Risk Assessment for Pharmaceuticals
Received: 26-Oct-2017 / Accepted Date: 30-Dec-2017 / Published Date: 06-Jan-2018
Keywords: Occupational exposure limits; Acceptable daily exposure; Permitted daily exposure; Risk assessment
Editorial
Manufacturing of active pharmaceutical ingredients (APIs) and products in pharmaceutical industry requires qualitative and/or quantitative health based risk assessment for occupational (workers involved) and product (relevant patient population) safety purposes [1]. Health based risk assessments are especially needed for potent compounds considering their potentially serious toxicity profiles. Appropriate application of health-based risk assessment principles can contribute in determining adequacy of controls, work practices and measures for worker and patient safety.
Application of quantitative occupational health-based risk assessment involves calculation of Occupational Exposure Limits (OELs) which permits to quantitatively assess worker exposure potential by monitoring of industrial air hygiene [2]. Alternatively, qualitative health-based risk assessment comprises categorization (“banding”) of API based on their toxicity and potency, which provides sufficient information about the relative hazard to decide applicable safety measures [3]. Occupational health categorization is then linked to task-specific safe handling practices for worker protection purposes which involves use of personnel protective equipment (PPEs) or especially designed/separate facilities in particular cases.
From a product safety perspective, it is essential in multi-purpose or shared manufacturing facilities to effectively clean product contact surfaces thus preventing cross-contamination of one product to the next. Scientifically justifiable health-based risk assessment approach is employed to establish an Acceptable Daily Exposure (ADE) for determining the amount of carryover of one material into next product. ADE or PDE (Permitted Daily Exposure) are synonymous terms and used by different regulatory authorities with similar purpose [4]. ADE/ PDE define limits at which a patient may be exposed daily for a lifetime with acceptable risks related to adverse health effects. ADE for product cross-contamination protection is then employed by the Quality Assurance department to establish a cleaning limit for the API and to establish quantitative objectives for analytical estimations. ADEs are also used to decide if dedicated facility or infrastructure is required for a particular API if the safe carryover value is below the cleaning capabilities of the facility [4-7].
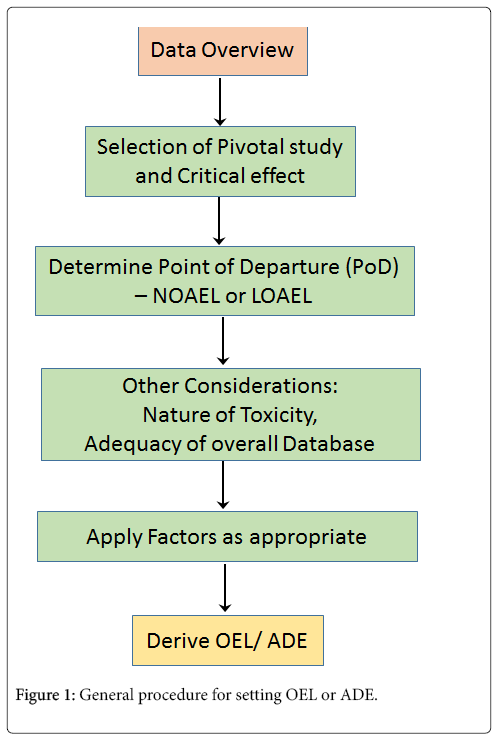
OEL and ADE determination procedure is almost similar because both require evaluation and interpretation of available/applicable pharmacological, toxicological and clinical data, selection of suitable pivotal studies/critical effect, determining point of departure (PoD) to estimate NOAEL (No Observed Adverse Effect Level) or LOAEL (Lowest Observed Adverse Effect Level), and extrapolation to acceptable levels from these studies using appropriate factors [5,8,9].
The general procedure for OEL/ADE determination is shown in Figure 1. In cases of unavailability of required pharmacological or toxicological data viz. for raw materials, starting materials, intermediates or API’s in early phase clinical trials, Quantitative Structure Activity Relationship (QSAR) and data from surrogate structures should be used to perform health-based risk assessment by toxicologists.
The most common approach used for calculation of OEL is NOEL/SF (No Observed Effect Level/ Safety Factor) approach. In this approach, a NOEL for the most sensitive effect is identified (Point of Departure) and modified by appropriate safety factors to accommodate for uncertainties and data gaps. This approach presumes that both carcinogenic and noncarcinogenic effects do not occur if exposure is kept below the NOEL. For genotoxic active substances with non-threshold mechanism of action, any level of exposure carries a risk. An acceptable level for non-threshold related genotoxicants has been established as Threshold of Toxicological Concern (TTC) of 1.5 μg/person/day, which is associated with a theoretical cancer risk of 1 additional cancer in 100,000 patients when exposed over a life time [10]. When additional information about mechanism of action of carcinogens became available, it became apparent that NOELs may exist for some carcinogens. This resulted in development of the concept of benchmark dose approach. In addition to the approaches described so far, several other approaches have been used to set OELs. For example, use of the therapeutic dose and the use of an incremental increase in some level of endogenous biologic activity for hormones [11]. In the case where OELs can be estimated by several approaches, one has to judge the most appropriate approach especially if the various approaches result in OELs that differ significantly [11,12].
The duty of toxicologist entrusted with setting OELs/ADEs is to derive a value that is safe for workers/patients yet without reaching so overly protective values that resources are unreasonably consumed.
References
- Ader AW, Kimmel TA, Sussman RG (2009) Applying health-based risk assessments to worker and product safety for potent pharmaceuticals in contract manufacturing operations. American Pharmaceutical Outsourcing, pp:48-53.
- Health and Safety Commission (2002)Discussion document on Occupational Exposure Limits (OEL) framework. Health and Safety Commission, UK.
- The Association of the British Pharmaceutical Industry (1995) Guidance on setting in-house occupational exposure limits for airborne therapeutic substances and their intermediates. ABPI, UK.
- Active Pharmaceutical Ingredients Committee (APIC)(2016) Guidance on aspects of cleaning validation in active pharmaceutical ingredient plants.
- European Medicines Agency (2014) Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities. 5.   European Medicines Agency, London, UK.
- Hayes EP, Jolly RA, Faria E, Barle EL, Bercu J, et al. (2016) A harmonization effort for acceptable daily exposure application to pharmaceutical manufacturing – Operational considerations. RegulToxicolPharmacol 79: S39-47.
- Sargent EV, Faria E, Pfister T, Sussman RG (2013) Guidance on the establishment of acceptable daily exposure limits (ADE) to support Risk-Based manufacture of pharmaceutical products. RegulToxicolPharmacol. 65: 242-250.
- Dankovic DA, Naumann BD, Maier A, Dourson ML, Levy LS (2015) The scientific basis of uncertainty factors used in setting Occupational Exposure Limits. J Occup Environ Hyg 12: S55-S68.
- European Medicines Agency (2006) Guideline on the limits of Genotoxic Impurities. CHMP, EMEA, London.
- Ku RH (2000) An overview of setting occupational exposure limits (OELs) for pharmaceuticals. Chemical Health and Safety 7: 34-37.
- Scientific Committee on Occupational Exposure Limits (SCOEL) (2013) Methodology for the derivation of Occupational Exposure Limits. Version 7.
Citation: Ahuja V (2018) Risk Assessment for Pharmaceuticals. World J Pharmacol Toxicol 1: e101.
Copyright: © 2018 Ahuja V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 6334
- [From(publication date): 0-2018 - Apr 06, 2025]
- Breakdown by view type
- HTML page views: 5342
- PDF downloads: 992