Risk Factors (Excluding Hormone Replacement Therapy) for Endometrial Hyperplasia: a Systematic Review
Received: 15-Feb-2016 / Accepted Date: 04-Mar-2016 / Published Date: 11-Mar-2016 DOI: 10.4172/2161-1165.1000229
Abstract
Objective: To conduct a systematic review of risk factors associated with the development of Endometrial Hyperplasia (EH).
Data sources: Ovid MEDLINE, EMBASE and Web of Science databases were searched from inception to 30 June 2015.
Study eligibility: Fifteen observational studies that reported on EH risk in relation to lifestyle factors (n=14), medical history (n=11), reproductive and menstrual history (n=9) and measures of socio-economic status (n=2) were identified. Pooled relative risk estimates and corresponding 95% confidence intervals (CI) were able to be derived for EH and Body Mass Index (BMI), smoking, diabetes and hypertension, using random effects models comparing high versus low categories.
Results: The pooled relative risk for EH when comparing women with the highest versus lowest BMI was 1.82 (95% CI 1.22–2.71; n=7 studies, I2=90.4%). No significant associations were observed for EH risk for smokers compared with non-smokers (RR 0.88, 95% CI 0.66-1.17; n=3, I2=0.0%), hypertensive versus normotensive women (RR 1.51, 95% CI 0.72–3.15; n=5 studies, I2=79.1%), or diabetic versus non-diabetic women (RR 1.77, 95% CI 0.79–3.96; n=5 studies, I2=31.8%) respectively although the number of included studies was limited. There were mixed reports on the relationship between age and risk of EH. Too few studies reported on other factors to reach any conclusions in relation to EH risk.
Conclusions: A high BMI was associated with an increased risk of EH, providing additional rationale for women to maintain a normal body weight. No significant associations were detected for other factors and EH risk, however relatively few studies have been conducted and few of the available studies adequately adjusted for relevant confounders. Therefore, further aetiological studies of endometrial hyperplasia are warranted.
Keywords: Endometrial hyperplasia; Endometrial cancer; Risk factors
164296Introduction
Endometrial Hyperplasia (EH) is a condition that is characterised by abnormal growth of the endometrium lining the uterus [1-3]. This condition is more prevalent among peri-menopausal and postmenopausal women [4]. While previously EH was classified into simple or complex EH, with or without atypia [2], the 2014 World Health Organisation classification simplifies this into EH without atypia and atypical hyperplasia [5]. Atypical hyperplasia is less common than other types, and results from observational studies suggest that it is the type which is more associated with the risk of progression to endometrial cancer [1-3]. The endometrial cancers which develop from EH are socalled type 1 endometrial cancers of endometrioid type [6].
The risk of progression for EH to endometrial cancer has been reported from a large population-based nested case-control study including 7,947 enrolees at a prepaid health plan in the USA. In that study, atypical EH was associated with a 14-fold increased risk of endometrial cancer, while the risk of progression for simple EH and complex (non-atypical) EH were significantly lower [7].
Given the potential for neoplastic progression, treatment options for EH include hysterectomy, and hormonal therapies; occasionally ‘watchful waiting’ is adopted for EH without atypia [8]. The need for such interventions, and potential psychological distress for women following an EH diagnosis [9]. highlight the importance of preventing EH where possible. Identification of modifiable risk factors for EH would enable women to make lifestyle changes that could reduce risk of this condition, and subsequent cancer risk [10]. EH, especially EH without atypia, develops as a consequence of excessive or prolonged exposure to oestrogen [11-13], and an imbalance between oestrogen and progesterone levels which usually occur as a result of insufficient progesterone in comparison with oestrogen level in a woman’s system [13], For premenopausal women, the balance between these hormones changes during a woman’s menstrual cycle each month. After menopause, the ovaries stop producing these hormones, but a small amount of oestrogen can be synthesized from androgen by the enzyme aromatase [14]. Given the predominant role of hormones in the development of EH, a Cochrane review on hormone therapy in postmenopausal women which included 45 trials and 38,702 participants found that unopposed oestrogen is associated with an increased risk of EH with relative risks of 3.20 (95% CI 2.02 – 5.26) and 10.09 (95% CI 4.90 – 20.80) for moderate and high doses of oestrogen respectively, although this increased risk was not observed with low doses of hormone replacement therapy (HRT) use [13].
Similar to known risk factors for endometrial cancer [15], it is possible that demographic and modifiable factors such as age, parity, oral contraceptive use, body fatness, physical activity, smoking and co-morbidities may play an aetiological role in the development of EH [1,12,16]. The aim of this systematic review and meta-analyses is to quantify the association between risk factors (excluding HRT, since this is incorporated in a Cochrane review 13 and development of EH.
Materials and Methods
Search strategy
Three electronic databases namely MEDLINE (US National Library of Medicine, Bethesda, Maryland), EMBASE (Reed Elsevier PLC, Amsterdam, Netherlands), and Web of Science (Thomson Reuters, USA) were systematically searched from inception up to 30 June 2015 for relevant studies that included one or more keyword(s) or Medical Subject Heading from each of the following groups of terms:
(i) endometrial hyperplasia, simple endometrial hyperplasia, complex endometrial hyperplasia, complex hyperplasia with atypia, simple hyperplasia with atypia, complex atypical endometrial hyperplasia, simple atypical endometrial hyperplasia;
(ii) risk factor(s), causality, association, predisposing factor(s), predisposing factor(s), parity, obesity, history of diabetes, ethnicity, race, socio-economic status, occupation, education, oral contraceptive use, tamoxifen use, NSAID use, aspirin use, age at first birth, miscarriage history, age at menarche, alcohol, smoking, PCOS, polycystic ovarian syndrome, family history of cancer, personal history of cancer, medications, BMI, body mass index, waist circumference, body weight, diet, body fatness, waist-hip ratio, physical activity, use of fertility treatments.
Review articles and animal studies were excluded and no language restriction was applied.
Data extraction
Titles and abstracts for potentially relevant articles were independently screened by two of three reviewers (OS, LM and HC). Then two reviewers (OS and HC) independently screened full text articles for the remaining studies to identify relevant studies that meet the pre-set inclusion criteria for the systematic review:
(i) Participants: Women aged 18 and above.
(ii) Interventions: Measurement of risk factors (excluding HRT) in the study population.
(iii) Comparators: Women without a diagnosis of EH.
(iv) Outcome: Risk of EH.
The full text of the remaining articles were independently screened by two reviewers (OS and HC) to identify relevant studies that meet the pre-set inclusion criteria for the systematic review. Articles reporting on less than 10 cases of EH were excluded from the review. Meetings were held between three reviewers (LJ, HC, OS) to resolve any discrepancies. The full protocol for this review can be found at https://www.crd. york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015016569#. VRfpRJMe5ME. Relevant information about study design, number of cases, controls or cohort size, menopausal status of the study population, age, and method used to diagnose EH, control definition, method used to measure exposure and adjusted confounders were extracted from full text articles. The Newcastle Ottawa Scale coding manual was used to assess quality of each study. Some studies reported EH risk as part of a combined EH and endometrial cancer risk estimate, and were retained for inclusion in the systematic review, and sensitivity analysis conducted removing such studies from overall pooled estimates. Studies that compared risk between different types of EH, and not in comparison with a true control group that did not have EH, were excluded. Attempts were made to retrieve additional information where required from a number of authors via e-mail contact [17-22].
Statistical analysis
Statistical analyses were conducted with STATA version 13 (StataCorp, College Station, TX, USA). Unadjusted and maximally adjusted relative risk (RR) estimates and corresponding 95% confidence intervals (CI) were extracted from published articles where possible. Random-effects models were used to derive pooled RRs [23] and CI. It was decided a priori to perform meta-analyses where at least three studies had reported risk estimates for a particular risk factor. When applying these criteria we were able to conduct meta-analyses of EH risk comparing high versus low for body mass index (BMI), smoking, hypertension, and diabetes.
Sensitivity analyses were conducted for EH risk in relation to BMI and diabetes removing individual studies; this was not possible for other risk factors as too few studies reported these. Sub-group analysis was performed where possible for EH with or without atypia in relation to BMI, diabetes and hypertension. We also assessed heterogeneity of studies included in meta-analyses using the I2 statistic [24,25]; I2 values of 25%, 50% and 75% are typically interpreted as low, moderate and high heterogeneity respectively. We investigated the likelihood of publication bias using the Egger’s test [26,27]. Combined RR were calculated before entry into final meta-analyses for studies that reported separate EH risk estimates only by different types of EH or different age categories. Specifically, one study reported separate EH risk estimates for complex EH and atypical EH [28].
Results
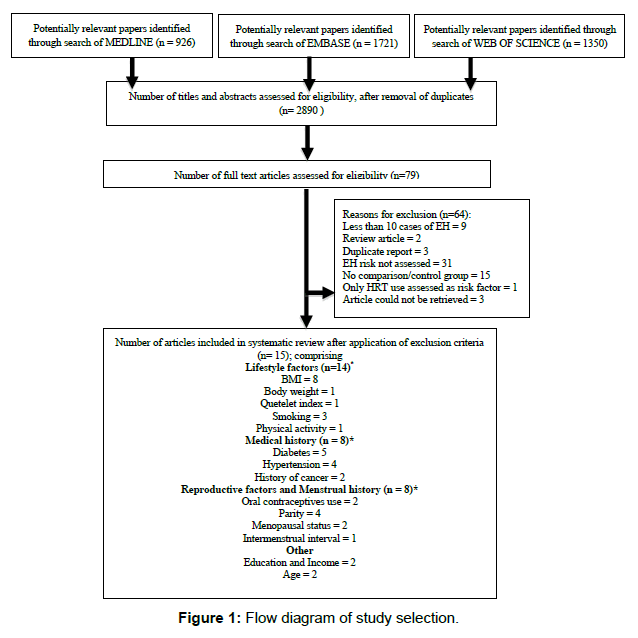
After application of our search strategy in the three databases, and removal of duplicates, a total of 2,890 titles and abstracts were reviewed in the first instance to determine potentially relevant studies for inclusion. After title and abstract review, 79 full text articles and abstracts were reviewed, and 15 full text articles were retained in the review (Figure 1). Included articles assessed lifestyle factors, menstrual history, age, medical history, reproductive history and socio-economic factors and their relationship with risk of developing EH.
Lifestyle factors
BMI
Eight studies examined BMI and risk of EH [12,28-34]. Four were case-control studies [12,28,30,32], two were cohort studies [31,34] and two were cross-sectional studies [29,33]. Characteristics of these studies are fully described in Table 1.
| Author, Year, Location | Study design | No.Cases | No.Controls/cohort size | Menopausal status | Age, years (range)Cases/ Controls | Case definition including EH type | Method of diagnosis | Control definition | Method of measuring body fatness | Adjusted confounders | Quality scale score(max. 9) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Body Mass Index | |||||||||||
| Balbi et al. (2012) [29], Italy | Hospital-based case-control | 167 | 282 | Pre-menopausal | 40-55 | Simple EH | Confirmed by 1 pathologist | Women attending gynaecologic unit of 2 hospitals for menstrual irregularities | Interview, medical history, general physical examination | Age, hypertension, diabetes, physical activity | 7 |
| Cheung et al. (2001)[30], Canada | Case-control | 45 | 36 | Pre-menopausal | 23-41/21-39 | Simple or complex EH with or without atypia | Histologically confirmed by pathologist | Consecutive women with PCOS and infertility due to anovulation | Not reported | Age, endometrial thickness, average inter-menstrual interval, menses biopsy interval, last OC use. | 5 |
| Cymbaluk et al.(2006) [31],Poland | Hospital-based case-control | 14 | 46 | Post -menopausal | 56.3/54.8[1] | EH with atypia | Curettage or hysteroscopy | Obese postmenopausal women referred for post-menopausal bleeding | Not reported | Not reported | 4 |
| Epplein et al.(2008) [27], USA | Population-based case-control | 45 | 446 | Pre- and post-menopausal | ≥ 18 | Complex EH or EH with atypia | Histologically confirmed by 3 pathologists | Randomly selected from the same health plan as cases | Not reported | Menopausal status, parity | 7 |
| Ricci et al.(2002)[12]Italy | Hospital-based case-control | 129 | 258 | Pre- and post- menopausal | 35-74 | Complex EH | Histologically confirmed | Non hysterectomized women selected form hospitals covering the same area as cases | Self-reported, Questionnaire | Age, education | 5 |
| Shan et al. (2014) [28] China | Cross-sectional | 194 | 39 | Pre-menopausal | 18-35 | Simple or complex EH with or without atypia | Histologically confirmed by at least 2 pathologists | Not reported | Height and weight measured to calculate BMI | Pregnancy, severe infection, CVD, breast cancer, reproductive cancers , HRT use, age, menopause | 7 |
| Topcu et al.(2014)[33], Turkey | Retrospective cohort | 13 | 203 | Pre- and postmenopausal | 41-69/ 40-84 | EH with or without atypia | Histologically confirmed | Not reported | Not reported | Not reported | 7 |
| Viola et al.(2007)[32], Brazil | Cross-sectional | 10 | 177 | Pre- and postmenopausal | 18-70 | Simple or complex EH with or without atypia | Not reported | Not reported | Weight and height measured to calculate BMI | Steroid hormone use, tamoxifen use, history of ovarian or endometrial tumour, history of endometriosis | 6 |
| Quetelet Index | |||||||||||
| Kreiger et al.(1986)[35], Canada | Population-based case-control | 149 | 248 | Pre- and post-menopausal | 40-74 | Adenomatous hyperplasia | Confirmed by 3 pathologists | Randomly selected from same neighbourhood as cases | Not reported | Menopausal status | 6 |
| Body weight | |||||||||||
| Farquhar et al. (1999)[34], New Zealand | Retrospective cohort | 46 | 1033 | Pre-menopausal | 17-50 | Simple or complex EH with or without atypia | Histologically confirmed by pathologist | Not reported | Patient record review | Not reported | 4 |
Table 1:Characteristics of studies included in the systematic review of Endometrial Hyperplasia and risk factor: body fatness.
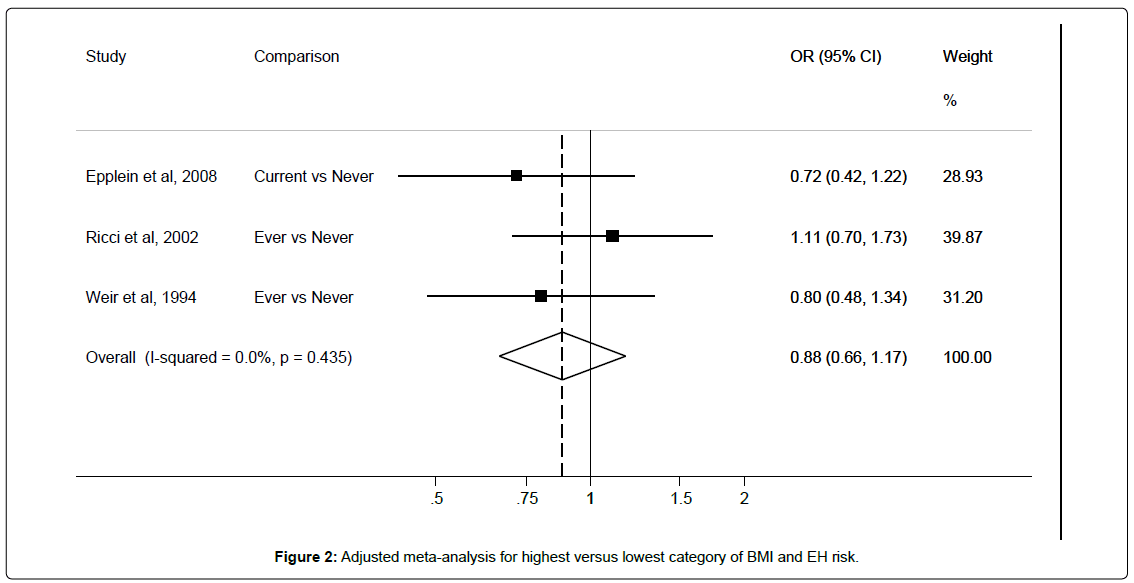
Six studies [12,28,29,32-34] provided or allowed unadjusted RRs to be calculated, and the pooled RR for EH when comparing women with the highest versus lowest BMI was 1.84 (95%CI: 1.18-2.88) with I2 62.9%. Results from seven studies [12,28-31,33,34] were pooled to derive maximally adjusted EH risk estimates for the highest versus the lowest category of BMI. As shown in Figure 2, high BMI was significantly associated with an increased EH risk (RR 1.82, 95%CI: 1.22-2.71) with an I2 of 90.4%. Egger’s test showed no significant evidence of publication bias (p=0.18). Heterogeneity remained consistently high after removal of individual studies, this may be due to the variability of adjusted confounders across studies (Table 2). Only the exclusion of Balbi et al. [30] reduced heterogeneity somewhat and markedly affected results. This study investigated simple EH risk only, and sub-group analysis between BMI and EH without atypia resulted in a non-significant positive association (RR 1.27, 95%CI 0.49-3.59) (Table 3).
| Number of studies included | References | Pooled risk estimate (95% CI) | I-squared (%) | p-value | |
|---|---|---|---|---|---|
| Body mass index | |||||
| Unadjusted | 6 | (12,27-30,32) | 1.84 (1.18 – 2.88) | 62.9 | 0.02 |
| Adjusted, excluding Balbi et al. 2012 | 6 | (12,27,28,30,32,33) | 1.20 (0.97 – 1.49) | 60.8 | 0.03 |
| Adjusted, excluding Ricci et al. 2002 | 6 | (27-30,32,33) | 1.88 (1.21 – 2.93) | 91.9 | 0 |
| Adjusted, excluding Epplein et al. 2008 | 6 | (12,28-30,32,33) | 1.78 (1.16 – 2.74) | 91.6 | 0 |
| Adjusted, excluding Cheung et al. 2001 | 6 | (12,27-29,32,32) | 2.29 (1.10 – 4.74) | 88.6 | 0 |
| Adjusted, excluding Shan et al. 2014 | 6 | (12,27,29,30,32,33) | 2.21 (0.96 – 5.07) | 91.8 | 0 |
| Adjusted, excluding Viola et al. 2007 | 6 | (12,27-30,33) | 1.78 (1.19 – 2.67) | 91.9 | 0 |
| Adjusted excluding Topcu et al. 2014 | 6 | (12,27-30,32) | 1.80 (1.19 – 2.71) | 91.9 | 0 |
| EH without atypia only | 3 | (12,27,28) | 1.27 (0.49 – 3.59) | 0 | 0.9 |
| Smoking | |||||
| Unadjusted | 3 | (12,27,36) | 0.98 (0.64 – 1.49) | 45.7 | 0.16 |
| Diabetes | |||||
| Unadjusted | 3 | (12,27,38) | 1.43 (0.79 – 2.57) | 0 | 0.96 |
| Adjusted, excluding Ricci et al. 2002 | 4 | (27,29,33,38) | 1.48 (0.47 – 4.64) | 46.3 | 0.13 |
| Adjusted, excluding Balbi et al. 2012 | 4 | (12,27,33,38) | 1.89 (0.82 – 4.37) | 40.6 | 0.17 |
| Adjusted, excluding Epplein et al. 2008 | 4 | (12,29,33,38) | 2.31 (1.10 – 4.85) | 11.6 | 0.34 |
| Adjusted, excluding Gol et al. 2001 | 4 | (12,27,29,33) | 1.89 (0.64 – 5.52) | 43.8 | 0.15 |
| Adjusted, excluding Topcu et al. 2014 | 4 | (12,27,29,38) | 1.37 (0.66 – 2.82) | 0 | 0.43 |
| EH without atypia only | 3 | (12,27,38) | 1.32 (0.31 – 5.70) | 0 | 0.78 |
| Hypertension | |||||
| Unadjusted | 3 | (12,27,39) | 1.33 (0.76 – 2.30) | 68.5 | 0.04 |
| Atypical EH only | 3 | (27,38,39) | 1.92 (0.57 – 6.53) | 70.3 | 0.04 |
| EH without atypia only | 3 | (12,27,38) | 1.17 (0.39 – 3.45) | 0 | 0.96 |
Table 2: Summary of unadjusted, subgroup and sensitivity analyses excluding individual studies from meta-analyses.
| Risk factor | No. of studies | References | Study design | Summary of results |
|---|---|---|---|---|
| Body weight | 1 | 34 | Retrospective cohort | n=1 study reported increased risk of complex EH with atypia amongst women weighing ≥90kg when compared with women weighing <90kg. |
| Quetelet index | 1 | 35 | Population-based case-control | n=1 study reported significant higher waist-hip ratio in EH cases when compared with controls. |
| Physical activity | 1 | 29 | Hospital-based case-control | n=1 study reported non-significant increased risk of EH amongst women who reported higher levels of physical activity. |
| History of cancer | 2 | 12,34 | 1 hospital-based case-control, 1 retrospective cohort study |
n=1 study reported non-significant 20% reduced risk of EH amongst women with a family history of EC. n=1 study reported significant increased risk of EH amongst women with a family history of EC or colon cancer |
| Oral contraceptive use | 2 | 12,27 | 1 population-based case-control, 1 hospital-based case-control | n=1 study reported a reduced risk of complex and atypical EH amongst women used OC 6months prior to abnormal vaginal bleeding. n=1 study reported non-significant increased risk of EH amongst women who had ever used OC compared with never-users. |
| Parity | 2 | 12,27 | 1 population-based case-control, 1 hospital-based case-control | n=1 study reported significant reduced risk of EH amongst women who had given birth to 3 or more children in comparison with women who had never given birth. n=1 study reported non-significant increase in risk of EH amongst women who had given birth to 2 or more children in comparison with nulliparous women. |
| Nulliparity | 2 | 34,37 | 1 hospital-based case-control, 1 retrospective cohort study | n=1 study reported significant increased risk of EH or EC amongst nulliparous women in comparison with multiparous women. n=1 study reported significant increased risk of EH amongst nulliparous women in comparison with multiparous women |
| Menopausal status | 2 | 12,28 | 1 hospital-based case-control, 1 cross-sectional study | n=1 study reported a non-significant reduced risk of non-atypical EH among post-menopausal women when compared with pre-menopausal women but non-significant increased risk of atypical EH was reported for postmenopausal women compared with pre-menopausal women. n=1 study reported a significant reduced risk of complex amongst post-menopausal women in comparison with pre- and peri-menopausal women. |
| Education and Income | 2 | 12,35 | 1 hospital-based case-control, 1 population-based case-control study | n=1 study reported higher level of education amongst EH cases compared with controls. n=1 study reported higher income for EH cases compared with controls. |
| Age | 2 | 12,37 | 2 hospital-based case-control studies | n=1 study reported significant increased risk of EH or EC amongst women ≥70 years compared with women 49-59 years old. n=1 study reported non-significant reduced risk of EH amongst women ≥65years in comparison with women <45years old. |
Table 3: Summary of results for risk factors for EH for which meta-analyses were not possible.
Other body fatness measures
Two studies have investigated other body fatness measures and EH risk, as summarised in Tables 1 and
One study among premenopausal women with abnormal menstrual bleeding reported a significant 7–fold increased risk for complex atypical EH and endometrial carcinoma combined (OR 7.3, 95%CI 3.2–16.8), comparing body weight >90kg versus <90kg [35].
In a further study, the Quetelet index was reported to be significantly higher in postmenopausal EH cases, compared with controls, leading to an increased risk (OR 3.8, 95%CI 1.27–11.40) when comparing >2.9 versus ≤2.9. In contrast, a protective effect for premenopausal women was noted (OR 0.25, 95% CI 0.07–0.95) [36].
Smoking
Two population-based [28,37,38] and one hospital-based 12 casecontrol studies examined the relationship between smoking and risk of EH. Descriptions of study characteristics are shown in Table 4.
| Author, Year, Location | Study design | No. Cases | No. Controls/cohort size | Menopausal status | Age, years (range) Cases/ Controls | Case definition including EH type | Method of diagnosis | Control definition | Method of measuring body fatness | Adjusted confounders | Quality scale score (max. 9) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoking | |||||||||||
| Epplein et al.(2008)[27], USA | Population-based case-control | 45 | 446 | Pre- and post-menopausal | ≥ 18 | Complex EH or EH with atypia | Histologically confirmed by 3 pathologists | Randomly selected from the same health plan as cases | Medical record review | Menopausal status, BMI, parity | 7 |
| Ricci et al.(2002)[12], Italy | Hospital-based case-control | 129 | 258 | Pre- and post- menopausal | 35-74 | Complex EH | Histologically confirmed | Non hysterectomized women selected form hospitals covering the same area as cases | Self-reported, Questionnaire | Age, education | 5 |
| Weir et al.(1994)[36], Canada | Population-based case-control | 177 | 530 | Pre- and post-menopausal | 40-74 | Adenomatous hyperplasia | Histologically confirmed by 3 pathologists | Randomly selected from same neighbourhood as cases | Interview | Age, obesity, oestrogen use | 6 |
| Physical activity | |||||||||||
| Balbi et al.(2012)[29], Italy | Hospital-based case-control | 167 | 282 | Pre-menopausal | 40-55 | Simple EH | Confirmed by 1 pathologist | Women attending gynaecologic unit of 2 hospitals for menstrual irregularities | Interview, medical history, general physical examination | Age, hypertension, BMI, diabetes | 7 |
Table 4: Characteristics of studies included in the systematic review of Endometrial Hyperplasia and risk factor: smoking and physical activity.
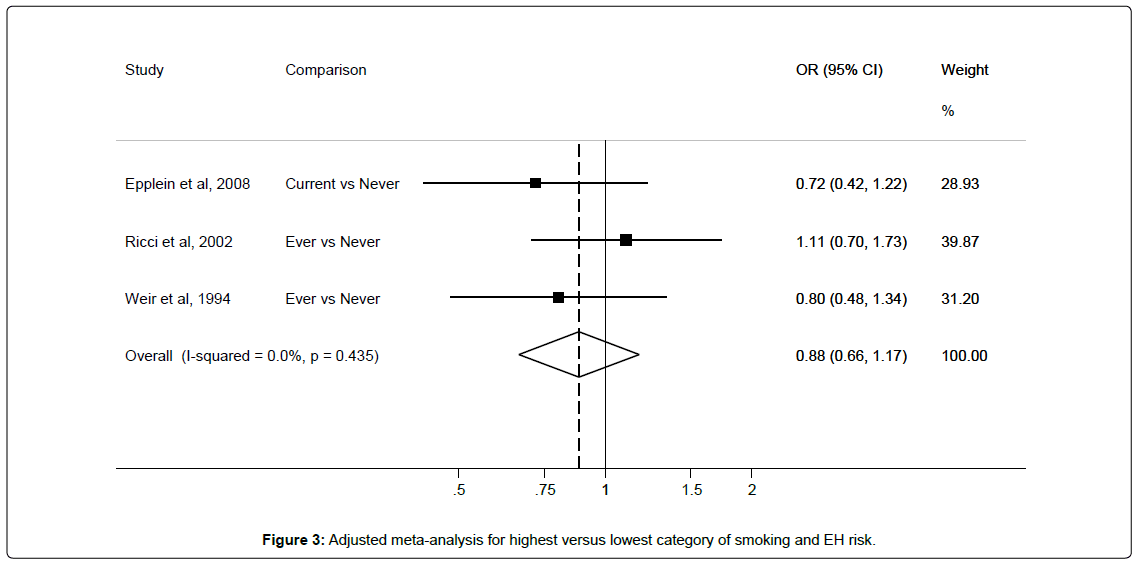
Results from these three studies were pooled to derive an unadjusted EH risk estimate of 0.98 (95%CI 0.64-1.49) with I2 45.7% for smokers compared with non-smokers. Adjusted pooled risk estimate was 0.88 (95%CI 0.66-1.17) with 0% heterogeneity for smokers when compared with non-smokers (Figure 3).
The moderate heterogeneity among studies disappeared after adjusting for confounders. However, only one study adjusted for HRT, and two adjusted for BMI as shown in Table 4.
Physical activity
One Italian hospital-based case-control study 30 reported nonsignificant increased risk of EH (OR 1.38 95%CI 0.50–3.77) among women who reported high levels of physical activity (≥60 minutes 3 times/week) when compared with those who reported lower levels of physical activity (Tables 1 and 4).
Medical history
Diabetes
Five studies evaluated the relationship between diabetes and EH risk. Three were hospital-based case-control [12,30,34], one was a population-based case-control,28 and one was a prospective cohort study [39]. Characteristics of the studies are shown in Table 5.
| Author, Year, Location | Study design | No.Cases | No.Controls/cohort size | Menopausal status | Age, years (range)Cases/ Controls | Case definition including EH type | Method of diagnosis | Control definition | Method of measuring medical history | Adjusted confounders | Quality scale score(max. 9) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes | |||||||||||
| Balbi et al.(2012)[29], Italy | Hospital-based case-control | 167 | 282 | Pre-menopausal | 40-55 | Simple EH | Confirmed by 1 pathologist | Women attending gynaecologic unit of 2 hospitals for menstrual irregularities | Interview, medical history, general physical examination | Age, BMI, physical activity | 7 |
| Epplein et al.27 (2008)[27], USA | Population-based case-control | 45 | 446 | Pre- and post-menopausal | ≥ 18 | Complex EH or EH with atypia | Histologically confirmed by 3 pathologists | Randomly selected from the same health plan as cases | Medical record review | Menopausal status, BMI, parity | 7 |
| Gol et al.(2001)[38], Turkey | Cohort | 30 | 556 | Post-menopausal | 52.5±6.6 | EH with or without atypia | Confirmed by pathologist | Not reported | Not reported | Not reported | 5 |
| Ricci et al.(2002)[12], Italy | Hospital-based case-control | 129 | 258 | Pre- and post- menopausal | 35-74 | Complex EH | Histologically confirmed | Non hysterectomized women selected form hospitals covering the same area as cases | Self-reported, Questionnaire | Age, education | 5 |
| Topcu et al.(2014)[33], Turkey | Retrospective cohort | 13 | 203 | Pre- and postmenopausal | 41-69/ 40-84 | EH with or without atypia | Histologically confirmed | Not reported | Not reported | Not reported | 7 |
| Hypertension | |||||||||||
| Balbi et al.(2012)[29], Italy | Hospital-based case-control | 167 | 282 | Pre-menopausal | 40-55 | Simple EH | Confirmed by 1 pathologist | Women attending gynaecologic unit of 2 hospitals for menstrual irregularities | Interview, medical history, general physical examination | Age, BMI, physical activity | 7 |
| Epplein et al.(2008)[27], USA | Population-based case-control | 45 | 446 | Pre- and post-menopausal | ≥ 18 | Complex EH or EH with atypia | Histologically confirmed by 3 pathologists | Randomly selected from the same health plan as cases | Medical record review | Menopausal status, BMI, parity | 7 |
| Ricci et al.(2002)[12], Italy | Hospital-based case-control | 129 | 258 | Pre- and post- menopausal | 35-74 | Complex EH | Histologically confirmed | Non hysterectomized women selected form hospitals covering the same area as cases | Self-reported, Questionnaire | Age, education | 5 |
| Vorgias et al. (2005)[39], Greece | Hospital-based case-control | 60 | 45 | Post-menopausal | 48-83 | Dilatation and curettage | Not reported | Women who underwent D&C at a tertiary cancer hospital | Blood pressure ≥150/100 mmHg, or daily use of antihypertensive medications | HRT use, obesity, tamoxifen use | 5 |
Table 5: Characteristics of studies included in the systematic review of Endometrial Hyperplasia and risk factor: medical history (diabetes, hypertension).
Three studies [12,28,38] were included in meta-analysis in order to derive unadjusted pooled EH risk estimate for diabetic versus non-diabetic women (RR 1.43, 95%CI 0.79–2.57; I2=0%). Five studies [12,28,30,34,38] were included in meta-analyses to derive adjusted pooled EH risk estimate (RR 1.77, 95%CI 0.79–3.96; I2=31.8%), as shown in Figure 4. Egger’s test showed no significant evidence of publication bias (p=0.34).
While risk estimates remained non-significant for the most part of sensitivity analyses, a significant positive association was observed when the study by Epplein et al. [28] was excluded. Heterogeneity however ranged from low to moderate throughout. Sub-group analysis by EH type showed non-significant positive association between EH without atypia and diabetes (RR 1.32, 95%CI 0.31-5.70) (Table 2). Two studies reported adjusting for BMI while none adjusted for HRT use as shown in Table 5.
Hypertension
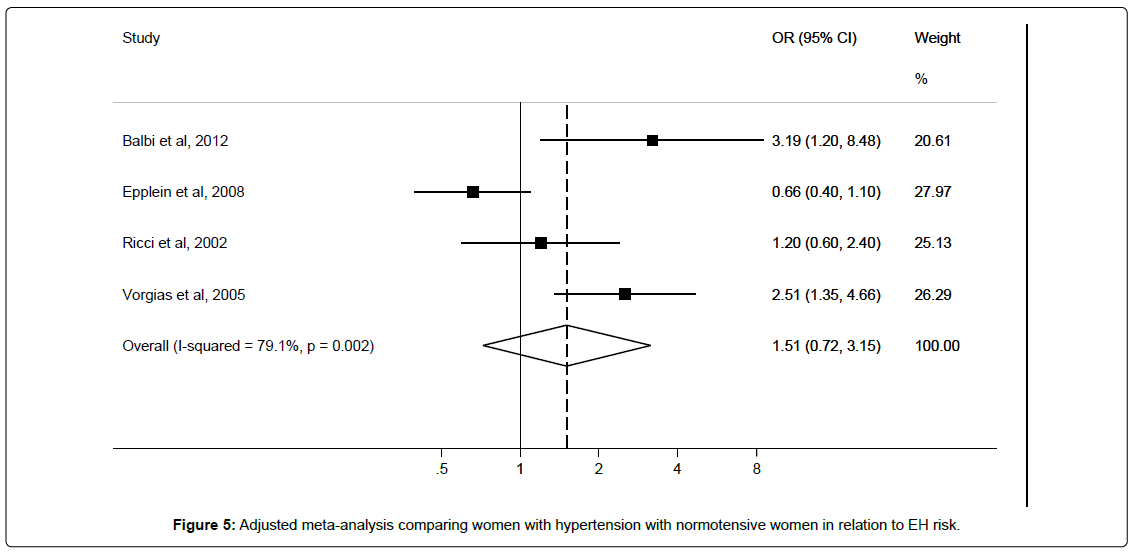
Four studies [12,28,30,39] reported on hypertension in relation to EH risk. Characteristics of the studies are shown in Table 5.
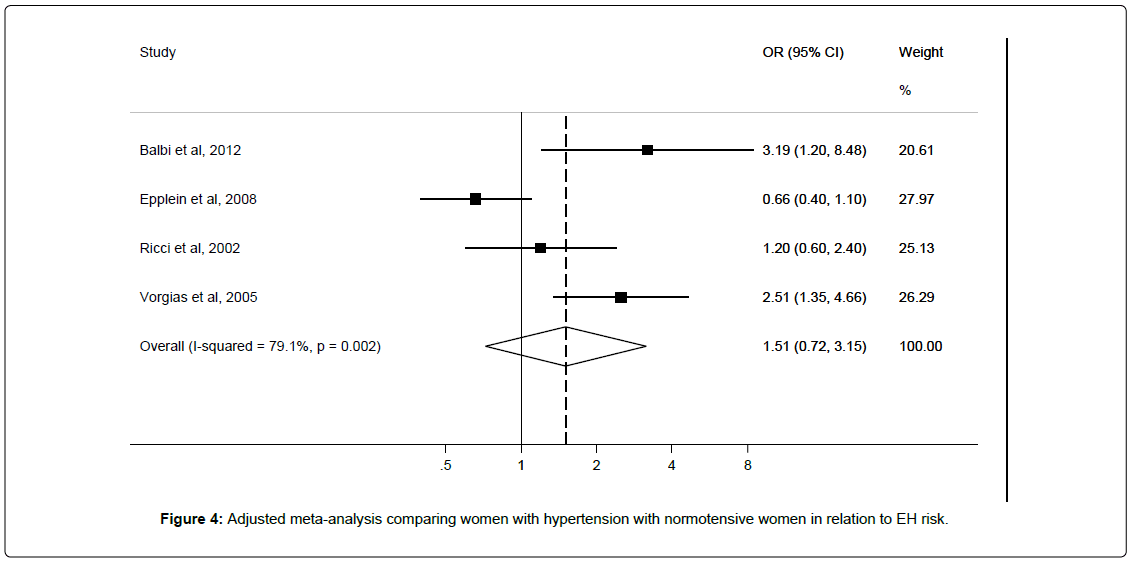
Three studies [12,28,39] were included in meta-analysis to derive an unadjusted pooled risk estimate of OR 1.33 (95%CI 0.76–2.30; I2=68.5%), and four studies [12,28,30,39] were included in meta-analysis to derive adjusted pooled risk estimate of OR 1.51 (95%CI 0.72–3.15; I2=79.1%) for hypertensive versus normotensive women (Figure 5). Egger’s test showed no significant evidence of publication bias (p=0.28).
Sub-group analyses showed non-significant positive associations between atypical EH (RR 1.92 95%CI 0.57-6.53) and EH without atypia (RR 1.17 95%CI 0.39-3.45) (Table 2) and hypertension status; only the latter showed reduced heterogeneity. Two studies adjusted for BMI while one reported adjusting for HRT as shown in Table 5.
Family History of cancer
Women with a family history of endometrial cancer were reported by Ricci et al. [12] to have around 20 percent reduced risk of developing EH , although not statistically significant (OR 0.8 95% CI 0.2 –2.6). Another study found women with abnormal bleeding who had a family history of colon cancer or endometrial cancer to be more likely to develop endometrial cancer or complex EH with atypia (OR 9.1, 95%CI 2.2 – 37.1;OR 5.8, 1.1 – 28.6, respectively) [35]. (Tables 1 and 5). None of the studies reported adjusting for BMI or HRT use as shown in Table 5.
Reproductive factors
Oral contraceptive use
Two studies [12,40] examined the relationship between oral contraceptive use and the risk of developing EH (Table 6). One population-based case-control study reported a reduced risk of complex and atypical EH among women who had used OC 6 months before presenting with abnormal bleeding (OR 0.2, 95%CI 0.0–0.6) after adjusting for BMI. However, a non-significant increased risk of EH was found in another study among women who had ever used OC versus those who had never used OC (OR 1.6, 95%CI 0.9–2.8) [12] (Table 1). The authors further assessed OC use and EH risk by duration of use and consistently found non-significant increased risks when they compared women who had used OC for more than 5 years, 13–60 months, 12 years or less with never users (OR 1.2, 95%CI 0.4–3.4;OR 1.4, 95%CI 0.5–3.6; and OR 2.0, 0.9–4.3, respectively). It should however be noted that BMI or HRT use was not adjusted for in the latter as shown in Table 6.
| Author, Year, Location | Study design | No. Cases | No. Controls/cohort size | Menopausal status | Age, years (range) Cases/ Controls | Case definition including EH type | Method of diagnosis | Control definition | Method of measuring medical history | Adjusted confounders | Quality scale score (max. 9) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reproductive factors | |||||||||||
| Epplein et al.(2009) [39], USA | Population-based case-control | 45 | 462 | Pre- and post-menopausal | ≥ 18 | Complex EH or EH with atypia | Histologically confirmed by 3 pathologists | Randomly selected from the same health plan as cases | Dispensed prescription record | Age, race, ZIP code, years enrolled in Group Health, BMI, diabetes, hypertension, smoking | 7 |
| Farquhar et al. (1999)[34], New Zealand | Retrospective cohort | 46 | 1033 | Pre-menopausal | 17-50 | Simple/complex EH with/without atypia | Histologically confirmed by pathologist | Not reported | Patients' records | Not reported | 4 |
| Feldman et al.(1995)[40], USA | Hospital-based case-control | 16 | 151 | Pre- and postmenopausal | 61.4/56.0[2] | Complex EH | Pathologic diagnosis | Women who received benign diagnosis following biopsy for abnormal vaginal bleeding | Structured questionnaire, interview | Age ≥70,prior use of oestrogen,hypertension, diabetes,menopause, nulliparity,historyof non-breast cancer,quetelet index | 5 |
| Ricci et al.(2002), [12]Italy | Hospital-based case-control | 129 | 258 | Pre- and post- menopausal | 35-74 | Complex EH | Histologically confirmed | Non hysterectomized women selected from hospitals covering the same area as cases | Self-reported, Questionnaire | Age, education | 5 |
| Menstrual history | |||||||||||
| Cheung et al. (2001)[30], Canada | Case-control | 45 | 36 | Pre-menopausal | 23-41/21-39 | Simple or complex EH with or without atypia | Histologically confirmed by pathologist | Consecutive women with PCOS and infertility due to anovulation | Not reported | Age, endometrial thickness, average inter-menstrual interval, menses biopsy interval, last OC use. | 5 |
| Ricci et al.(2002) [12], Italy | Hospital-based case-control | 129 | 258 | Pre- and post- menopausal | 35-74 | Complex EH | Histologically confirmed | Non hysterectomized women selected form hospitals covering the same area as cases | Self-reported, Questionnaire | Age, education | 5 |
| Shan et al.(2014),[28] China | Cross-sectional | 194 | 39 | Pre-menopausal | 18-35 | Simple or complex EH with or without atypia | Histologically confirmed by at least 2 pathologists | Not reported | Height and weight measured to calculate BMI | Pregnancy, severe infection, CVD, history of breast cancer, malignancies in the reproductive system, HRT use, age, BMI | 7 |
2Mean
3Investigated menopausal status and age at menopause
4Investigated menopausal status
Table 6: Characteristics of studies included in the systematic review of Endometrial Hyperplasia and risk factor: reproductive factors (oral contraceptive use and parity) and menstrual history (menopausal status and age at menopause).
Parity
When comparing women who had given birth to two or more babies with nulliparous women, Ricci et al. [12] found an almost significant 2-fold (OR 1.8, 95%CI 0.9–3.6) increase in risk of complex EH (Table 1) after adjusting for age and education [12]. In contrast, Epplein et al. [28], found significant reduced risk (OR 0.29, 95% CI 0.07–0.51) of EH among women who had given birth to three or more babies when compared with nulliparous women, after adjusting for BMI [28].
Two studies which compared nulliparous women with multiparous women found a significant increased risk of EH in nulliparous women (OR 3.7, 95%CI 1.2-10.9) 35 and (OR 2.8, 95% CI 1.3-6.1) (after adjusting for prior use of oestrogen) [41], respectively. Meta-analysis was not performed for these four studies, summarised in Table 6, due to differences in the reference groups analysed. While two studies Farquhar et al and Feldman et al used multiparous women as reference group, Epplein et al and Ricci et al. [12] used nulliparous women as reference group.
Menstrual history
Menopausal status
Two hospital-based case-control studies [12,29] evaluated menopausal status in relation to EH risk (Table 6). One study among Chinese women 29 found that postmenopausal women were less likely to develop EH without atypia (OR 0.65, 95%CI 0.17–2.50) but they were more likely to develop EH with atypia (OR 2.40, 95%CI 0.43–13.27) when compared with premenopausal women, although these estimates did not achieve statistical significance. Similarly, another study 12 also reported a significant reduced risk of complex EH among postmenopausal women in comparison with pre- and perimenopausal women (OR 0.2, 95%CI 0.1–0.5). The authors also found a non-significant 20% increased risk of complex EH among women who reported menopause at ≥53 years versus <50 years at menopause [12] (Table 1). One of the studies reported adjusting for BMI and HRT use as shown in Table 6. One further study suggested that polycystic ovarian syndrome patients with longer intermenstrual intervals have a significant increased risk of developing EH (OR 1.43, 95%CI 1.78- 1.15) after adjusting for confounders including last oral contraceptive use [31].
Other factors
Age
Two hospital-based case-control studies reported risk estimates for age with regards to EH [12,41]. Characteristics and results from these studies are shown in Tables 1 and 7 . One study reported a significant increased risk of EH or EC amongst women ≥70 years old versus women 49-59 years old after adjusting for confounders including prior use of oestrogen, and another study reported a non-significant decreased risk of EH amongst women ≥65 years old versus <45 years old. Meta– analyses were not conducted because only two studies [12,41] provided risk estimates for age in relation to development of EH, reports from the studies were mixed (Table 1). One of the studies adjusted for quetelet index, a measure of body fatness as shown in .
Socio-economic status
Two studies [12,36] examined education and income in relation to EH risk (Table 7). In one study, a positive association was observed among women who had ≥12 compared with <7 years of education (OR 2.8 95% CI 1.70–4.80).
| Author, Year, Location | Study design | No. Cases | No. Controls/cohort size | Menopausal status | Age, years (range) Cases/ Controls | Case definition including EH type | Method of diagnosis | Control definition | Method of measuring medical history | Adjusted confounders | Quality scale score (max. 9) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||||
| Feldman et al.(1995)[40], USA | Hospital-based case-control | 16 | 151 | Pre- and post-menopausal | 61.4/56.0[1] | Complex EH | Pathologic diagnosis | Women who received benign diagnosis following biopsy for abnormal vaginal bleeding | Structured questionnaire, interview | Prior use of oestrogen, hypertension, diabetes, menopause, nulliparity, history of non-breast cancer, quetelet index | 6 |
| Ricci et al.(2002)[12], Italy | Hospital-based case-control | 129 | 258 | Pre- and post- menopausal | 35-74 | Complex EH | Histologically confirmed | Non hysterectomized women selected form hospitals covering the same area as cases | Self-reported, Questionnaire | Education | 5 |
| Socio-economic status | |||||||||||
| Kreiger et al.(1986)[35], Canada | Population-based case-control | 149 | 248 | Pre- and post-menopausal | 40-74 | Adenomatous hyperplasia | Confirmed by 3 pathologists | Randomly selected from same neighbourhood as cases | Self-reported | Menopausal status | 6 |
| Ricci et al.(2002)[12], Italy | Hospital-based case-control | 129 | 258 | Pre- and post- menopausal | 35-74 | Complex EH | Histologically confirmed | Non hysterectomized women selected form hospitals covering the same area as cases | Self-reported, Questionnaire | Age | 5 |
Table 7: Characteristics of studies included in the systematic review of Endometrial Hyperplasia and risk factor: Age and Socio-economic status (income and education).
Similarly, women who earned ≥$30,000 were found to have higher chances of developing EH when they were compared with women who earned less than $30,000 in a Canadian study (Table 1). The observed association was significant for premenopausal women (OR 1.85, 95%CI 1.16 – 2.96) but not for postmenopausal women (OR 1.15, 95%CI 0.78 – 1.69) [36]. Neither of the studies reported adjusting for HRT use or BMI as shown in Table 7.
Comment
Main findings
In this novel systematic review of risk factors for EH (excluding hormone replacement therapies), meta-analyses suggested a significant positive association between increased BMI and risk of EH; no significant associations were detected between smoking, hypertension or diabetes and EH risk in pooled analyses of a limited number of studies. However, there was paucity of high quality, consistent evidence for the aforementioned and other factors in the review. There was also inadequate adjustment for relevant confounders, namely HRT and BMI, in some of the included studies.
The importance of pooling risk estimates is demonstrated by the expected finding that higher BMI is positively associated with EH compared with lower BMI, considering that only three out of six studies which reported a positive association between BMI and EH risk showed statistical significance. EH is an oestrogen-driven disease. From a biologically plausible viewpoint, it is well known that oestrogen can be synthesized from adipose tissue, this increases the level of circulating oestrogen which in turn stimulates growth of the endometrium. Reduction in high heterogeneity which occurred when the study of simple EH 30 was excluded during sensitivity analyses suggests that the relationship between BMI and EH may differ according to the presence of atypia. Due to the role of body fatness in the development of EH and endometrial cancer, it is important for women to maintain a healthy weight [15].
Pooled analysis of studies that investigated hypertension showed non-significant positive association between hypertension and EH risk. Some authors have reported that hypertension is positively associated with EH 40 or endometrial cancer [42]. However, this association was found among overweight or obese women compared with lean women [42], this observation should therefore be viewed with caution as it is likely to be confounded, considering the association between obesity and hypertension. Hypertension has previously been linked to insulin-like growth factor 1 (IGF-1), and measures of body fatness such as waist-hip ratio and obesity were reported to be higher among hypertensive patients than controls [43,44]. IGF-1 is known to be related to cell growth and cancer progression [45].
We found a non-significant increased EH risk among diabetic versus non-diabetic women. Although the mechanism for a potential association between diabetes and EH is not very clear, diabetes has been linked to IGF-1 [46]. Low levels of IGF-1were found to be positively associated with diabetes after adjusting for confounders including BMI [46]. In a rat model, Type 1 diabetes was also been shown to induce EH development, potentially mediated by oestrogen receptor alpha and p16 expression [47]. Several authors have reported overweight/obesity as one of the most important modifiable risk factors for diabetes [48,49]. Despite the well-known relationship between obesity and diabetes, few of the studies included in our meta-analysis adequately adjusted for this confounder.
Meta-analysis of three studies showed no association between tobacco smoking and risk of EH. An earlier literature review suggested that smoking has an anti-oestrogenic effect, which can reduce the rate of androgen-oestrogen conversion [50]. Smoking has also been linked to early menopause [51-53]. Women who undergo menopause early are less exposed to oestrogen than women who are older at menopause. However, smoking has been consistently linked to the development of many neoplastic conditions and is certainly not advised [54,55]. It is plausible that known carcinogenic effects of smoking may be countered by the aforementioned anti-oestrogenic effect, explaining the observed null association for tobacco smoking and EH risk.
One study reported a non-significant increased EH risk for women with self-reported higher levels of physical activity compared to those who reported lower levels of physical activity. However, as with all selfreported information of desirable lifestyle factors, this result should be interpreted with caution. Physical activity has previously been shown to be protective against endometrial carcinoma, given that physical activity may modulate metabolism, and excretion of endogenous sex hormones such as oestrogen which is also known to be responsible for development of EH [56]. Interestingly the previously described EH diabetic rat model did observe a significant reduction in oestrogenreceptor alpha and p16 expression for those rats undertaking aerobic exercise [48].
Contrasting results were reported for parity and EH risk by individual studies included in this review, although the majority reported protective effects of child-bearing for EH risk. Nulliparity is known to be associated with an increased risk of endometrial cancer [57] - a possible mechanism for this is that during pregnancy, a woman is exposed to larger amounts of progesterone as opposed to oestrogen. Contrasting reports were also observed for the two studies investigating OC use and EH risk. It should however be noted that OC usage has consistently been found to reduce EC risk among users when compared with non-users [58,59]. Biologically, this is related to the low dose of oestrogen in relation to progestin contained in OC, which inhibits endometrial proliferation [60,61].
A significant decreased risk of complex EH was reported among postmenopausal versus pre- and peri-menopausal in an Italian study. Conversely, findings from a further study included in our review suggesting an increased risk of atypical EH among postmenopausal versus premenopausal women, which may indicate that HRT use, a well-known risk factor for EH, has more of a propensity to invoke atypical than non-atypical EH. We however did not assess use of HRT in this review due to an earlier Cochrane review which assessed the effects of different hormone therapy regimens on the postmenopausal endometrium. The reviewers found unopposed oestrogen to be associated with increased risk of all types of EH at all doses, in line with the existing literature. Although the reviewers did not perform subgroup analysis for the different types of EH, they found no difference in the risk of EH in women who took low dose oestrogen combined with progestogen compared with controls who took placebo [13].
It is notable that one study reported an increased risk of complex EH in women with higher versus lower level of education while another reported the same association amongst women with higher versus lower income. Measures of social class have been implicated in the development of neoplasms due to the differing medical attention seeking behaviour of the different groups [62,63].
Finally, two studies in the review suggest a link between family history of endometrial or colon cancer in relation to EH risk. This points to a shared genetic or environmental risk factor in EH development. Families with a history of Lynch syndrome have been found to have between 1.5–3 fold increased risk of developing endometrial cancer [64]. Indeed endometrial cancer is more common than colonic cancer in patients with Lynch syndrome.
Strengths and limitations
This is the first systematic review examining the risk factors (excluding HRT use) for EH, the review has a number of strengths and limitations. A major strength of this review was the evaluation of three databases and the robust methodology and adherence to a previously published protocol. This included the strict exclusion of several studies that included simple EH cases in control groups. Although several studies that were included reported on EH combined with other outcomes such as benign endometrial polyps and/or carcinoma, we were careful to consider those separately in our interpretations, as their risk estimates would be distorted. We were also able to perform subgroup analyses for atypical and non-atypical EH in relation to BMI, diabetes and hypertension. Importantly, our collective assessment of EH risk factors has highlighted the general paucity of data available for this condition, but does suggest that EH could be potentially prevented through maintenance of normal body weight.
Limitations of this systematic review largely relate to insufficient data which would have been helpful in increasing precision of risk estimates for the different risk factors evaluated. Pooled risk estimates could not be derived for important risk factors such as age, parity and menopausal status due to insufficient number of studies (<3) providing risk estimates, in accordance with our protocol. Also, many hospitalbased studies were included, which limits applicability of results to the general population. In addition, very few studies adjusted for HRT use and BMI in their statistical models. HRT use is known to play a significant role in the development of EH, its use at all doses in the treatment of menopausal symptoms has been found to be associated with an increased risk of EH [65]. It is still plausible that additional risk factors may exist for EH and could be identified in future, high-quality studies.
The relationship between a high BMI and EH cannot be overemphasized as is shown in this review. Notably, no studies evaluated nutrition or dietary factors in relation to EH risk, even though several aspects of diet, for example coffee and high glycaemic load intake, have been associated with endometrial cancer risk [15]. We hope that this review stimulates further work in this area in an effort to identify more modifiable, preventative factors for EH.
Conclusion
In conclusion, body fatness was found to be associated with an increased risk of EH, therefore women should be encouraged to maintain a normal body weight. No significant associations were detected for other factors and EH risk. However, relatively few studies have been conducted and further aetiological studies which might help identify other non-modifiable risk factors for EH are warranted.
Funding
OS is being funded by a QUB International PhD studentship
References
- Linkov F, Edwards R, Balk J (2008) Endometrial hyperplasia, endometrial cancer and prevention: Gaps in existing research of modifiable risk factors. Eur J Cancer 44:1632-1644.
- Kurman RJ, Norris HJ (1982) Evaluation of criteria for distinguishing atypical endometrial hyperplasia from well-differentiated carcinoma. Cancer 49: 2547-2559.
- Lacey JV Jr, Ioffe OB, Ronnett BM, Rush BB, Richesson DA, et al. (2008) Endometrial carcinoma risk among women diagnosed with endometrial hyperplasia: the 34-year experience in a large health plan. Br J Cancer 98: 45-53.
- Reed SD, Newton KM, Clinton WL, Epplein M, Garcia R, et al. (2009) Incidence of endometrial hyperplasia. Am J ObstetGynecol 200: 678.
- Kurman RJ, Shih IeM (2016) Seromucinous Tumors of the Ovary. What's in a Name? Int J GynecolPathol 35: 78-81.
- Horn L, Schnurrbusch U, Bilek K, Hentschel B, Einenkel J (2004) Risk of progression in complex and atypical endometrial hyperplasia: Clinicopathologic analysis in cases with and without progestogen treatment. International Journal of Gynecological Cancer 14:348-353.
- Lacey JV Jr, Sherman ME, Rush BB, Ronnett BM, Ioffe OB, et al. (2010) Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J ClinOncol 28: 788-792.
- Ferenczy A (1983) Cytodynamics of endometrial hyperplasia and neoplasia, Part II: in vitro DNA histoautoradiography. Hum Pathol 14: 77-82.
- Stein CJ, Colditz GA (2004) Modifiable risk factors for cancer. Br J Cancer 90: 299-303.
- Reed SD, Newton KM, Garcia RL, Allison KH, Voigt LF, et al. (2010) Complex hyperplasia with and without atypia: clinical outcomes and implications of progestin therapy. ObstetGynecol 116: 365-373.
- Ricci E, Moroni S, Parazzini F, Surace M, Benzi G, et al. (2002) Risk factors for endometrial hyperplasia: results from a case-control study. Int J Gynecol Cancer 12: 257-260.
- Furness S, Roberts H, Marjoribanks J, Lethaby A, Hickey M, et al. (2009) Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. The Cochrane Library.
- Agorastos T, Vaitsi V, Pantazis K, Efstathiadis E, Vavilis D, et al. (2005) Aromatase inhibitor anastrozole for treating endometrial hyperplasia in obese postmenopausal women. European Journal of Obstetrics & Gynecology and Reproductive Biology 118:239-240.
- Endometrial cancer: A report on updated evidence for endometrial cancer.
- MacMahon B (1974) Risk factors for endometrial cancer. GynecolOncol 2: 122-129.
- Acmaz G, Aksoy H, Albayrak E (2014) Evaluation of endometrial precancerous lesions in postmenopausal obese women - A high risk group? Asian Pacific Journal of Cancer Prevention 15:195-198.
- Kacalska-Janssen O, Rajtar-Ciosek A, Zmaczynski A (2012) Higher premenopausal serum androgen levels and higher postmenopausal estrogen levels in women with endometrial hyperplasia. PrzegladMenopauzalny 11:309-318.
- Bobrowska K, Kamiński P, Cyganek A, Pietrzak B, Jabiry-Zieniewicz Z, et al. (2006) High rate of endometrial hyperplasia in renal transplanted women. Transplant Proc 38: 177-179.
- Heller DS, Mosquera C, Goldsmith LT, Cracchiolo B (2011) Body mass index of patients with endometrial hyperplasia: comparison to patients with proliferative endometrium and abnormal bleeding. J Reprod Med 56: 110-112.
- Drakontaidis A, Konstadatou A, Cumashi E, Pavlakis E, Skolarikos P (2012) Endometrial polyps and risk factors for malignancy in postmenopausal women. Maturitas71:S78.
- Gregoriou O, Konidaris S, Vrachnis N, Bakalianou K, Salakos N, et al. (2009) Clinical parameters linked with malignancy in endometrial polyps. Climacteric 12: 454-458.
- DerSimonian R, Kacker R (2007) Random-effects model for meta-analysis of clinical trials: an update. ContempClin Trials 28: 105-114.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557-560.
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539-1558.
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629-634.
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, et al. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343: d4002.
- Epplein M, Reed SD, Voigt LF, Newton KM, Holt VL, et al. (2008) Risk of complex and atypical endometrial hyperplasia in relation to anthropometric measures and reproductive history. Am J Epidemiol 168: 563-570.
- Shan W, Ning C, Luo X(2014) Hyperinsulinemia is associated with endometrial hyperplasia and disordered proliferative endometrium: A prospective cross-sectional study. GynecolOncol 132:606-610.
- Balbi G, Napolitano A, Seguino E (2012) The role of hypertension, body mass index, and serum leptin levels in patients with endometrial hyperplasia during premenopausal period. ClinExpObstetGynecol 39:321-325.
- Cheung AP (2001) Ultrasound and menstrual history in predicting endometrial hyperplasia in polycystic ovary syndrome. ObstetGynecol 98: 325-331.
- Cymbaluk A, Chudecka-Glaz A, Rzepka-Gorska I (2008) Leptin levels in serum depending on body mass index in patients with endometrial hyperplasia and cancer. European Journal of Obstetrics Gynecology and Reproductive Biology 136:74-77.
- Viola AS, Gouveia D, Andrade L, Aldrighi JM, Viola CF, et al. (2008) Prevalence of endometrial cancer and hyperplasia in non-symptomatic overweight and obese women. Aust N Z J ObstetGynaecol 48: 207-213.
- Topcu HO, Erkaya S, Guzel AI (2014) Risk factors for endometrial hyperplasia concomitant endometrial polyps in pre- and post-menopausal women. Asian Pacific journal of cancerprevention: APJCP 15:5423-5425.
- Farquhar C, Lethaby A, Sowter M, Verry J, Baranyai J (1999) An evaluation of risk factors for endometrial hyperplasia in premenopausal women with abnormal menstrual bleeding. ObstetGynecol 181:525-529.
- Kreiger N, Marrett LD, Clarke EA, Hilditch S, Woolever CA (1986) Risk factors for adenomatous endometrial hyperplasia: a case-control study. Am J Epidemiol 123: 291-301.
- Weir HK, Sloan M, Kreiger N (1994) The relationship between cigarette smoking and the risk of endometrial neoplasms. Int J Epidemiol 23: 261-266.
- Gol K, Saracoglu F, Ekici A, Sahin I (2001) Endometrial patterns and endocrinologic characteristics of asymptomatic menopausal women. Gynecological Endocrinology 15:63-67.
- Vorgias G, Strigou S, Varhalama E, Savvopoulos P, Dertimas B, et al. (2006) The effect of hypertension and anti-hypertensive drugs on endometrial thickness and pathology. European Journal of Obstetrics, Gynecology, & Reproductive Biology 125:239-242.
- Epplein M, Reed SD, Voigt LF, Newton KM, Holt VL, et al. (2009) Endometrial hyperplasia risk in relation to recent use of oral contraceptives and hormone therapy. Ann Epidemiol 19: 1-7.
- FELDMAN S, COOK E, HARLOW B (1995) Predicting endometrial cancer among older women who present with abnormal vaginal bleeding. GynecolOncol56: 376-381.
- Weiderpass E, Persson I, Adami H, Magnusson C, Lindgren A, et al. (2000) Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (sweden). Cancer Causes & Control 11:185-192.
- Galderisi M, Vitale G, Lupoli G (2001) Inverse association between free insulin-like growth factor-1 and isovolumic relaxation in arterial systemic hypertension. Hypertension 38:840-845.
- Galderisi M, Caso P, Cicala S (2002) Positive association between circulating free insulin-like growth factor–1 levels and coronary flow reserve in arterial systemic hypertension. American journal of hypertension 15:766-772.
- Nagamani M, Urban RJ (2003) Expression of messenger ribonucleic acid encoding steroidogenic enzymes in postmenopausal ovaries. J SocGynecolInvestig 10: 37-40.
- Teppala S, Shankar A (2010) Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care 33:2257-2259.
- Al-Jarrah M, Matalka I, Aseri HA, Mohtaseb A, Smirnova IV, et al. (2010) Exercise training prevents endometrial hyperplasia and biomarkers for endometrial cancer in rat model of type 1 diabetes. J Clin Med Res 2: 207-214.
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, et al. (2009) The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9: 88.
- Nguyen NT, Nguyen XT, Lane J, Wang P (2011) Relationship between obesity and diabetes in a US adult population: Findings from the national health and nutrition examination survey, 1999–2006. Obesity Surg 21:351-355.
- Tankó LB, Christiansen C (2004) An update on the antiestrogenic effect of smoking: a literature review with implications for researchers and practitioners. Menopause 11: 104-109.
- Midgette AS, Baron JA (1990) Cigarette smoking and the risk of natural menopause. Epidemiology 1: 474-480.
- Cramer DW, Barbieri RL, Muto MG, Kelly A, Brucks JP, et al. (1994) Characteristics of women with a family history of ovarian cancer. II. Follicular phase hormone levels. Cancer 74: 1318-1322.
- Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, et al. (1983) Cigarette smoking, relative weight, and menopause. Am J Epidemiol 117: 651-658.
- Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, et al. (2008) Tobacco smoking and cancer: a meta-analysis. Int J Cancer 122: 155-164.
- Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, et al. (2008) Smoking and colorectal cancer: a meta-analysis. JAMA 300: 2765-2778.
- Thune I, Furberg AS (2001) Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc 33: S530-550.
- Schonfeld SJ, Hartge P, Pfeiffer RM, Freedman DM, Greenlee RT, et al. (2013) An aggregated analysis of hormonal factors and endometrial cancer risk by parity. Cancer 119: 1393-1401.
- Benshushan A, Paltiel O, Rojansky N, Brzezinski A, Laufer N (2002) IUD use and the risk of endometrial cancer. Eur J ObstetGynecolReprodBiol 105: 166-169.
- Parslov M, Lidegaard O, Klintorp S, Pedersen B, Jønsson L, et al. (2000) Risk factors among young women with endometrial cancer: a Danish case-control study. Am J ObstetGynecol 182: 23-29.
- Anderson KM, Shah NR, Davis MA (2015) Beyond fertility: The safety of ovarian preservation in women with complex endometrial hyperplasia with atypia. GynecolOncol137:82-83.
- Archer DF, Furst K, Tipping D, Dain MP, Vandepol C (1999) A randomized comparison of continuous combined transdermal delivery of estradiol-norethindrone acetate and estradiol alone for menopause. CombiPatch study group. ObstetGynecol 94:498-503.
- Kelsey JL, LiVolsi VA, Holford TR, Fischer DB, Mostow ED, et al. (1982) A case-control study of cancer of the endometrium. Am J Epidemiol 116: 333-342.
- Elwood JM, Cole P, Rothman KJ, Kaplan SD (1977) Epidemiology of endometrial cancer. J Natl Cancer Inst 59: 1055-1060.
- Parazzini F, La Vecchia C, Moroni S, Chatenoud L, Ricci E (1994) Family history and the risk of endometrial cancer. Int J Cancer 59: 460-462.
- Gruber SB, Thompson WD (1996) A population-based study of endometrial cancer and familial risk in younger women. cancer and steroid hormone study group. Cancer Epidemiol Biomarkers Prev 5:411-417.
Citation: Sanni OB, Kunzmann AT, Murray LJ, McCluggage WG, Coleman HG (2016) Risk Factors (Excluding Hormone Replacement Therapy) for Endometrial Hyperplasia: A Systematic Review. Epidemiol 6:229. DOI: 10.4172/2161-1165.1000229
Copyright: © 2016 Sanni OB et al., This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11040
- [From(publication date): 4-2016 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 10149
- PDF downloads: 891