Research Article Open Access
Rifampicin Resistant Tuberculosis in a Secondary Health Institution in Nigeria, West Africa
| Shittu O Rasaki1*, Akanbi II A AJibola2, Sanni A Musa3, Alabi K Moradeyo4, Odeigah LO4, Sule G Abdullateef5, Wale Adeoti6 and Isiaka-Lawal Salamat7 | ||
| 1Department of Family Medicine, Kwara State Specialist Hospital, Sobi, Kwara State, Nigeria | ||
| 2Department of Microbiology, University of Ilorin Teaching Hospital, Kwara State, Nigeria | ||
| 3Department of Haematology, Kwara State Specialist Hospital, Sobi, Ilorin, Nigeria | ||
| 4Department of Family Medicine, University of Ilorin Teaching Hospital, Kwara State, Nigeria | ||
| 5Department of Family Medicine, Ahmadu Bello University, Teaching Hospital, Zaria, Nigeria | ||
| 6Chest clinic, Kwara State Specialist Hospital, Sobi, Ilorin, Kwara State, Nigeria | ||
| 7Department of obstetrics and gynaecology, Kwara State Specialist Hospital, Sobi, Ilorin, Nigeria | ||
| Corresponding Author : | Shittu RO Head of Department of Family Medicine Kwara State Specialist Hospital Sobi, Ilorin, Kwara State, Nigeria Tel: +2348035062687 E-mail: oorelopehospital@gmail.com |
|
| Received March 17, 2014; Accepted April 22, 2014; Published April 26, 2014 | ||
| Citation: Rasaki SO, AJibola AA, Musa SA, Moradeyo AK, LO O, et al. (2014) Rifampicin Resistant Tuberculosis in a Secondary Health Institution in Nigeria, West Africa. J Infect Dis Ther 2:139. doi: 10.4172/2332-0877.1000139 | ||
| Copyright: © 2014 Rasaki SO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Background: Rifampicin-dependent tuberculosis is an unrecognized and potentially serious treatment issue. Rifampicin resistance is a risk factor for poor outcome in tuberculosis. It is prevalent in Nigeria. Therefore, we sought to examine the pattern of rifampicin resistance tuberculosis in Nigeria, West Africa.
Method: One hundred and forty tuberculosis cases were referred to the chest clinic of Sobi Specialist Hospital from January to December 2013. Sputum samples were obtained from them, smeared on glass slides, stained using Ziehl Neelsen Stain and later observed under light microscopy. The GeneXpert MTB/RIF assay was used to simultaneously detect TB and rifampicin resistance.
Result: The minimum age of the patients was 18years, while the maximum was 83. The mean age was 38.39± 13.75. There was male preponderance 84(60%), compared to 56(40%) female. The secondary health institution made the highest referral. Forty eight (34.3%) had smear-positive TB, while 92(65.7%) were sputum negative. Thirty two (38.1%) male out of 84 and 12(21.4%) female out of 56 were sensitive to Rifampicin, while 6(7.1) male out of 84 and 4 (7.1%) female out of 56 were resistant to it. Forty four (31.4%) were MD-TB positive with a prevalence of 31.4%. Ten (7.2%) were Rifampicin resistant; this included 6 males and females. This was statically significant.
Conclusion: Our study highlights that physicians should have high index of suspicion for rifampicin resistant tuberculosis in patients refractory to anti-TB treatment. The MTB/RIF test is a useful method for rapid diagnosis of TB and detection of RIF-resistance strains. There is need for increasing effort to interrupt the transmission of RIF-TB.
| Keywords | |
| Rifampicin resistant tuberculosis; Secondary health institution; Nigeria; West Africa | |
| Introduction | |
| According to World Health Organization [1], nearly 2 billion people, one-third of the world’s population have T.B. Almost 95% of T.B cases are found in the developing world. Nigeria currently ranks 10th among the 22 tuberculosis high burden countries in the world. | |
| According to the World Health Organization, 650,000 people are infected by worldwide [2]. In Africa, 1.9% of new cases and 9.4% of diagnosed and treated patients are infected by a MDR strain [2]. The WHO estimates a Multi-Drug Resistant (MDR)-TB prevalence rate of 3.1% and 1% among new and retreatment TB patients respectively in Nigeria [3]. Multi-drug resistance (MDR) TB is defined as tuberculosis disease caused by a strain of M. tuberculosis that was resistant to at least isoniazid and rifampicin [4]. Emerging Multidrug-Resistant Tuberculosis-TB (MDR) is one of the major concerns of the Health policy [5]. Few studies had documented the presence of disease in Nigeria, with prevalent rates ranging from 4%-76.3% [6-9]. | |
| In a case study conducted in infectious Diseases Hospital, Kano, out of 80 patients sampled, 52 were diagnosed to be Acid Fast Bacilli (AFB) positive and 28 were Acid Fast Bacilli negative; 61.5% and 38.5% prevalence were found in male and female patients respectively, with age group 31-40years having the highest prevalence of 28.8% [10]. Sputum smear microscopy remains the most common way to diagnose pulmonary TB. Depending on the report and method used, smear microscopy can accurately detect TB in 20% to 80% (using fluorescence microscopy methods) of TB cases [11]. | |
| The acronym means: Multi-drug resistant TB, that is, TB in patients whose infecting isolates of M. tuberculosis are confirmed to be resistant in vitro to both isoniazid (INH) and RIF. The categories of suspects are: failure to category 1 treatment, failure to convert at the end of intensive phase of CAT 2 or failure to CAT 2 treatment, Old cases (RAD, relapse, other etc), known symptomatic contacts of DR-TB, TB/HIV coinfected. | |
| The GeneXpert MTB/RIF assay is a molecular-based rapid test with potential to revolutionalize TB diagnosis. The regions of the target gene associated with RIF resistance (rpoB gene) is PCR amplified Table 4. 5 wild type (A-E) sites of the rpoB gene are present. Five molecular beacons are present and they bind only to the wild type rpoB gene. It can simultaneously detect Mycobacterium tuberculosis (MTB) complex DNA and mutations associated with rifampicin (RIF) resistance (a reliable proxy for) directly from sputum specimens in less than 2 hours, and it minimizes staff manipulation and bio safety risk [12]. Moreover, its ability to detect smear-negative TB provides a significant advantage, especially for PLHIV. In December 2010, the World Health Organization (WHO) endorsed Xpert for the rapid and accurate detection of TB, particularly among PLHIV and people suspected of having [12]. | |
| There is currently no country wide drug resistance survey hence, the exact data on the prevalence and pattern of rifampicin resistance tuberculosis is unknown. Moreover, there is paucity of data on the prevalence of in Nigeria in general and North-Central Nigeria in particular, hence the need for this study. | |
| Materials and Methods | |
| This study was conducted at the Chest Clinic of Kwara State Specialist Hospital, Ilorin, Nigeria. The target populations were tuberculosis suspects referred from the primary, secondary and tertiary health care facilities in Kwara State in particular and Nigeria in general. The chest clinic project of Sobi Specialist Hospital Ilorin, Kwara State was commissioned on Thursday 7th of June, 2012. It was donated by star deep water petroleum limited. The aim was to assist in the diagnosis of Tuberculosis and Drug Resistance Tuberculosis and to serve as referral center for tuberculosis cases. The hospital caters for infectious diseases such as HIV and tuberculosis. | |
| Ethical approval was obtained from the Ethical Review Committee of the Kwara State Ministry of Health before commencement of the study. The respondents were adequately informed about the nature of the study and its benefits. An interviewer administered questionnaire was used. However, for subjects who do not understand English, a local dialect version of the instrument was used. | |
| The inclusion criteria are tuberculosis (TB) patient who previously received Category 1 (new case) treatment and was declared cured or completed a full course of treatment and has once again development sputum smear-positive tuberculosis, or a TB patient who while on treatment remain, or becomes again smear positive five months or later after commencement of treatment, or a TB patient who completed at least one month of treatment and returns smear positive after at least 8 weeks of interruption of treatment, or a TB patient already registered for treatment in one local government area (LGA) who is transferred to another LGA where(s) he continues treatment, or a TB patient who does not easily fit into one of the above case definitions. The exclusion criteria is a TB patient who has never had treatment for TB or who has taken anti-TB drugs for less than 4 weeks. A total of 140 Drug Resistance Tuberculosis Suspects cutting across age groups and of both sex were referred between January and December, 2013. | |
| Respondent were grouped into different occupational classes using Oyedeji’s classification [13] viz; | |
| I. Senior public servants, professionals, managers, large scale traders, business-men and contractor, senior military officers. | |
| II. Intermediate grade public servants, and senior school teachers, non-academic professionals e.g. Nurses, owners of medium sized business, secretaries. | |
| III. Non-manual skilled workers including clerks, typists, telephone operators, junior school teachers, drivers, artisans. | |
| IV. Petty traders, labourers, messengers, lower cadre civil servants. | |
| V. Unemployed, full-time house wives, students, subsistence farmers. | |
| The predictive value of a positive test is calculated as follow: | |
| A/(A+B)X100, where A represents those with oral lesions, B represents those without oral lesions and A + B, the total sample respondents. | |
| Three sputum specimens were collected from each TB individual. The first sample was taken on the spot, the second sample was also an early morning sample and the third was taken on the spot when the client returns to the consulting room with the second sample. | |
| The sputum was collected into a dry, clean, transparent, widemouthed leak proof container. The sides of the specimen containers were labeled correctly with the patient’s name and referral Centre. The smear was heat-fixed with flame after complete air drying. Carbol fuchsin was applied onto the slides and heated gently for 5 minutes, then washed and drained. Later decolorized for three minutes, washed thoroughly, and covered with methylene blue for one minute. The slides were then washed, drained and ready for microscopy after drying [14]. The stained and dried smear was viewed under oil as a fine red rod against blue background. Interpretation of results was done using WHO guidelines [14]. Presence of pinkish red rods indicated presence of acid fast bacilli while absence of pinkish red rods meant absence of acid fast bacilli. AFB counts, recording and reporting were as follows: Table 1. | |
| GeneXpert was used to diagnose TB and to test for rifampicin (RIF) resistance which was used as proxy for MDR-TB. The specimen for Gene Xpert was collected in an open field fresh. The patient was instructed to inhale deeply 2-3 times, breathe out each time, cough deeply from the chest, place the open container close the mouth to collect the specimen. Appropriate specimen used for Xpert was sputum, minimum of 1ml without food particles and stored 40C max 10 days. Report results were as follows: MTB detected, MTB not detected, RIF resistance detected, RIF resistance not detected, RIF resistance indeterminate and Error/ invalid. In December 2010, the World Health Organization (WHO) endorsed Xpert for the rapid and accurate detection of TB, particularly among PLHIV and people suspected of having [15]. | |
| Completed questionnaire and measurements were entered into a computer data base. The data were analyzed using the epidemiological information (Epi-info) 2005 software package of Center for Disease Control and Prevention (CDC). The 2 by 2 contingency tables were used to carry out Chi-square test and to find out the level of significance and values less than 0.05 were regarded as statistically significant. | |
| Results | |
| One hundred and forty patients consisting of 84 males; and 56 females with cough more than two weeks were investigated during the studied period. The minimum age of respondent was 18years, while the maximum was 83. The mean age was 38.39 ± 13.75. | |
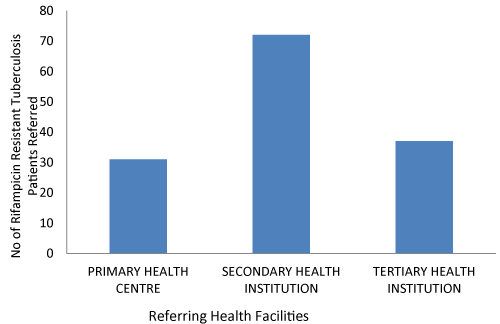
| Figure 1 shows the pattern of referral of the TB suspects with the secondary health institution making the highest referral, while the primary health centre made the least. | |
| Discussion | |
| Demographic characteristic analysis showed that age group 31-40 had the highest drug resistance TB. This was comparable to the previous study in Nigeria [16], but differed from Imam and Oyeyi [17], Nigeria, where 15.29 age group had the highest percent distribution. | |
| There was male preponderance, 84(60%) as against 56(40%) female; this was also in agreement with the previous studies [16-18]. and in concord with the work of Taura et al. [19], where male subjects had prevalence of 61.5% as against 38.5% of females. This disparity could be due to the fact that male subjects were more exposed to risk factors of TB infection such as smoking, which could make them more susceptible. | |
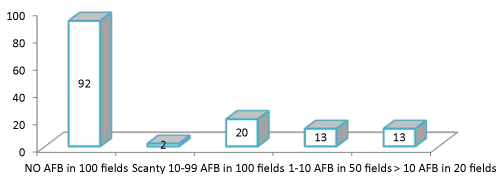
| Forty eight (34.3%) had smear-positive TB. Comparable to 31(18.2%) obtained in the study by Lawson et al. [20] but lower than (8%) smear-positivity reported by Boyer et al. [21]. For TB diagnosis in sputum smear-positive samples, studies showed sensitivities ranging from 93% to 98% and specificities of 83% to 99% [22,23]. Sputum smear microscopy has some limitations because it can only be used to diagnose TB when sputum has sufficient bacillary load, and it cannot detect drug resistance. Thus, HIV-associated TB often goes undetected because people living with HIV (PLHIV), especially those with severe immune suppression generally have very low numbers of bacilli [24]. A more sensitive approach to diagnosis is to culture sputum samples, which can include testing for drug resistance. However, such techniques require expensive and sophisticated laboratory equipment and staff, and it take weeks or months to obtain results. Realistically, most people who need culture the tests to diagnose their TB will not have access to the test results on time, in other to commence medication on time there by preventing complication and spread to others. In this regard, GeneXpert assay is more sensitive than sputum smear microscopy in detecting TB, and it has similar accuracy as culture (Figure 2). | |
| Eighteen (12.9%) were HIV positive and commoner among males than females. Age group 31-40 (33.57%) was more pronounced. This could be attributed to the social life style in the study area. Many of them lives a polygamous way of life, as well as wife inheritance as influenced by their religion. Alcohol use and misused was another contributing factor. Social class-V constituting 8(12.9%) of the respondents were unemployed. Majority of them were full time house wives, and some of them were subsistence farmers. They were found to statistically significant (Table 2). | |
| In this study HIV co-infection was not found to be statistically significant with anti-TB drug resistant, as found in other studies [25,26]. The current meta-analysis provides strong evidence to affirm that HIV co-infection may not have an influence on the development of anti-TB drug resistance. Additionally, with the exception of a report from Latvia and Ukraine, several reports from different parts of the world had shown that HIV infection had no statistically significant association with [27]. This was however in contrast to studies done about two decades ago, which showed that 90% of cases of occurred in HIV-co-infected individual [28]. Similarly, in the United State, it was reported that HIV infection was associated with [29]. Anastasis et al. expressed concern regarding the development of drug-resistant TB as the HIV epidemic progressed [30]. | |
| In our study, 44(31.4%) were rifampicin sensitive, while 10(7.2%) were rifampicin resistant (Table 3). This agreed with the findings of Olusoji et al. [16] where (8.6%) were resistant to rifampicin, but lower to the study of Lawson et al. [20] where (19%) isolates were resistance to rifampicin. This level of resistance was in keeping with the findings of Idigbe et al. [31] who reported 2% resistance to rifampicin in Lagos, Nigeria, but contrary to the study of Akaninyene and co-workers18 where no strain of rifampicin resistant was reported. Mycobacterium TB is more liable to undergo mutation when it is exposed to rifampicin than to many of the second line anti-TB drugs [32]. | |
| Forty four (31.4%) were positive and this was comparable to rates published in previous studies from India [33,34], but much lower than 77.4% reported by Olusoji et al. [16]. Few studies had documented the presence of in Nigeria, with prevalent rates ranging from 4%-76.3% [6-9]. According to the World Health Organization 650,000 people are infected by Worldwide and 12 million suffer from tuberculosis. In Africa, 1.9% of new cases and 9.4% of diagnosed and treated patients are infected by MDR strain [2]. The 2002-2007 global survey conducted by WHO also showed that the highest prevalence of among newly diagnosed and previously treated TB cases were 22% and 60% respectively [35]. The World Health Organization (WHO), without an actual survey, estimated a much lower burden of 1.9 and 9.3% for new and previously treated patients, respectively [1]. This could be attributed to lack of record keeping. It is probable that WHO probably underestimates Nigeria’s burden. This re-echoes the dire need for good clinical management practices and a prospective countrywide DST survey and adequate record keeping. Idigbe et al. [31] reported that 56% of the strains recovered from 96 patients not responding to anti- TB treatment were resistant to one or more of the drugs used, with 38% being resistant to isoniazid, although only 2% were resistant to rifampicin at that time [9] and did not find an association with HIV infection [10]. There is a need to establish more National Reference Laboratories (SRL) in Nigeria for technical support, quality assurance and surveillance. Several factors might be responsible to the development of MDR-TB. These include patient factors such as poor adherence of patients to first line anti-TB drugs, inappropriate treatment regimen, dosage and duration for treatment and non-compliance to national guidelines of TB treatment protocol by clinicians. | |
| Since drug-resistance is a dynamic phenomenon, it is pertinent to monitor the trend of drug-resistance periodically among patients presenting with TB symptoms. Adequate enlightenment should be given to patients for proper drug compliance, because inadequate dosage intake on non-compliance on the part of the patients and clinician as well should be encourage to abide with the National Tuberculosis Protocol (NTP) in other to reduce the TB surge. | |
| Conclusion | |
| Rifampicin resistant TB is factor to be considered in refractory cases, hence the need for continuous monitoring of drug resistance trends, in order to assess the efficacy of current interventions and their impact on the TB epidemic. | |
| Strengths | |
| GeneXpert has the potential to significantly increase TB case detection where traditional diagnostics were woefully inadequate – people with suspected HIV-associated TB and MDR-TB. | |
| Limitations | |
| First of all, this is a hospital-based study, hence there could have been significant referral bias involved in patients selection. GeneXpert machine is recommended to test for RIF resistance, which is used as proxy for. Every RIF-resistant case should have also culture/DST to determine resistance against other first and second line drugs. However, GeneXpert is not a panacea, its implementation presents major challenges, particularly related to cost and infrastructure, which call for a thoughtfully phased and careful introduction. | |
| Acknowledgement | |
| We are extremely grateful to the entire staff of the chest clinic project of Sobi Specialist Hospital, Ilorin, Kwara State, especially Mrs. Owolewa Omoshalewa Binta, Oyawoye Oluwaseun Tabitha and Messer’s. Abdulbaki Balogun Abdulrahman, Alao Salihu O as well as Dr. Erubu Saad Ayodeji and to the star deep water petroleum limited for donating the GeneXpert machine. | |
| Conflict of Interest Statement | |
| We declare that we have no conflict of interest | |
References
- WHO (2008) TB and Children in: Communicable diseases: tuberculosis fact sheet.
- WHO (2008) Epidemiology Fact Sheet on HIV and Aids: core data on epidemiology and response/ Djibouti. Who globalatlas.
- World Health Organization (WHO) (2012). Global tuberculosis report 2012. WHO/HTM/TB/2008.394).
- World Health Organization (WHO) (2000). Anti-Tuberculosis drug resistance in the world: the WHO/IUATLD Global Project on Anti-tuberculosis drug Resistance Surveillance (Fourth global report) (WHO/HTM/TB/2008.394).
- WHO (2011) Global Tuberculosis Control.
- Dosumu EA, Osagie K, Shuaib A, Lawson L (2008). Multidrug resistant tuberculosis at the national hospital, Abuja Nigeria. Afr J Respir Med 4: 22-23.
- Ani AE, Idoko J, Dalyop YB, Pitmang SL (2009) Drug resistance profile of Mycobacterium tuberculosis isolates from pulmonary tuberculosis patients in Jos, Nigeria. Trans R Soc Trop Med Hyg 103: 67-71.
- Kehinde AO, Obaseki FA, Ishola OC, Ibrahim KD (2007) Multidrug resistance to Mycobacterium tuberculosis in a tertiary hospital. J Natl Med Assoc 99: 1185-1189.
- Daniel O, Osman E (2011) Prevalence and risk factors associated with drug resistant TB in South West, Nigeria. Asian Pac J Trop Med 4: 148-151.
- Taura Dw, Sale IT, Muhammed Y (2008) The prevalence of Tuberculosis in patients Attending the infectious Disease Hospital, Kano, Nigeria Int. Jor. P. App. Scs. 2: 63-69.
- Steingart KR, Ramsay A, Pai M (2007) Optimizing sputum smear microscopy for the diagnosis of pulmonary tuberculosis. Expert Rev Anti Infect Ther 5: 327-331.
- Helb D, Jones M, Story E, Boehme C, Wallace E, et al. (2010) Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 48: 229-237.
- Oyedeji GA Socioeconomic and Cultural Background Hospitalized Children in Ilesha, Nigeria. Journal of Paediatrics 1985;12111-117.
- WHO (2000). Tropical Diseases Research. World Health Organization, Geneva, Switzerland.
- World Health Organization (2011) Rapid implementation of the Xpert MTB/RIF diagnostic test. Geneva: WHO.
- Olusoji D, Eitayeb O, Olanrewaju O,Olapade GD (2013) Global Advanced Research Journal of Microbiology 2: 022-025.
- Imam TS, Oyeyi TI (2008) A retrospective study of pulmonary tuberculosis (PTB) Prevalence amongst patients attending infectious diseases hospital (IDH) in Kano, Nigeria. Bayero Journal of Pure and Applied Sciences 1: 10-15.
- Otu A, Umoh V, Habib A, Ameh S, Lawson L, et al. (2013) Drug Resistance among Pulmonary Tuberculosis Patients in Calabar, Nigeria. Pulm Med 2013: 235190.
- Taura DW, Sale IT, Mohammed Y (2008) The prevalence of tuberculosis in patients attending the infectious diseases hospital, Kano, Nigeria. Int. Jor.P. App.Scs 2: 63-69.
- Lawson L, Habib AG, Okobi MI, Idiong D, Olajide I, et al. (2010) Pilot study on multidrug resistant tuberculosis in Nigeria. Ann Afr Med 9: 184-187.
- Boyer-Cazajous G, Martinaud C, Déhan C, Hassan MO, Gaas Y, et al. (2014) High prevalence of multidrug resistant tuberculosis in Djibouti: a retrospective study. J Infect Dev Ctries 8: 233-236.
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, et al. (2010) Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363: 1005-1015.
- Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, et al. (2011) Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 377: 1495-1505.
- Barnes PF, Bloch AB, Davidson PT, Snider DE Jr (1991) Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med 324: 1644-1650.
- Zhao P, Li XJ, Zhang SF, Wang XS, Liu CY (2012) Social behaviour risk factors for drug resistant tuberculosis in mainland China: a meta-analysis. J Int Med Res 40: 436-445.
- Suchindran S, Brouwer ES, Van Rie A (2009) Is HIV infection a risk factor for multi-drug resistant tuberculosis? A systematic review. PLoS One 4: e5561.
- Gethum H, Gunneberg C, Granich R, Nunn P. Hiv infection-associated tuberculosis: a challenge in the management of tuberculosis. Afr J Health Sci 2008; 15: 6-13.
- Snider DE Jr, Roper WL (1992) The new tuberculosis. N Engl J Med 326: 703-705.
- Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen GM, et al. (1993) The emergence of drug-resistant tuberculosis in New York City. N Engl J Med 328: 521-526.
- Anastasis D, Pillai G, Rambiritch V, Karim SSA (1997) A retrospective study of human immunodeficiency virus infection and drug-resistant tuberculosis in Durban, South Africa, 2002-2008. Int J Tuberc Lung Dis 16: 967-973.
- Idigbe O, Sofola T, Akinosho R, Onwujekwe D, Odiah F, et al. (1998) Initial drug resistance tuberculosis amongst HIV seropositive and seronegative prison inmates in Lagos, Nigeria. Int Conf AIDS 12: 137.
- Getahun H, Gunneberg C, Granich R, Nunn P (2010) HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 50 Suppl 3: S201-207.
- Trivedi SS, Desai SG (1988) Primary antituberculosis drug resistance and acquired rifampicin resistance in Gujarat, India. Tubercle 69: 37-42.
- Jain NK, Chopra KK, Prasad G (1992) Initial and acquired isoniazid and rifampicin resistance to Mycobacterium tuberculosis and its implication for treatment. Indian J Tuberc 39: 121-124.
- WHO/IUATLD: Global Project on Anti-tuberculosis Drug Resistance Surveillance 2002-2007. Anti-tuberculosis drug resistance in the world: report 4
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 16492
- [From(publication date):
June-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 11910
- PDF downloads : 4582
