Case Report Open Access
Rifampicin-Resistance Tuberculous Meningitis in a Patient with Cerebral Lupus Diagnosed Using Cerebrospinal Xpert Mtb/Rif Test
| Mohamed Faisal Abdul Hamid1*, Soo Chun Ian1, Andrea Ban Yu-Lin1, Mohd Shahrir Mohamed Said1 and Roslina Abdul Manap2 | |
| 1Respiratory Unit, University Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia | |
| 2Rheumatology Unit, Universiti Kebangsaan Malaysia Medical Centre, Malaysia | |
| Corresponding Author : | Mohamed Faisal Abdul Hamid Respiratory Unit, Universiti Kebangsaan Malaysia Medical Centre Kuala Lumpur, Malaysia Tel: +60391455555 Fax: +60391456679 E-mail: arabinose@hotmail.com |
| Received March 17, 2015; Accepted Novmber 15, 2015; Published Novmber 20, 2015 | |
| Citation:Hamid MFA, Ian SC, Yu-Lin AB, Said MSM, Manap RA, (2015)Rifampicin- Resistance Tuberculous Meningitis in a Patient with Cerebral Lupus Diagnosed Using Cerebrospinal Xpert Mtb/Rif Test. J Neuroinfect Dis 6:194. doi:10.4172/2314-7326.1000194 | |
| Copyright: © 2015 Hamid MFA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Tuberculous (TB) meningitis or tubercular meningitis is a Mycobacterium tuberculosis infection of the meninges—the system of membranes which envelop the central nervous system. Molecular tests like the automated rapid molecular assay Xpert® MTB/RIF greatly expedites the detection of M. tuberculosis complex and rifampicin resistance. We report a 22-year old man with cerebral lupus and concurrent rifampicin resistance tuberculous meningitis which was detected by Xpert® MTB/RIF test in the cerebrospinal fluid. The rifampicin resistance in this patient was due to poor compliance. The patient took one tablet of akurit-4 throughout the intensive phase of treatment. This report highlights the usefulness of molecular testing which allowed a faster diagnosis than a bacteriologic confirmation of drug resistant tuberculosis. This is important as early diagnosis and treatment leads to better outcome in TBM.
| Keywords |
| Rifampicin-resistance (RR) tuberculosis; Cerebral lupus; Xpert® MTB/RIF; Meningitis |
| Introduction |
| Multidrug-resistant tuberculosis (MDR-TB) is caused by organisms that are resistant to at least isoniazid and rifampicin, the backbone of current first-line treatment regime [1]. Rifampicin-resistant TB (RR-TB) is caused by organisms that are resistant to rifampicin with or without resistance to other drugs [1]. MDR-TB and XDR-TB are both forms of RR-TB. Drug-resistant TB commonly occurs in pulmonary tuberculosis; extra-pulmonary involvement particularly tuberculous meningitis (TBM) is rare and is associated with high mortality. We would like to report the successful treatment of a patient with underlying central nervous system lupus with co-existing MDR-TBM which was diagnosed by a positive cerebrospinal fluid (CSF) Xpert® MTB/RIF assay. He showed marked improvement following commencement of second line anti-TB therapy. |
| Case Report |
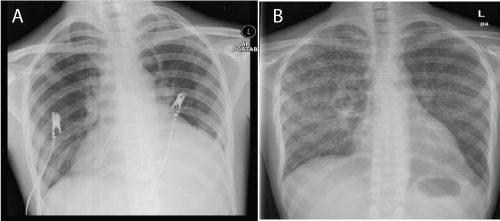
| A 22-year-old man presented with prolonged fever, photosensitivity rash and multiple joint pains for 1 month duration. Clinical examination revealed malar rash, oral ulcers and alopecia. Full blood count showed bicytopenia (Hb 13.1 g/dL, white cells 1.3 X 10^9 /L, platelets 103 X 10^9/L). Renal profile and liver function test were both normal. ESR was 66 mm/hr, and CRP was 6 mg/ dL. There was evidence of mild pericardial effusion on transthoracic echocardiogram. His chest radiograph (CXR) during that admission was normal (Figure 1a). No mantoux test was performed. A clinical diagnosis of active SLE was made. Further blood tests confirmed this diagnosis. ANA 1: 640 (speckled) Anti-dsDNA was negative, and low complement levels. (C3 55.2 mg /dL, C4 15.9 mg/ dL.) He developed altered sensorium leading to aggressive behaviour. We did not get consent for a lumbar puncture. On the basis of the investigations available, SLE with hematological, serositis, musculoskeletal and cerebral involvement was made. He was treated with methylprednisolone, followed by cyclosphomide, and rituximab, and discharged with oral prednisolone 30 mg twice a day. |
| A month later, he presented again with fever, cough and whitish sputum for 3 days. His CXR (Figure 1b) showed features of miliary tuberculosis and sputum for acid-fast bacilli (AFB) was positive. Anti-TB using fixed-dose combination, Akurit-4 (3 tablets daily) was started. A scheduled second dose of rituximab was withheld in view of evidence of active tuberculosis. He was maintained with oral prednisolone for SLE and subsequently discharged. He was subsequently lost to our follow-up. |
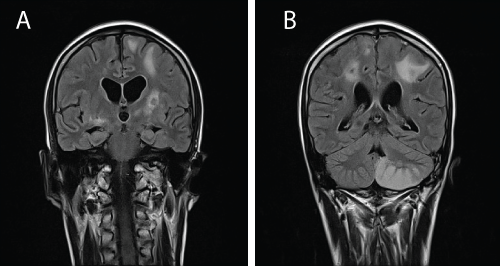
| Four months later, he presented with generalised tonic-clonic seizures. We were worried of treatment interruption but he claimed he had continued anti-TB treatment at a clinic near his home. At that point of presentation he was on the maintenance phase of anti-TB (isoniazid + rifampicin). A lumbar puncture revealed an increased protein level (3029 g/dL) and an MRI brain showed multiple enhancing lesions at the basal ganglia and grey-white matter junction suggestive of tuberculoma (Figure 2a and 2b). Further questioning revealed non-compliance to medications with a history of taking only 1 tablet of Akurit-4 throughout the intensive phase of treatment. Treatment was restarted with the addition of dexamethasone and streptomycin. He showed improvement clinically and was discharged well. |
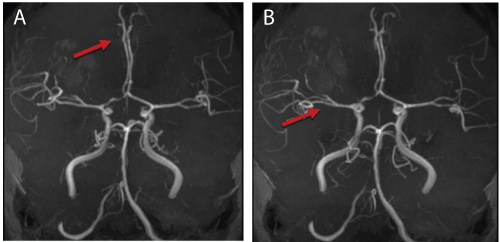
| Six months later, while on maintenance phase of anti-TB, he presented with a 5-day history of left-sided body weakness, low grade fever and lethargy. Clinically, the power was 0/5 for both left upper and lower limb, and there was hyperrreflexia. Blood investigations were unremarkable. The CXR showed resolution of miliary changes. An MRI brain showed improvement of the previously seen enhancing lesions. However the MRI showed evidence of vasculitis involving the right middle cerebral artery and the right anterior cerebral artery. (Figure 3a and 3b). Based on the MRI findings, he was diagnosed as a flareup of cerebral lupus. High-dose methylprednisolone was administered followed by cyclophosphamide. He developed drug induced hepatitis and was switched to bridging therapy of streptomycin, ethambutol and ciprofloxacin. |
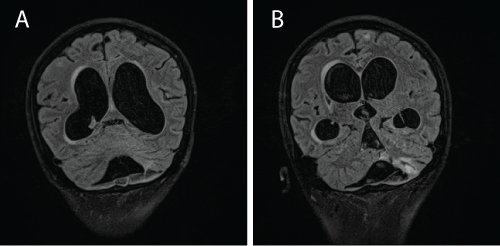
| Despite receiving treatment, he became progressively disinhibited and drowsy. A repeated MRI revealed hydrocephalus (Figure 4a and 4b). A lumbar puncture showed a raised CSF protein > 6 g/dL with negative AFB smear and bactec culture for Mycobacterium tuberculosis was negative. A lumbar drained was inserted. |
| At this point, we entertained the possibility of MDR-TBM and CSF sample was sent for Xpert® MTB/RIF assay. This test was performed at a separate centre. Within a day, the result of Mycobacterium tuberculosis resistant to rifampicin was detected. The patient was labelled as MDR-TB and treatment regime was changed to pyrazinamide 1 gram daily, ethambutol 800 mg daily, amikacin 600 mg 5 times per week, moxifloxacin 400 mg od, ethionamide 500 mg morning and 250 mg in the evening, and clofaxamine 150 mg daily. The hydrocephalus was corrected with a ventricular-peritoneal shunt. A repeated MRI brain performed 3 months later showed resolution of the hydrocephalus (Figure 5a and 5b). He was planned for a total duration of second line anti-TB of 24 months. 2 months post treatment, CSF test for Xpert® MTB/RIF was negative and CSF culture sent earlier showed resistance to isoniazid, rifampicin and streptomycin. Hence, the exact diagnosis of MDR-TBM (a form of RR-TB). Currently he remains in remission of his SLE and has not shown any signs of relapse of TBM. His lungs are clear but he has grade 4/5 weakness of both left arms and legs. He has normal mental status. |
| Discussion |
| To our knowledge this is the first reported case of RR-TB meningitis in a patient with underlying cerebral lupus. Estimated proportion of new cases with MDR-TB is 3.5%. Eastern Europe and Central Asian countries have the highest levels of MDR-TB reaching 35% of new cases and 75% of previously treated cases.In Malaysia the rates of RR-TB have been estimated at 0.3% in 2005 and 1.3% in 2011 [2]. TB meningitis is associated with significant morbidity. CSF smears have a low sensitivity and cultures require a prolong period of time. This makes RR TBM a difficult diagnosis to confirm. To rely on cultures means there will be a delay in initiation of treatment. TBM from drugresistant TB has been reported worldwide and in South Africa, 8.6% of a series of 350 TBM cases were found to harbour an MDR strain [3]. |
| Factors that contribute to TB resistance are erratic drug supply, prescription of inadequate chemotherapy, poor patient management and non-adhenrence to therapy and treatment interruptions. As in our patient, development of MDR-TB was secondary to a monotherapy treatment regime. |
| The Xpert® MTB/RIF assay is an automated nucleic acid amplification test that can simultaneously identify M. tuberculosis and rifampicin resistance within two hours. This test is currently only available in less than 3 centres in our country. In TBM, the sensitivity and specificity (95% CI) of uncentrifuged Xpert® MTB/RIF was reported as 51% (35%–68%) and 94% (82%–99%) [4]. The result of Xpert® MTB/RIF assay in our patient changed the management in this patient rapidly. Following guidelines, all GeneXpert positive patients with resistance to rifampicin should be started on MDR-TB treatment [5]. |
| There are no uniform recommendations on how to treat RRTBM. Treatment depends on the succeptibility pattern of the isolate and previous treatment history of the patient. Second line drugs with good CSF penetration should be preferred. In this patient, selection of second-line agents was based on WHO guidelines 2014. Pyrazin- amide and ethambutol were chosen from the available 1st-line agents followed by an injectable agents (group 2) and a fluroquinolone (group 3) where amikacin and moxifloxacin were used respectively. Moxifloxacin being a newer agent with better CSF penetration was preferred over levofloxacin [6]. Among group 4 agents, ethionamide was selected; possible neuropsychiatric side effects prevented the usage of cycloserine [6]. To strengthen the regiment, clofazimine (group 5) was added. We decided that the duration of intensive phase treatment in our patient would be 8 months while the maintenance phase was 16 months with total treatment duration of 24 months. To avoid further non-compliance, this patient is currently undergoing Directly Observed Treatment Short course (DOTS). |
| Conclusion |
| This case highlights the value of gene Xpert testing of cerebrospinal fluid (CSF) leading to a rapid detection of drug-resistant MTB in TBM. There is an urgent need for rapid diagnostic techniques to be made available as delay in treatment will lead to high case fatality. Our patient has responded to treatment but he has some sequelae of body weakness which we attribute to the underlying vasculitis from CNS lupus. |
References
- http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf
- Patel VB, Padayatchi N, Bhigjee AI, Allen J, Bhagwan B, et al. (2004) Multidrug-resistant tuberculous meningitis in Kwazulu-natal, South Africa. Clinical Infectious Diseases 38: 851-856.
- Patel VB, Theron G, Lenders L, Matinyena B, Connolly C, et al. (2013) Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med 10: e1001536.
- http://www.hst.org.za/publications/management-drug-resistant-tuberculosis-policy-guidelines
- Marx GE, Chan ED (2011) Tuberculous meningitis: diagnosis and treatment overview. Tuberculosis Research and Treatment 2011.
- Sharma B, Handa R, Nagpal K, Prakash S, Gupta PK, et al. (2014) Cycloserine-induced psychosis in a young female with drug-resistant tuberculosis. Gen Hosp Psychiatry 36: 451.e3-454.
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 11568
- [From(publication date):
December-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10423
- PDF downloads : 1145
