0) | 8 (1.41) | 0.75 |
| Poly-microbial (%) | 2 (28.57) | 24 (4.23) | <0.001* |
| MRSA (%) | 2 (28.57) | 48 (8.47) | 0.098 |
| Timetable (mean)(SD) | |||
| Operation from admission date (day) | 11.52 (12.75) | 1.84 (2.02) | 0.09 |
| Duration of overall antibiotics (day) | 60.71 (4.39) | 40 (8.33) | 0.112 |
VO: Vertebral Osteomyelitis; PJI: Periprosthetic Joint Infection; eGFR: estimated Glomerular Filtration Rate; CRP: C-Reactive Protein; IQR: Inter-Quartile Range; SD: Standard Deviation; ER: Emergency Room; SIRS: Systemic Inflammatory Response Syndrome; HCV: Hepatitis C Virus; HBV: Hepatitis B Virus; COPD: Chronic Obstructive Pulmonary Diseases; CV: Cardiovascular; AF: Atrial Fibrillation; CAD: Coronary Artery Disease; DAIR: Debridement and Irrigation; MRSA: Methicillin-Resistant Staphylococcus Aureus.*p value<0.05.
Table 1: Comparison of demographic data between patients with and without metachronous VO.
Risk factors were evaluated using a logistic regression model to address multifactorial issues, including host comorbidities, treatment methods used for PJI, intraoperative parameters, and causative pathogens identified. The risk factors that we identified for the development of metachronous VO following PJI include SIRS, substance abuse, polymicrobial PJI, and 3rd stage resection arthroplasty or more (Odds Ratios (ORs):1.43, 52.98, 55.83 and 29.78, respectively) (Table 2).
| Multivariate Model results | ||
|---|---|---|
| Variables | Adjusted Odds ratio (95% CI) | p |
| Admission from ER | 0.38 (0.721-1.343) | 0.321 |
| Blood culture positive | 0.92 (0.788-1.063) | 0.248 |
| HCV carrier | 3.32 (0.451-66.409) | 0.324 |
| Atrial fibrillation | 2.25 (0.7034-28.8325) | 0.234 |
| 2nd Stage resection arthroplasty (static spacer) | 7.33 (0.4007-99.899) | 0.221 |
| Repeated 2nd stage resection arthroplasty | 8.21 (0.734-87.234) | 0.323 |
| Fungus | 13.6 (0.769-117.945) | 0.218 |
| SIRS | 1.43 (1.246-1.644) | <0.001* |
| Drug abusers | 52.98 (6.221-608.213) | 0.003* |
| Poly-microbial PJI | 55.83 (7.506-415.328) | <0.001* |
| 3rd Stage resection arthroplasty or more | 29.78 (4.490-197.453) | <0.001* |
VO: Vertebral Osteomyelitis; ER: Emergency Room; HCV: Hepatitis C Virus; SIRS: Systemic Inflammatory Response Syndrome; OR: Odds Ratio; CI: Confidence Interval; *p-value<0.05.
Table 2: Results of multivariate logistic regression analysis of variables associated with metachronous VO.
Clinical outcomes of metachronous VO following PJI
Of the seven patients with metachronous VO following PJI, the following significant clinical outcomes were observed (Table 3). Six patients (85.7%) developed SIRS, five patients (71.4%) yielded positive blood cultures, and four patients (57.1%) had negative tissue cultures. Six patients (85.7%) had recurrent infections and underwent multiple complicated debridement surgeries, and three patients (42.9%) developed metachronous VO within one month of PJI diagnosis with negative tissue cultures.
| Case | Positive blood culture | SIRS | Procedures | Region | Date of infection | Period between PJI and vertebral osteomyelitis (days) | Recurrent infection | Organism | Same species | Final outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | 3rd stage resection arthroplasty | Right hip | 11/20/2012 | 281 | Yes (2) | Candida Metapsilosis Staphylococcus epidermidis | - | Amputation |
| L4-S1 | 8/28/2013 | 0 | Moraxella Osloensis Strepcoccus. Agalactiae | TLIDF | ||||||
| 2 | + | + | 3rd stage resection arthroplasty | Right knee | 11/10/2006 | 28 | 0 | Staphylococcus aureus | - | Revision arthroplasty |
| L3-L4 | 12/8/2006 | 0 | No growth | Prolonged antibiotics usage | ||||||
| 3 | - | + | 2nd stage with mobile spacer | Right knee | 4/7/2013 | 736 | Yes (4) | No growth | - | Revision arthroplasty with recurrent PJI |
| L3-L4 | 4/13/2015 | Yes (2) | Klebsiella pneumoniae | TLIDF with recurrent VO | ||||||
| 4 | - | - | 3rd stage resection arthroplasty | Left knee | 11/6/2012 | 887 | Yes (2) | No growth | - | Revision arthroplasty |
| T10-12 | 4/12/2015 | 0 | No growth | TTIDF | ||||||
| 5 | + | + | 2nd stage with mobile spacer | Left knee | 7/1/2014 | 28 | Yes (2) | Strepcoccus. agalactiae | - | Permanent spacer |
| L2-L4 | 7/29/2014 | Yes (2) | No growth | TLIDF with recurrent VO | ||||||
| 6 | + | + | 2nd stage with mobile spacer | Left knee | 5/8/2013 | 29 | Yes (4) | No growth | - | Permanent Spacer with recurrent PJI |
| L3-L4 | 6/6/2013 | 0 | No growth | TLIDF | ||||||
| 7 | + | + | 3rd stage resection arthroplasty | Left knee | 9/28/2011 | 358 | Yes (3) | Candida Metapsilosis Staphylococcus epidermidis Strepcoccus. Agalactiae | + | Permanent Spacer |
| L4-L5 | 9/20/2012 | 0 | Strepcoccus. agalactiae | TLIDF |
TLIDF: Transforaminal Lumbar Interbody Debridement and Fusion; PJI: Periprosthetic Joint Infection; VO: Vertebral Osteomyelitis; TTIDF: Transforaminal Thoracic Interbody Debridment and Fusion.
Table 3: Significant findings in patients with metachronous Vertebra Osteomyelitis (VO) following PJI.
Discussion
Background and rationale
In this retrospective study, we investigated the potential risk factors that may link two seemingly unrelated diseases, and these included a history of SIRS, drug abuse, polymicrobial PJI and ≥ 3rd stage resection arthroplasty. Various risk factors associated with the development of PJI or VO has been identified. These include host conditions, such as advanced age, American Society of Anesthesiologists score, obesity, diabetes mellitus, rheumatoid arthritis, substance abuse, acute or chronic infection (especially HIV), long-term systemic steroid use, poor nutritional status, immunologic incompetence, and previous surgery [13]. Second, microorganisms such as Staphylococcus aureus, Coagulase Negative Staphylococci (CoNS), streptococci, enterococci, aerobic gram negative bacilli, and fungi have all been identified as causative pathogens in PJI or VO [14]. Although overlaps may exist between the two diseases, to our knowledge, no study has explored this possibility. In this study, we considered the development of synchronous PJI as a separate clinical entity and thus excluded such cases from analysis.
The possible pathogenesis of the development of metachronous VO following PJI may be bacteremia and it may be warranted for clinicians to evaluate patients for this condition specifically, and tailor treatment plans accordingly [15]. Approximately 25%–59% of cases of bacteremia can be detected by obtaining two sets of aerobic and anaerobic blood cultures [16]. The detection rate may increase to 70% if patients have not been treated with antibiotics [17]. Many clinical procedures can lead to infection and even bacteremia when infection is not contained locally, allowing bacteria to enter the bloodstream. Oral hygiene and dental procedures, such as chewing, brushing, teeth cleanings, tooth extractions, or root canal treatments may be associated with an increased risk of bacteremia, necrotizing fasciitis, and bacterial endocarditis [18]. The likelihood of developing bacteremia depends on the clinical setting, and bacteremia is a clinically significant entity that likely plays a role in the pathogenesis of metachronous VO following PJI. Nevertheless, few studies have evaluated bacteremia in patients with PJI, and the rates of bacteremia are likely underestimated for numerous reasons. First, the consensus PJI diagnostic criteria do not include blood cultures [19]. A high false-positive rate can lead to an unnecessary financial burden on healthcare systems. Studies have shown that the percentage of positive blood cultures in patients with PJI is in the range of 4.3%–7.3% [20]. It was earlier proposed that a positive blood culture in patients with PJI can contribute to treatment failure by lowering the treatment success rate to 65.1% [21]. In our study, although 71.4% of patients yielded positive blood cultures, it is difficult to prove that this is a significant risk factor for the development of metachronous VO after PJI. One reason might be the possibility of high false-positive blood culture rates in patients with PJI. Moreover, it is challenging to establish whether metachronous VO is caused by the same organism that caused PJI. To address this challenge, we have analyzed pathogen genotypes using multilocus sequence typing and pulsed-field gel electrophoresis at our institute since 2018. However, much of this genotyping information was not available in the present study given that we mostly used a database predating the availability of such data at our facility.
The patient’s clinical condition may contribute to increased susceptibility for developing metachronous VO after PJI and is often neglected during preoperative assessment. We identified SIRS and drug abuse as two significant patient factors in the treatment of the index PJI. SIRS might be a sequela of bacteremia and is potentially a more reliable measure than blood cultures given their high false positive rate, as mentioned earlier. In our study, six patients (85.7%) exhibited SIRS during PJI, and we identified SIRS as an independent risk factor for the development of metachronous VO after PJI. A history of drug abuse during PJI treatment was identified as the strongest risk factor for the development of metachronous VO in our study. The international consensus group on periprosthetic joint infection recommends postponing arthroplasty until after at least one year of substance use cessation to minimize the chances of infection [22]. Additionally, illicit drug use also puts patients at a higher risk of contracting HIV, HCV, and candida infections. In patients with PJI who abuse substances, the gold standard of care is to perform 2nd stage resection arthroplas. However, due to their immunocompromised status, such patients may have bacteremia [23].
Compared to monomicrobial PJIs, polymicrobial PJIs are rare, accounting for 4%–27% of all PJI cases. The rates of polymicrobial PJIs in patients with THA and TKA range from 10.5% to 19% and 9% to 12.3%, respectively [24]. With more arthroplasties being performed every year, a 37% increase in polymicrobial PJI cases has been documented [25]. Polymicrobial PJIs occur more frequently in the early postoperative period, and this is associated with the presence of highly virulent pathogens, such as Staphylococcus aureus, streptococci, and enterococci [26]. Staphylococcus epidermidis and other CoNS are particularly common in polymicrobial PJIs, and the most common co-pathogen with Staphylococcus epidermidis is Enterococcus faecalis [27]. Therefore, empiric broad spectrum antibiotics should be administered. Additionally, polymicrobial PJIs occur frequently in patients with soft tissue defects or wound dehiscence (OR:5.9), sinus discharge (OR:5.0), and age ≥ 65 years (OR:2.8) [28]. Studies have also shown that using DAIR as a mainstay treatment is inadequate to treat polymicrobial PJIs, as higher hospital readmission rates and deep surgical site infections have been reported. Approximately 23% of patients with polymicrobial PJIs require implant removal within 12 months of follow-up [29]. We found that patients with polymicrobial PJIs who undergo 2nd stage resection arthroplasty with implant removal, as per the treatment protocol described earlier, had poor clinical outcomes, which could contribute to higher susceptibilities for bacteremia and possibly, the development of metachronous VO.
The overall PJI rate has been reported as slightly higher in patients who undergo TKA than in those who undergo THA (1.41% vs. 0.92%), and the correlation is significant in PJIs that develop late, with reported incidence rates of 0.080% vs. 0.05% (CI:0.45–0.69) per prosthesis year (p=0.006) [30]. Moreover, the eradication rate of 2nd stage resection arthroplasty is distinctly different for the hip versus the knee. The success rate for knee PJI has been reported as 72%–95%, compared to hip PJI with an eradication rate of 87%–100% [15]. A possible explanation is the anatomical differences between the knee and hip joints. In a 12 years study of 320 patients, significantly more patients suffered from symptoms of infection in affected TKA joints than in THA joints. Knee joint inflammation may be more symptomatic and apparent earlier during infection because of the thin layer of soft tissue surrounding the knee, but more research is required to investigate this hypothesis [31].
Risk factors for the development of metachronous VO following PJI
In our cohort, 85.7% (6/7) of the patients with subsequent metachronous VO had PJIs of the knee. A higher failure rate in the treatment of knee PJIs may be more likely to induce bacteremia and to the development of metachronous VO in high risk patients. However, the actual mechanism still needs to be elucidated through genetic analysis of the bacteria cultured from PJI, blood, and VO samples.
Clinical outcomes of metachronous VO following PJI
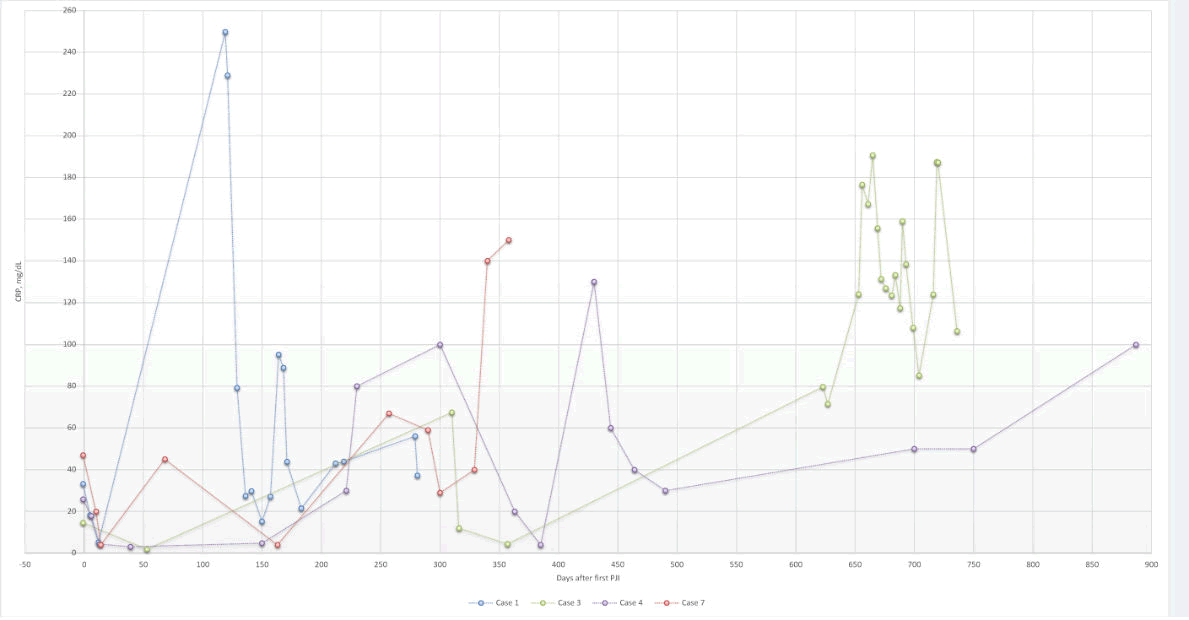
We divided patients with metachronous VO in our study into two categories: short and long interval metachronous VO. For those who developed metachronous VO one month after PJI diagnosis (short interval), bacteremia could be a possible etiology. However, most spinal tissue samples had negative cultures, which limit our ability to make inferences about possible etiologies. Negative tissue cultures might have occurred due to prolonged antibiotic therapy utilized in our PJI treatment protocols. In contrast, the clinical course of cases with long intervals could be more readily analyzed and evaluated. We noticed that these cases had recurrent PJIs during the treatment period, with fluctuating CRP levels (Figure 1). For this reason, even if pathogenic bacteria were present in such cases, it is difficult to culture these organisms given the long-term antibiotic therapy. Indeed, it was difficult to obtain conclusive tissues cultures in both short-and long-interval metachronous VO cases. Furthermore, we were unable to identify whether the causative bacteria in PJI, blood, and spine samples were identical because of the unavailability of genetic analysis during most of the period in review; we were only able to identify identical bacterial species in the PJI and spinal samples in case 7.
Limitations
This study had several limitations. First, it was a retrospective case-control study, which likely includes selection bias and missing data. We attempted to minimize bias using the same treatment protocols and rehabilitation programs. Second, clinical outcomes need to be established with longer follow-up periods.
Conclusion
The incidence of VO following PJI was 1.2% in the present study. The risk factors associated with developing VO were the presence of SIRS during PJI, drug abuse, polymicrobial PJI, and ≥ 3rd stage resection arthroplasty. Further prospective research with genetic analysis of causative pathogens is needed to determine the pathophysiology and clinical outcomes of this clinical entity.
Acknowledgements and Affiliations
We thank to Chun-Chieh Chen and Hsin-Nung Shih for their contributions of patients and Uni-edit for editing and proofreading this manuscript.
References
- Cram P, Lu X, Kates SL, Singh JA, Li Y, et al. (2012)Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries. JAMA 308:1227-1236.
- Sanders TL, Maradit Kremers H, Schleck CD, Larson DR, Berry DJ (2017) Subsequent Total Joint Arthroplasty After Primary Total Knee or Hip Arthroplasty: A 40-Year Population-Based Study. J Bone Joint Surg Am 99:396-401.
- Kapadia BH, Banerjee S, Cherian JJ, Bozic KJ, Mont MA (2016) The economic impact of periprosthetic infections after total hip arthroplasty at a specialized tertiary-care center. J Arthroplasty 31:1422-6.
- Hartzler MA, Li K, Geary MB, Odum SM, Springer BD (2020) Complications in the treatment of prosthetic joint infection when do they occur? Bone Joint J 102:145-50.
- Komnos GA, Manrique J, Goswami K, Tan TL, Restrepo C, et al. (2020) Periprosthetic Joint Infection in Patients Who Have Multiple Prostheses in Place: What Should Be Done with the Silent Prosthetic Joints. J Bone Joint Surg Am 102:1160-8.
- Jafari SM, Casper DS, Restrepo C, Zmistowski B, Parvizi J, et al. (2012) Periprosthetic joint infection: are patients with multiple prosthetic joints at risk? J Arthroplasty 27:877-80.
- Luessenhop CP, Higgins LD, Brause BD, Ranawat CS (1996) Multiple prosthetic infections after total joint arthroplasty. J Arthroplasty 11:862-868.
- Murray R, Bourne M, Fitzgerald RJ (1991) Metachronous infections in patients who have had more than one total joint arthroplasty. J Bone Joint Surg A 73:1469-1474.
- Wigren A, Karlstrom G, Kaufer H (1980) Hematogenous infection of total joint implants: a report of multiple joint infections in three patients. Clin Orthop Relat Res 152:288-291.
- Stüer C, Stoffel M, Hecker J, Ringel F, Meyer B (2013) A staged treatment algorithm for spinal infections. Journal of Neurological Surgery Part A: Central European Neurosurgery. J Neurol Surg A Cent Eur Neurosurg 74:087-95.
- J Arthroplasty (2011) Workgroup Convened by the Musculoskeletal Infection S. New definition for periprosthetic joint infection. J Arthroplasty 26:1136-1138.
- Tsukayama DT, Estrada R, Gustilo RB (1996) Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am 78:512-23.
- Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W,et al.(2013) Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1-e25.
- Eka A, Chen AF (2015) Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med 3:233.
- Tande AJ, Patel R (2014) Prosthetic joint infection. Clinical microbiology reviews. mBio 27:302-345.
- Fleege C, Wichelhaus T, Rauschmann M (2012) Systemic and local antibiotic therapy of conservative and operative treatment of spondylodiscitis. Der Orthopade 41:727-735.
- Cheung W, Luk KD (2012) Pyogenic spondylitis. Int Orthop 36:397-404.
- Nolla JM, Ariza J, Gómez-Vaquero C, Fiter J, Bermejo J, et al.(2002) Spontaneous pyogenic vertebral osteomyelitis in nondrug users. Semin Arthritis Rheum 31: 271-278
- Tomás I, Alvarez M, Limeres J, Potel C, Medina J,et al.(2007)Prevalence, duration and aetiology of bacteraemia following dental extractions. Oral Dis 13:56-62.
- Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, et al. (2018) The 2018 definition of periprosthetic hip and knee infection: an evidence based and validated criteria. Multicenter Study 33:1309 1314.
- Coburn B, Morris AM, Tomlinson G, Detsky AS (2012) Does this adult patient with suspected bacteremia require blood cultures? JAMA 308:502-511.
- Klement MR, Siddiqi A, Rock JM, Chen AF, Bolognesi MP,et al.(2018) Positive blood cultures in periprosthetic joint infection decrease rate of treatment success. J Arthroplasty 33:200-204.
- Aggarwal VK, Tischler EH, Lautenbach C, Williams GR, Abboud JA, et al.(2014) Mitigation and education. J Arthroplasty 29:19-25.
- Lentino JR (2003) Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis 36:1157-1161.
- Moran E, Masters S, Berendt A, McLardy-Smith P, Byren I, et al.(2007)Guiding empirical antibiotic therapy in orthopaedics: the microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J Infect 55:1-7.
- Zimmerli W, Trampuz A, Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351:1645-54. [Crossref]
- Flurin L, Greenwood-Quaintance KE, Patel R (2019) Microbiology of polymicrobial prosthetic joint infection. Diagn Microbiol Infect Dis94:255-9.
- Marculescu CE, Cantey JR (2008) Polymicrobial prosthetic joint infections: risk factors and outcome. Clin Orthop Relat Res 466:1397.
- Hooshmand B, Youssef D, Riederer KM, Szpunar SM, Bhargava A (2019) Clinical Outcome of Polymicrobial Prosthetic Joint Infection Managed with Debridement, Antibiotics, and Implant Retention (DAIR). Open Forum Infect Dis 6: S198-S198.
- Huotari K, Peltola M, Jämsen E (2015) The incidence of late prosthetic joint infections: a registry-based study of 112,708 primary hip and knee replacements. Acta Orthop 86:321-325.
- Zajonz D, Wuthe L, Tiepolt S, Brandmeier P, Prietzel T, et al.(2015) Diagnostic work-up strategy for periprosthetic joint infections after total hip and knee arthroplasty: a 12-year experience on 320 consecutive cases. Patient safety in surgery 9:1-9.
Citation: Lin YC, Luo AJ, Lee SH, Kao FC, Tai AS, et al. (2022) Risk Factors for Metachronous Vertebral Osteomyelitis after Periprosthetic Joint Infections: A Minimum Five-Years Retrospective Analysis. J Clin Infect Dis 7:153.
Copyright: © 2022 Lin YC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 2865
- [From(publication date): 0-2022 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 2410
- PDF downloads: 455