Rift Valley Fever in Chronic Carrier and Liver Manifestations
Received: 17-Feb-2018 / Accepted Date: 19-Mar-2018 / Published Date: 25-Mar-2018 DOI: 10.4172/2157-2526.1000161
Abstract
Background: Rift Valley Fever virus (RVFV) causes certain type of hemorrhagic fever. In Egypt, during September 2017, RVFV was detected in samples from imported cattle (country of origin: the Sudan). Samples were collected from imported cattle from different localities in Cairo during October, November, December 2017.
Material and methods: RVFV isolation in Swiss mice (6 days old) was by intra-peritoneal inoculation (I/P) with tissue homogenates, positive results were seen in second passages. Detection of RVFV antigen in samples was done by Agar Gel Precipitation test (AGPT) and the Immunofluorescence Technique (IF). However; reverse transcription real time polymerase chain reaction (RT-PCR) was applied and showed negative results for both samples and positive control Egyptian strain (Menya /Sheep/258) indicating non indicative information (false negative). IF tests were applied on paraffin sections and tissues impressions.
Results: The pathological investigations showed chronic infection in the form of necrotic foci (necrogranulomas) and IF test was showing RVFV antigen in hepatocytes, lymphocytes, Kupffer cells (histiocytes of liver), polymorphnuclear cells and in macrophages. RVFV is necrotizing virus that use histiocytes to produce its destructive effects on infected tissues (hepatocytes eating virus), it has its specific and unique pathologic profile inside hepatic tissues. The infected cells produce the virus in large number which leaves cells in groups of millions of viral particles enclosed in certain type of capsule or membrane. The severity of RVFV infections of the liver indicates the virulence status of the virus.
Conclusion: The pathologic changes indicate chronic disease. This study proves that RVFV causes chronic diseases and carriers are present in the endemic areas.
Keywords: Rift valley fever; Rift valley fever virus; Liver infections
Abbreviations
RVFV: Rift Valley Fever Virus; RT-PCR: Reverse Transcriptase - Polymerase Chain Reaction; RNA: Ribonucleic Acid; H&E: Haematoxyline and Eosin Stain; H&F: Haematoxylin and Fuchsin Stain; IF: Immune Florescent; IgG: Immune Globulin G; N protein: Nucleoprotein; NSM and NSs: Non-Structural Proteins; SsRNA: Single Stranded RNA; Gn and Gc: Envelope Glycoproteins; NGs: Necrogranulomas, Necrotic-Granulomas; RBCs: Red Blood Cells; PMNC: Polymorphnuclear Cells
Introduction
This study proves that RVFV causes chronic diseases and carriers are present in the endemic areas. In Egypt, during September 2017, Rift Valley Fever virus (RVFV) was detected in imported cattle comes from the Sudan. This study was carried out on liver samples of RVFV infected cattle. Rift valley fever (RVF) is a febrile illness, acute or sub-acute and zoonotic, that sometimes in severe conditions causes’ hemorrhagic fever and death. Rift valley fever virus, is an enveloped, icosahedral, segmented (Medium, Small, Large), small sized RNA virus belongs to genus Phlepovirus; and the family Bunyaviridae [1]. The RVFV main arthropod hosts are mosquitoes. Man and wide range of mammal’s species are susceptible of variables degree. Cattle are highly susceptible. RVFV that isolated during 1977 epizootic in Egypt showed spherical viral particles, 100 nm in diameter [2]. The genome of RVFV is composed of three single stranded RNA segments. The large one (L) codes for large polymerase protein, the medium sized segment (M) codes for the two major glycoproteins (G1 and G2) while the small segment (S) codes for the N protein. Two non-structural proteins NSM and NSS are encoded by the M and S segments respectively [3]. RVFV has three segments, negative or ambisense single stranded RNA (SsRNA) genome. The small (S) segment encodes for a nucleoprotein (N), the non-structural protein (NSs), which supposed to help the virus to evade the host immune system, the polymerase encoded on the large (L) segment. The medium (M) segment encodes two non-structural proteins, NSm1 and NSm2, and two envelope glycoproteins, (Gn and Gc) [4,5]. A serious epidemic of RVF occurred in Egypt in 1977, in which camels, sheep, cattle, buffaloes and goats were affected [6,7]. In October 1977, RVF appeared clinically in Egypt, the disease presented itself in the beginning as dengue like fever of man at Belbeis area, Sharkia province [8]. In the 18th October 1977, RVF virus was isolated for the first time from a human case in fever hospital at Zagazig, Sharkia province. The disease was observed clinically in animal in July 1977 in Aswan province which adjoins the Sudan, after that the epizootic had been reported in other provinces [9]. An officially reported outbreak of RVF occurs in Aswan province (Egypt) in man and domestic animals in late May, 1993[10]. Another outbreak occurred in Egypt in Aswan and Assiut provinces between April and August 1997 [11]. And during year 2003 an epizootic of RVFV reported officially in Egypt [12]. The wild vertebrates and invertebrates aid in spreading of RVFV to Egypt during 1977 epizootic [13]. Where rodents, birds, dogs, and domesticated animals including camels have a significant role in RVF virus epidemiology as reservoirs or amplifying hosts during this epizootic [14]. Rift Valley fever virus (RVFV) lead to cell death by mechanisms of necrosis directed by histiocytes, white blood cells, macrophages, and apoptosis. The disease is fatal in man with unhealthy liver and immunocompromised patients are more liable to the disease. Outbreaks of RVF virus in Africa are characterized by distinct spatial and temporal patterns that are directly related to specific environmental parameters associated with mosquitoes vector that play a role in the maintenance (enzootic) and transmission (epizootic) cycle of the virus [15,16]. Daubney et al. [1] were the first to describe the pathological and postmortem changes of RVF in lambs and sheep in details with more particularly to the liver lesion [1]. This study showing that RVFV can cause chronic hepatic diseases in endemic areas. And diagnosis of chronic infections showed applied by IF tests and pathological investigations, otherwise false negative results are expected in serological tests or RT-PCR which supplied as commercial kits.
Materials and Methods
Reference sera and reference RVFV antigens, were kindly supplied by research sector holding company for vaccines and sera Egypt (VACSERA). This research was performed in AHRI and VACSERA Egypt.
Animals
Swiss mice (6 days old) were supplied by VACSERA (Egypt), and subjected for isolation of RVFV from the samples which were collected from the imported Cattle. Some samples were collected from abattoirs in Cairo, Giza.
Samples for virological investigation
Sera, whole blood, swaps were collected from live animals; however, cattle in this study were not vaccinated against RVFV. In addition to samples from hepatic blood that collected from the portal veins. Tissue specimens (kidneys) were collected from abattoirs and butchers. Sera samples were inactivated at 56 C for 30 minutes before being used in the serum neutralization test (SNT) according to Edwin and Nathalie,1979 [17].
Histopathology studies
Tissue specimens were undergoing pathological and virological examinations. The tissue specimens were divided into two parts one for virology study that kept in -20°C and the other part kept in 10% formalin solution for subsequent pathological studies [18,19].
Tissue culture cell lines
VERO cell line were prepared and provided by VACSERA Egypt. The VERO cells were used for isolation, adaptation of RVFV, virus titration as well as serum neutralization test (SNT) [19].
RVFV strain
RVFV pantropic Menya Strain (Menya/Sheep/258) was kindly provided by applied research sector VACSERA, Egypt. RVFV was of infectivity titre in the order of 7.5log (10)/ml [19].
RVFV titration and determination of infectious dose (ID50)
VERO cell lines were used for determination of virus’ infectivity titer. 50% tissue culture infectious dose of a virus (TCID50) was carried out by traditional methods of virus quantification. Viral infectivity titer was evaluated according to the method adopted by Reed and Muench [20].
Trial for RVF virus isolation
Mice aged 6 days were inoculated intra-peritoneal by tissue homogenates samples collected from imported cattle according to Findaly and Howard [14]. Two mice were used for every prepared buffy coat sample and observed daily for 8 days post-inoculation. 0.2 ml from each buffy coat sample was inoculated into mice using i/p injection (2 mice for each sample) and observed daily for day 8 post- inoculation, or appearance of clinical symptoms or death (paralysis and death) [14].
Tissue culture inoculation: VERO used for isolation of RVF virus. Each prepared buffy coat sample was inoculated into VERO cell line tissue culture (3 tissue culture tube used for each sample). Detecting maintenance medium before inoculation 0.1 ml per each tube from each sample was inoculated into cell culture tube and left for one hour for adsorption. 100 μl maintenance medium containing 2% inactivated bovine serum was added. The tube were incubated at 37°C and observed daily for 5 days post-inoculation for evidence of cytopathic effect [19].
Virus neutralization test (VNT), plaque test
Serial dilutions of heat inactivated test serum are prepared in a 96 well plate and are incubated with a set amount (100 TCID50) of infectious virus. Following this incubation, virus susceptible cells are added to the virus-serum mixture, and the final virus/serum/cell combination is incubated for a period of 2-3 days. After this incubation period the test is read by examining each well of the plate for the presence of viral infection. Depending on the virus, this may be by direct microscopic examination of the plate for evidence of viral Cyto-pathic effect (CPE) or, if the virus causes little or no CPE, by immune fluorescent staining of wells for the presence of viral antigen in the cell monolayer [19].
Immune fluorescent antibody technique (IF)
In our study, buffy coats, impression smears, mice liver tissue sections (that used for isolation of RVFV from the samples), portal blood smears and liver tissue sections of cattle were subjected for IF tests. The detection of RVFV antigen in infected and control was applied by direct Immunofluorescence Technique (IF) according to [19]. The control negative, control positive monolayer tissue cultures, and the control tissue sections were kindly provided by VACSERA Egypt.
Agar gel precipitation tests (AGPT)
This test was applied on buffy coats, portal blood, tissue homogenates, and the tissue culture isolates. The reference polyclonal antiserum against RVFV was kindly provided by VACSERA Egypt. The principle for the AGPT is the migration of soluble antigens and soluble antibodies toward each other through the agar gel matrix. RVFV antigen and the known specific antibodies would complexes to form a precipitate which is trapped in the gel matrix and produces a visible line [19].
Reverse transcription real time polymerase chain reaction (RT-PCR)
RVFV genome extraction was performed by PowerPrep™ Viral DNA/RNA Extraction Kit Protocol. RT-PCR kits primers and probes were provided by the genetic PCR solutions™, Spain. The reference RVFV Egyptian strain (Menya /Sheep/258) used as another positive control of this investigation.
Results
This study was conducted on the cattle imported from the Sudan which are usually a free grazing breeds (African breed) living in south Sudan. The environmental conditions and the endemic status of country of origin are serious factors for the spreading of infectious diseases by living animal transportation. Cattles in the Sudan were not receiving RVFV vaccine.
Agar gel precipitation tests (AGPT)
RVFV antigen and the known specific antibodies were complexes and form a precipitate which is trapped in the gel matrix and produces a visible line.
Histopathology results
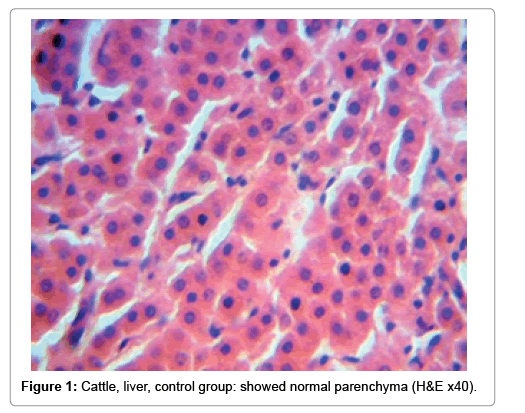
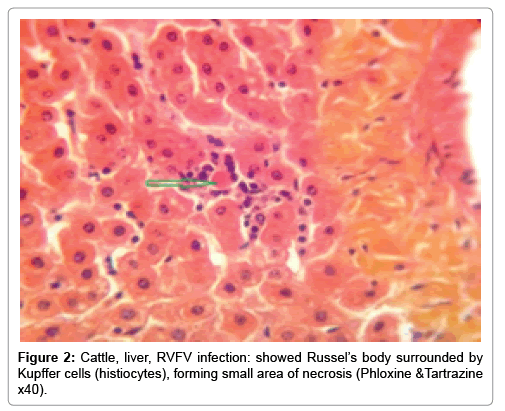
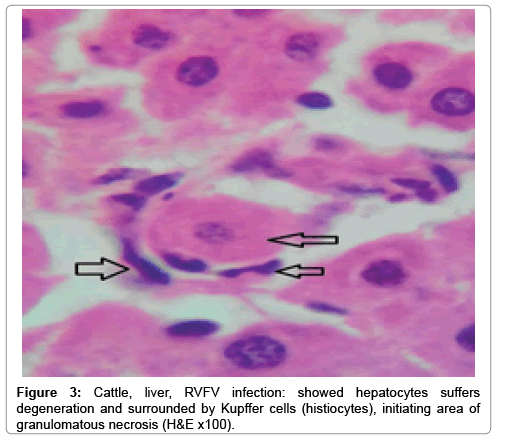
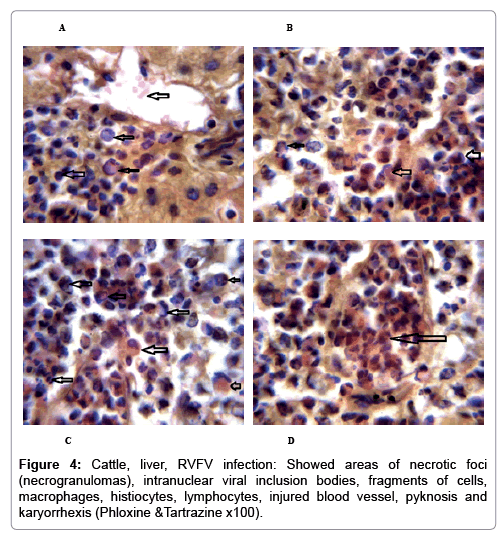
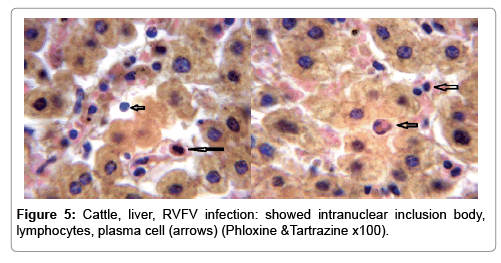
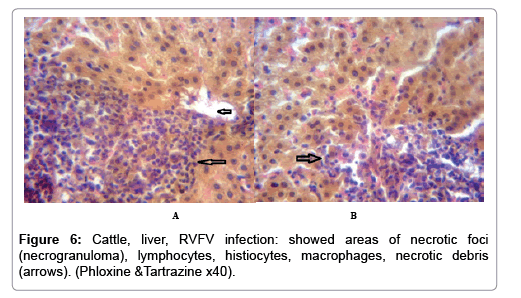
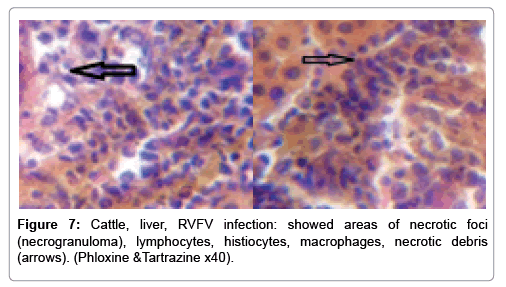
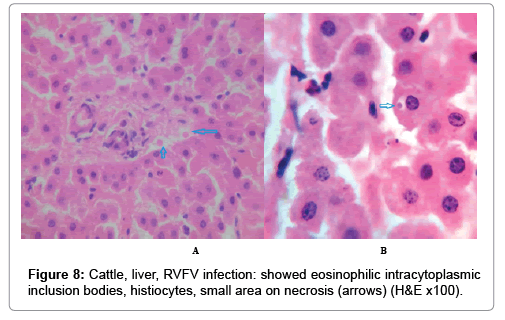
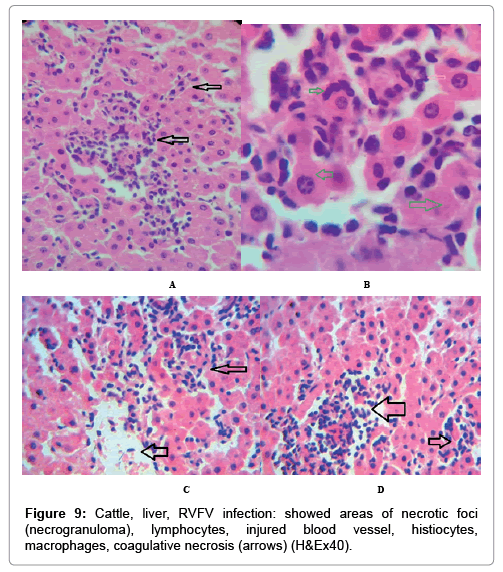
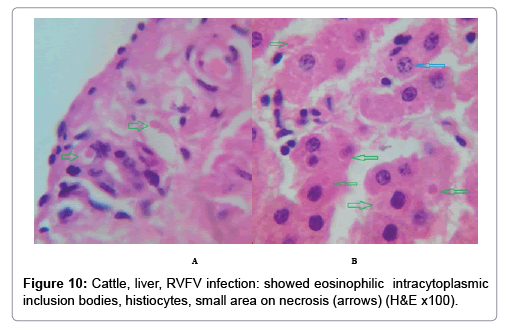
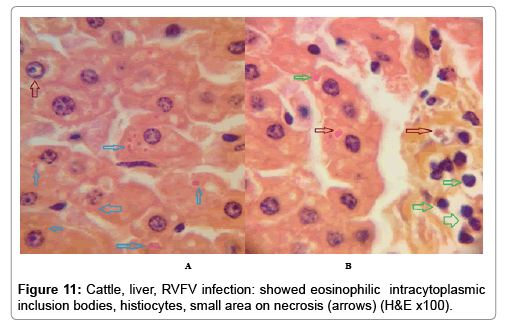
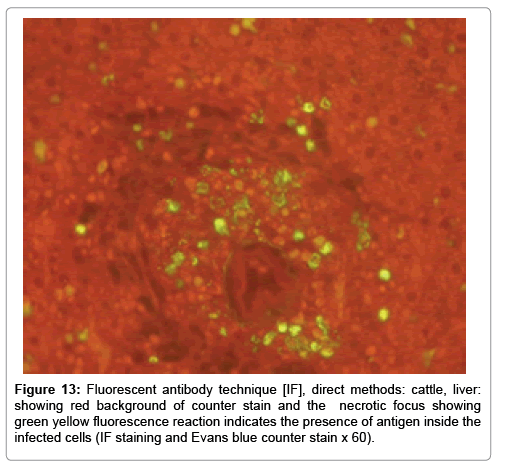
In examining the pathological picture caused by the virus, we note the following: The method or technique used by the virus to cause the disease begins with liver cells that appear to be suicidal (apoptosis). Lymphocytes travel through the liver and are attached to the cells or trapped inside them. They also gather in large numbers around hepatic cells struggling to die. The virus multiplies inside the liver cells in both the nucleus and cytoplasm and then large numbers of viruses accumulates inside the infected cells forming inclusion bodies. These inclusions were seen in the cytoplasm and nuclei of hepatocytes. The free inclusions that encountered in naturally infected animals, indicating that RVFV reproduction inside mammalian cells and especially hepatocytes arise from cells in the form of very large numbers surrounded by delicate membranes that protected from being fragile in case virions are moving as single particles. The liver phagocytic cells (Kupffer cells) which present around and lining the hepatic sinusoids plays major role of hepatic necrosis, besides apoptosis, macrophages, fibroblasts, lymphocytes. The microscopic examinations were showing the RVFV histopathological changes (Figures 1-11). The characteristic necrotic foci were encountered in hepatic parenchyma. The Haematoylin and Eosin stain (H&E) (Figures 8-10) and the special stains for viral inclusion bodies (Phloxine & Tartrazine) were showing numerous intracytoplasmic (Figures 2 and 11) and intranuclear inclusions bodies (Figures 4 and 5). The most outstanding findings are the necrogranulomas (NGs) or the necrotic-granulomas [aggregations of lymphocytes, macrophages, histiocytes, polymorph nuclear cells (PMNC), necrotic parenchyma, cellular debris, red blood cells (RBCs), plasma cells, and exudates]. NGs were seen substituted large areas of hepatic tissue. Activated proliferated histiocytes along with lymphocytes and macrophages forming NGs which strangling the hepatocytes. It resembles invading troops, or floods that destruct all things come their ways. Viral inclusion bodies were seen inside nuclei of hepatocytes, macrophages, histiocytes and Russel’s bodies or councilman like bodies were seen inside some necrotic foci. Hemorrhages and coagulative necrosis was encountered near injured blood vessels of liver.
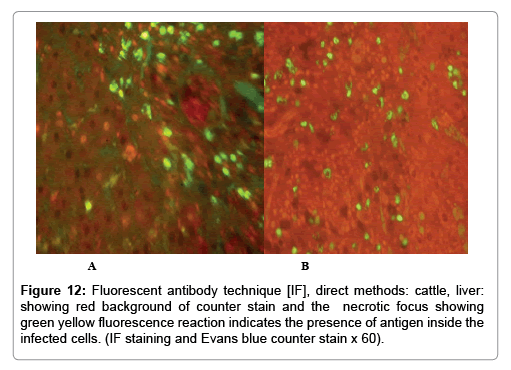
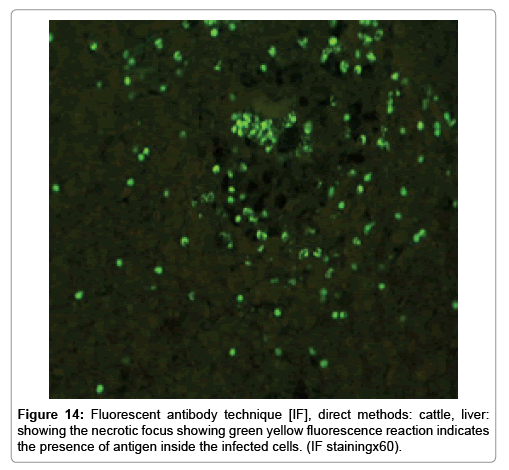
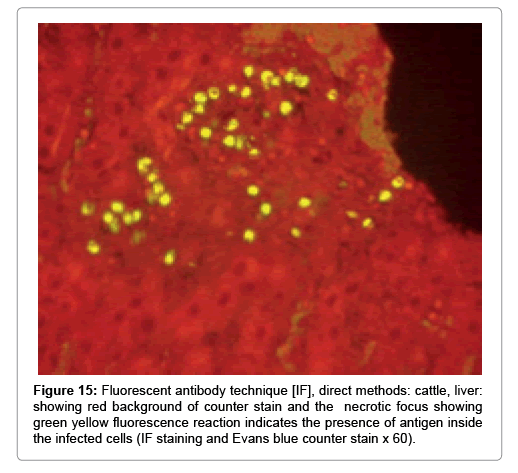
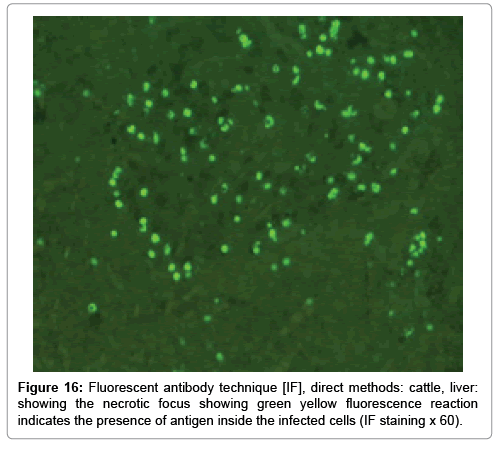
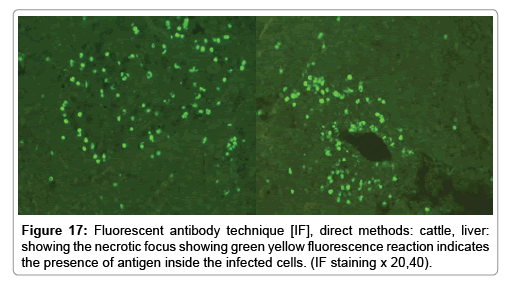
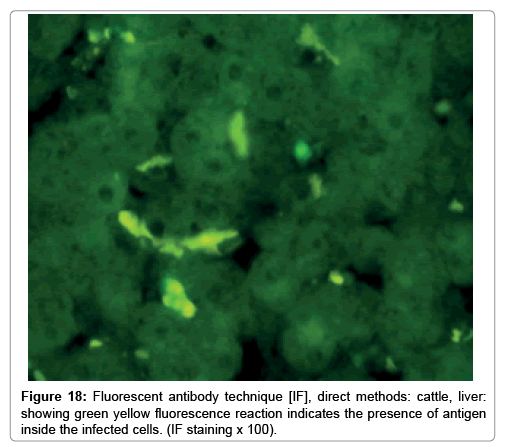
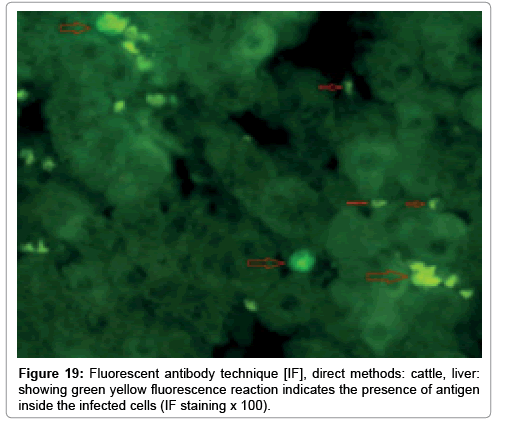
Immunofluorescence examination
In liver (Figures 9-14) the viral inclusion bodies exhibits prominent green florescence (positive reaction). The RVFV antigen was seen inside cytoplasm of histiocytes, macrophages, lymphocytes, endothelial lining of blood vessels, and epithelial lining of bile ducts and inside the necrotic foci. The RVFV antigen in mice was observed in brain, histiocytes, endothelial lining of blood vessels, intestine, hepatocytes and bile ducts. The viral intracellular inclusion bodies in the histopathological sections were showing strong IF reaction, indicating that inclusion bodies are the aggregates of viral particles (virions) protected inside a membranous formations.
RT-PCR findings
Both samples and positive control reference RVFV [Egyptian strain (Menya /Sheep/258)] were giving negative results (Supplementary pictures).
Discussion
Our findings proves that RVFV is necrotizing virus that use histiocytes of liver (Kupffer cells) to produce the destructive effects on infected tissues (RVFV a hepatocytes eating virus), it has its specific and unique pathologic profile inside hepatic tissues. It was observed that RVFV infected cells produce the virus in large number which leave cells in groups of millions of viral particles enclosed in certain type of capsule or membrane (Figures 2 and 8-10). RVF cause hepatitis and subcutaneous hemorrhage in acute form. The multiplication of the virus in various tissues of the body causes a hemorrhage in various organs including the brain, eye, kidneys and intestines. It is expected to cause many health complications. The Rift Valley virus needs to acquire additional factors to cause the disease in a way that we can see with the naked eye and those virtual factors such as the transmission of the virus from one animal to another and the transition from mosquitoes to eggs to mosquitoes and then to the animal and so in an integrated circle [1-16]. The pathological examinations showed the Kupffer cells (histocytes) proliferate and attacking the hepatocytes. So that, these cells plays major role in RVFV pathogenesis (Figures 8B and 9C). Moreover, IF indicates that these cells (histiocytes) have the viral antigen (Figures 9D-15). Intranuclear and intracytoplasmic viral inclusions were detected inside these cells (Figures 3-5). It was observed that viral antigens detected by IF test were numerous inside necrotic foci, blood vessels and around bile ducts (Figures 9D-14) and scattered in liver in the form of round masses resembles the Russel’s bodies and the inclusions seen in histological sections (Figures 15 and 16). The spread of the disease naturally without human intervention must precede the transformation of the virus through mosquitoes and the surrounding environment. If infected animals enter a new country, the spread of the virus does not occur directly. But requires an unknown period of time in which mosquitoes play the main role. The transmission of long-lived animals from a country infected by the virus, in which mosquitoes and bloodsucking insects multiply, is a major factor in the natural progression of the disease [21-26]. The presence of antibodies in human blood should be considered an infection. Indicating that the virus is present in their environment and in mosquitoes and that mosquitoes are infected with the virus that is multiplying within [27,28]. In endemic area heavy rainfall, floodwater Aedes mosquitoes serve as vectors, and the virus could be transmitted into offspring transovarially [9,10]. However, rivers and fresh water channels increases the number of permanent fresh water species of mosquitoes such as Culex pipens, which play a role in amplifying RVFV among mosquitoes, ruminants and humans [10-15]. Mice are one of the most susceptible animal species to RVFV infection [6,8] and RVF pathology in infected mice mimics the pathological findings in newborn lambs [6]. Our findings are in accordance with; Tacke [29], Dixon et al. [30] Russell and James [31]: who mentioned that liver macrophages are Kupffer cells and monocytederived macrophages. Kupffer cells are resident and non-migratory histiocytes. Liver injury is activating Kupffer cell, leading to the release of inflammatory chemotactic factors (cytokine and chemokine). This leads to infiltration of monocytes into liver. Liver macrophages are very plastic and adapt their phenotype according to signals derived from the hepatic microenvironment [29]. Kupffer cells, as well as other cells of the innate immune system, including natural killer, natural killer-T cells, and dendritic cells, reside within the sinusoid. Our findings in this research and the pathological changes refer to chronic viral infection and are in accordance with [29]. Who mentioned that “under hepatic disease conditions, the Kupffer cell shifts to a pathologically activated state that is a characteristic of chronic inflammatory diseases”. And agree with [30,31]. They stated that when Kupffer cells affected by a harmful interventions in the hepatic microenvironments may result in hepatocellular injury and damage. As in the present investigation, the Kupffer cells plays major role in the exhibition of pathological changes and hepatocytes necrosis. However, Kupffer cells are the major phagocytic cell of the liver, detoxify endotoxins, tolerate foreign material and/or substances. The disease conditions or induced factors which affects the ability of the Kupffer cells to perform their roles will result in injury to hepatic parenchymal cells and many vital body functions (Figures 2,3 and 9B). The chronic disease caused by RVFV characterized by the presence of acute infections of RVFV are very short as mentioned by recent investigation who stated that the replication of RVFV genome segments in cell level starts locally at the site of fusion of the virion with the endosome, and within 4-6 hours, it continue to proceed throughout the cytoplasm. After the replication phase, genome segments are recruited to the Golgi. However, the co-infection with complementing particles may result in productive infection; moreover, that virions containing antigenomic-sense RNPs may also contribute to the RVFV infection cycle (Figures 17-20) [32,33].
RT-PCR findings
The test was using commercial kits which were not providing the sequence and type of primers and probes. So that, RVFV diagnosis by RT-PCR in this research have certain cautions, and negative results may be a false result. Moreover positive results should be in accordance with other laboratory findings. Both samples and positive controls samples [reference RVFV Egyptian strain (Menya /Sheep/258)] were giving negative results (Supplementary pictures) indicating that this technique is not in accordance with other findings and it is false negatives because reference virus samples (control) were also negative. This point need more investigations, that different primer should prepared from known sequences and by applications of variables trials.
Moreover: it is worth to mention that:
1. Positive control was Egyptian strain (Menya /Sheep/258), which used as reference virus during this study, so that; it is not consider from kits’ components.
2. RT-PCR use primers and probes which provided in the kits component, with masked information about sequences and which segments they are the target for RVFV.
3. In scientific research, the investigator should know the sequence of primers and probes, and apply many and different choices for genome segments, as RVFV is RNA virus with three segments, but the test kits that provided by a company that masked the information, shouldn’t applied and the results obtained were for comparison and proving its non-usefulness. In this study, isolation of the causative agents is our target, and confirmation by lab tests was applied.
4. The tests materials were provided by governmental institutions of ministry of health (VACSERA) and ministry of agricultural, AHRI, (reference laboratories of EGYPT).
5. The findings also showed the chronic condition of this disease, which is a significant value in field of virology, unless it proven by histopathology.
Conclusion
This research proves that the Immune florescence test [IF] is the most reliable test, as antigen is seen by microscope. It is economic and safe and can use as rapid and highly sensitive technique for RVFV diagnosis. This study showing that RVFV can cause chronic hepatic diseases in endemic areas and diagnosis of RVFV chronic infections better performed by IF tests and pathological investigations, otherwise false negative results are expected in serological tests or RT-PCR which are usually supplied as commercial kits.
References
- Daubney R, Hudson JR, Garnham PC (1931) Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep cattle and man from East Africa. J Pathol Bacteriol 34: 545-579.
- Meegan JM (1979) The Rift Valley fever epizootic in Egypt 1977-1978 1. Description of the epizootic and virological studies. Trans R Soc Trop Med Hyg 73: 618-623.
- Knipe DM, Howley P (2013) Fields virology. Lippincott Williams & Wilkins, Philadelphia.
- Gommet C, Billecocq A, Jouvion G, Hasan M, Zaverucha do, et al. (2011) Tissue tropism and target cells of NSs-deleted Rift Valley fever virus in live immunedeficient mice. PLoSNegl Trop Dis 5:e14-21.
- Calisher C (1991) Classification and nomenclature of viruses fifth Ed. Report of the International Committee on Taxonomy of viruses. Arch Virol, pp:273-283.
- Meegan JM, Khalil GM, Hoogstrall H, Adham FK (1980) Experimental transmission and field isolation studies implicating Culexpipens as a vector of RVF in Egypt. Am J Trop Med Hyg29: 1405-1410.
- Hoogstraal H,Meegan JM, Khalil GM, Adham FK (1979) The Rift Valley fever epizootic in Egypt 1977-1978 2. Ecological and entomological studies. Trans R Soc Trop Med Hyg 73:624-629.
- Imam IZE, Darwish MA, El-Karamany R, Omar F (1977)A preliminary report on an epidemic of Rift valley fever (RVF) in Egypt. J Egypt Public Health Assoc 52: 417-18.
- World health organization (1978) Rift valley fever in Egypt. Weekly epidemiological records53: 107-198.
- Arthur RR, El-Sharkawy MS, Cope SE, Botros BA, Oun S, et al. (1993) Recurrence of Rift valley fever in Egypt. Lancet342: 1149-1150.
- Abdel- Rahim IH, Abdel- Hakim U, Hussein M (1999) An epizootic of Rift valley fever in Egypt in 1997. RevSci Tech 18: 741-748.
- World Health Organization (2003) Disease outbreak reported: Rift Valley fever in Egypt. Weekly Epidemiological Record 36: 5.
- Meegan JM, Niklasson B, Bengtsson E (1979) Spread of Rift valley fever virus from continental Africa. Lancet 314: 1184-1185.
- Imam IZE, El-Karamany R, Darwish MA (1979)An epidemic of rift valley fever in egypt.I. Diagnosis of Rift valley fever in man. Bulletin of the WHO 57: 437-439.
- Linthicum KJ, Davies FG, Kairo A (1985) Rift Valley Fever virus (family Bunyaviridae, genus Phlebovirus). Isolation from Diptera collected during an inter-epizootic period in Kenya. J Hyg 95:197-209.
- Schmaljohn CS (1996) Fundamental virology (3rd edn.). Raven publishers, Philadelphia, USA.
- Edwin HL, Nathalie JS (1979) Micro-neutralization test in cell cultures.
- Lillie RD, Fullmer HM (1976) Histopathology technique and practical histochemistry (4th edn.). McGrew Hill Book Company, USA.
- Payment P, Trudel M (1993) Methods and techniques in virology. Marcel Dekker INC, USA.
- Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoint. Am J Hyg 27: 493-497.
- Terasaki K, Makino S (2015) Interplay between the virus and host in rift valley fever pathogenesis. J Innate Immun 7:450-458.
- Coatzer JAW (1977) The pathology of Rift Valley Fever 1. Lesions occurring in natural cases in new-born lambs. Onderstepoort J Vet Res 44:205-212.
- Coetzer JA (1982) The pathology of Rift Valley fever. II. Lesions occurring in field cases in adult cattle, calves and aborted fetuses. Onderstepoort J Vet Res 49:11-17.
- Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ (2009) Rift Valley fever virus. J Am Vet Med Assoc 234:883–893.
- Bird BH, BawiecDA ,KsiazekTG , Shoemaker TR , Nichol STÂ (2007) Highly sensitive and broadly reactive quantitative reverse transcription-PCR assay for high-throughput detection of Rift Valley fever virus. J Clin Microbiol 45:3506-3513.
- Van Velden DJ, Meyer JD, Olivier J, Gear JH, Mcintosh B (1977) Rift Valley fever affecting humans in South Africa - a clinicopathological study. S Afr Med J 51:867-871.
- Nicholas DE , Jacobsen KH , Waters NM (2014) Risk factors associated with human Rift Valley fever infection: Systematic review and meta-analysis. Trop Med Int Health 19:1420-1429.
- Tacke F (2017) Targeting hepatic macrophages to treat liver diseases. J Hepatol 66: 1300-1312.
- Dixon LJ, Barnes M, Hui Tang, Pritchard MT, Nagy LE (2013)Kupffercells in the liver. Compr Physiol 3: 785–797.
- Russell CC, James AP(2002) Organ-specific toxicologic pathology in handbook of toxicologic pathology.
- Bouloy M (1991) Bunyaviridae: Genome organization and replication strategies. AdvVir Res 40: 235-266.
- WichgersSchreur PJ, Kortekaas J (2016) Single-molecule FISH reveals non-selective packaging of rift valley fever virus genome segments. PLOS Pathogens 12: e1005800.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11195
- [From(publication date): 0-2018 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 10297
- PDF downloads: 898