Research Article Open Access
Ribosomal Internal Transcribed Spacer (ITS) DNA Variation in Millepora
Craig S Tepper1* and Sophia C Gaynor1,2
1Department of Biology, Cornell College, Mt. Vernon, IA 52314, USA
2Interdisciplinary Graduate Program in Genetics, University of Iowa, Iowa City, IA 52242, USA
- *Corresponding Author:
- Craig S. Tepper
Department of Biology; Cornell College
Mt. Vernon, IA
USA, 52314
Tel: +3198954376
E-mail: ctepper@cornellcollege.edu
Received date: September 07, 2015; Accepted date: December 04, 2015; Published date: December 10, 2015
Citation: Tepper CS, Gaynor SC (2015) Ribosomal Internal Transcribed Spacer (ITS) DNA Variation in Millepora. J Marine Sci Res Dev 6:177. doi:10.4172/2155-9910.1000177
Copyright: © 2015 Tepper CS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Two main growth forms of Millepora (fire coral) are present around the islands of The Bahamas: one exhibits a strong, blade-like structure, Millepora complanata, and the other possesses a delicate branch-like structure, Millepora alcicornis. The phylogenetic relationship of these corals has been under considerable debate for over a century due to the existence of a wide-range of intermediate growth forms. Recent genetic analysis using ribosomal DNA (rDNA) suggests the existence of two distinct reproductively isolated cryptic clades that are independent of morphology [1]. However, using repeated rDNA sequences for phylogenetic construction can lead to false phylogenies if repeated sequences have not undergone concerted evolution, a process involving homogenization of individual repeats of a multigene family. We analyzed twenty rDNA clones isolated from a single bladed Millepora colony and found that although variant rDNA sequences were present, rDNA appears to be largely homogenized.
Abstract
Two main growth forms of Millepora(fire coral) are present around the islands of The Bahamas: one exhibits a strong, blade-like structure, Millepora complanata, and the other possesses a delicate branch-like structure, Millepora alcicornis. The phylogenetic relationship of these corals has been under considerable debate for over a century due to the existence of a wide-range of intermediate growth forms. Recent genetic analysis using ribosomal DNA (rDNA) suggests the existence of two distinct reproductively isolated cryptic clades that are independent of morphology [1]. However, using repeated rDNA sequences for phylogenetic construction can lead to false phylogenies if repeated sequences have not undergone concerted evolution, a process involving homogenization of individual repeats of a multigene family. We analyzed twenty rDNA clones isolated from a single bladed Millepora colony and found that although variant rDNA sequences were present, rDNA appears to be largely homogenized.
Keywords
rDNA ITS (Internal transcribed spacer); Millepora; Concerted evolution; Phenotypic plasticity; Intragenomic sequence variation; DGGE (Denaturing gradient gel electrophoresis)
Introduction
For centuries, biologists have attempted to group organisms based on shared characteristics in order to understand the evolutionary relationships of the tremendous diversity of life. Phylogenic relationships are estimated using morphological, behavioral, and other phenotypic characters. However, these characters may not accurately represent evolutionary relationships because evolution is not always divergent [2,3]. Two species can independently evolve the same features due to similar habitats and favored adaptations. Therefore, two species that are not closely related may end up more phenotypically similar to each other than to their closest relatives.
The problem of understanding evolutionary relationships between organisms is particularly acute in reef-building coral assemblages that serve as the foundation of complex reef ecosystems [4]. The evolutionary history and current speciation in this diverse class of animals remain paradoxical. For example, although some corals reproduce during non-synchronous spawning, most corals reproduce in annual, synchronous mass spawning events that provide numerous opportunities for interspecific hybridization [5]. Vollmer and Palumbi [6] examined Acropora cervicornis, A. palmata, and A. prolifera. They demonstrated that A. prolifera was a first-generation hybrid descendent of A. cervicornis and A. palmata and did not deserve a separate species designation. Hence, coral diversity can occur not only by conventional species formation, but also through inter-species hybridization. Further compounding the problem is that morphological traits used to construct phylogenies are not always useful for corals [4]. Coral taxonomic classification (as well as our current understanding of coral evolution) is based upon morphological characters of the calcareous skeleton [7]. Unfortunately, the calcareous skeleton of many marine organisms shows considerable phenotypic plasticity. The architecture of the coral skeleton is affected by environmental factors such as underwater irradiance, water motion, water temperature, and sedimentation [8]. Additionally, calcification rates are affected by lunar, diurnal and seasonal fluctuations [9].
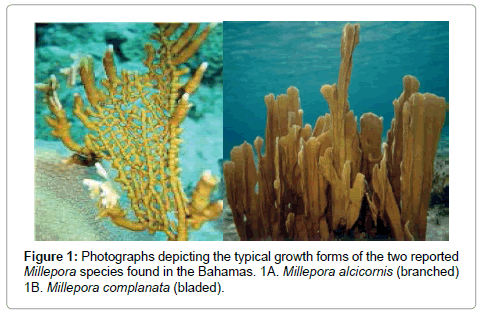
The coral phylogenetic issue is particularly problematic in the calcareous hydrozoan coral, Millepora, which is one of the most common skeleton-forming animals on reefs. This group of corals, known as fire coral because of its painful sting, is represented by multiple species and is nearly ubiquitous on reefs in the Atlantic, Indian and Pacific Oceans [10]. Millepores are important reef framework builders, second only to the scleractinia corals [10]. The morphology of the Millepores is highly variable and shows phenotypic plasticity [1,4,11]. Stearn and Riding [4] reported that the various growth forms of Millepora in the Caribbean range from thinly encrusting sheets and delicate dendroid branches for M. alcicornis, to thicker, rigid bladed forms for M. complanata (Figure 1). It is this variation in morphology that has led to constant controversy about Millepore classification. Currently, species designation within the genus is mainly based on growth form, geographical distribution and morphological differences such as surface texture, nematocyst structure, and the size and shape of pores [10].
Tepper et al. [1] examined the evolutionary relationship of the two commonly found Millepores (M. complanata and M. alcicornis) in the northern Caribbean off the coast of San Salvador, The Bahamas. In addition to these two recognizable morphologies, they reported the existence of numerous intermediate forms, of which the specific species status is questionable. Because of the wide range of growth forms (Figure 2), the question arises whether the blade (M. complanata) and branching (M. alcicornis) forms are separate species or represent phenotypic variations of one highly variable species complex.
The inherent uncertainty in using morphological characters as a way to establish phylogenetic relationships can be aided by using genetic markers to distinguish between closely related growth forms. Among the most widely used genetic markers are the internal transcribed spacer (ITS) regions of ribosomal DNA (rDNA). Ribosomal RNA genes are organized in clusters of hundreds to thousands of tandem repeats per chromosome, each of which consists of three coding regions (18S, 5.8S and 28S), two internal transcribed spacer regions (ITS-1 and ITS-2), one external transcribed spacer (ETS), and one non-transcribed spacer (NTS) region [12].
The two ITS regions have been used for detecting differences between conspecifics and are useful in studying closely related individuals due to their higher mutation rates [13]. Takabayashi et al. [14] reported that the ITS region varies from 2 to 31% in different coral species, making this region ideal for comparative analyses between populations. Meroz-Fine et al. [15] utilized DNA sequence information from the ITS regions of the Red Sea Fire Coral, Millepora dichotoma, to show that the currently recognized single species with two growth forms (blade and branching) was instead two distinct species. Forsman et al. [16] reported that they were unable to use morphological characters to identify species diversity in the coral genus Porites. Using rDNA ITS regions and mitochondria gene markers, they revealed numerous cryptic patterns of species diversity in Porites.
Tepper et al. [1] used rDNA ITS regions and identified the existence of two Millepore clades. Each clade contained members of all three morphologies (bladed, branched, and intermediate growth forms). Their analysis suggests the existence of two cryptic clades that are independent of morphology and reproductively isolated.
Although phylogenetic analysis based on rDNA has helped untangle evolutionary relationships, the use of rDNA sequences has proven to be problematic because of the existence of variability among the repeated rDNA units, which may cause extensive differentiation even within single individuals [17]. The problem concerns the assumption that these repeated sequences have homogenized via concerted evolution, a process involving homogenization of individual repeats of a multigene family [18-22]. Concerted evolution results in the production of uniform sequences in all repeats in a given species. Two mechanisms, unequal crossover and gene conversion, have been proposed to contribute to the process of concerted evolution [18]. However, evidence exists that concerted evolution may not be complete in all species. Vollmer and Palumbi [17] showed that concerted evolution did not completely homogenize rDNA arrays, thereby accounting for the intragenomic variation they observed in rDNA repeats for the scleractinian coral Acropora leading to the construction of false phylogenies. LaJeunesse and Pinzón [23], using a single colony of Acropora valida, reported the existence of 23 unique ITS-2 sequences out of 29 that were sequenced, some differing by up to 28%. Dorado and Sánchez [24], using the gorgonian coral Pseudopterogorgia bipinnata, reported an ITS-2 sequence variation of 14.4% among 37 samples. However, phylogenetic analyses of the ITS regions for corals in the genera Pavona, Platygyra, Poritesand Siderastrea [25-27] demonstrated clear phylogenetic relationships due to the lack of intragenomic variability within ITS regions. These results indicate that sequences of rDNA ITS regions are useful for phylogenetic analysis as long as the level of intragenomic variability is assessed.
Sequencing cloned rDNA ITS regions to generate phylogenetic trees, as was done by Tepper et al. [1] for the Millepores, can lead to to the detection of variants in repeated sequences resulting in construction of false phylogenies unless repeated rDNA arrays have been homogenized [28]. In order to determine if the two cryptic Millepore clades identified by Tepper et al. [1] are valid, we are assessing intragenomic rDNA sequence variation.
Here we report that although minor rDNA sequence variation is present, Millepore intraspecific rDNA sequence divergence is consistently lower than interspecific sequence divergence. Since other investigators have reported high levels of rDNA intragenomic variation using single colonies of coral [17,23,24], our results imply that the rDNA regions appear to be homogenized in Millepores residing in patch reefs around San Salvador, The Bahamas.
Materials and Methods
Field-site description
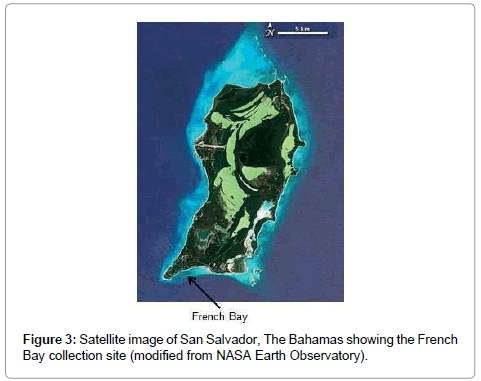
Millepore colonies used in this study were collected in May 2010 from a patch reef at French Bay (23°56’59”N, 74°32’50”W), located on the southern end of San Salvador, The Bahamas (Figure 3). San Salvador is located on the eastern edge of the Bahamian island chain and is characterized by its karst and hyper saline lakes. Colonies were held in a flow-through seawater tank for no more than two days before coral DNA was isolated.
DNA extraction, PCR amplification and DNA sequencing
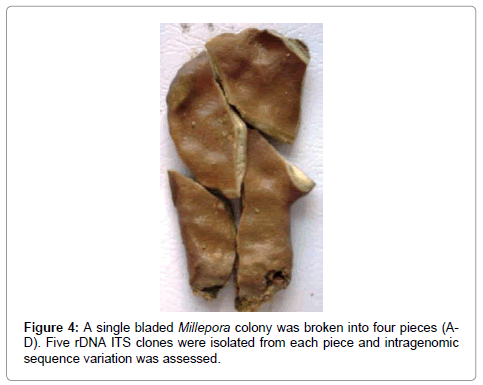
A single bladed form of Millepora was broken into four pieces (Figure 4) and DNA was extracted, PCR amplified, cloned, and sequenced as described below. Five clones were isolated and sequenced from each coral fragment.
Genomic DNA was isolated from colonies of Millepora using a procedure modified from Rowan and Powers [29] and Lopez et al. [30] and described in Tepper et al. [1].
ITS rDNA PCR amplification was performed as described in Tepper et al. [1] using the coral specific primer A18S (5’GATCGAAC-GGTTTAGTGAGG3’), and the universal primer ITS 4 (5’TCCTCCGCTTATTGATATGC3’) [14]. The primers amplify approximately 781 nucleotides [1].
Amplified PCR products were run on 1.2% (w/v) low melting agarose gels, excised, ligated into pGEM-T vectors (Promega) and transformed into competent JM109 E. coli host cells [1]. Following blue-white selection, positive colonies were harvested and plasmid DNA was isolated using the Zyppy Plasmid Miniprep Kit (Zymo Research). Plasmids containing ITS rDNA inserts were sequenced in both directions with fluorescently-labeled M13 forward and reverse primers [1] using a LI-COR 4300 DNA Analyzer (LI-COR, Lincoln, NE). Sequence reaction products were analyzed using e-Seq V3.0 (LI-COR). A BLAST query of the National Center of Biological Information’s (NCBI) sequence database confirmed that the sequences were most similar to other Millepora rDNA samples.
Intragenomic sequence variation analysis
Sequences were manually aligned to correspond with published alignments. Percent divergence and unrooted phylogenetic reconstructions (based on Maximum Parsimony) were implemented in ClustalW and ClustalX version 2.0 [31]. Percent divergence calculations were based on pair-wise treatments of sequences. Ribosomal DNA sequence from M. exaesa was used as the outgroup [32].
Denaturing gradient gel electrophoresis (DGGE)
Amplification and primers for PCR-DGGE analysis were as described above except the primer ITS 4 was modified with a 39 bp GC clamp [33] (5’CGCCCGCCGCGCCCCGCGCCCGTC CCGCCGCCCCCGC CCTCCTCCGCTTATT GTATG3’). In order to determine if the PCR amplified rDNA products were heterogeneous or homogeneous, PCR products from one bladed and one branched form of Millepora were run on 6% polyacrylamide denaturing gels containing a 40- 80% gradient (2.8M urea/16% formamide to 5.6M urea/32% formamide). Gels were pre-run at 90 volts and 55°C for 30 minutes. Once PCR products were loaded, the gels were pre-heated at 60°C for 10 minutes without voltage followed by 150 volts at 60°C for 20-24 hrs. The gels were stained for 1-2 hrs in TAE (40 mM tris, 10 mM sodium acetate, 1 mM EDTA, pH 8) containing ethidium bromide (5 μg/mL) and destained in fresh TAE buffer for 30 minutes. Following staining, the gels were photographed under UV light.
Results
Ribosomal DNA variation within individuals and between species
Twenty different clones were isolated and sequenced from a single colony of bladed Millepora (Figure 3). Of the 20 rDNA clones, 11 (55%) were identical in sequence. The amplified rDNA fragment (781 bp, not including primers) contained partial fragments of the 18S (127 bp) and 28S (39 bp) rDNA genes, the entire sequence of the 5.8S (158 bp) gene, and the entire sequence of the ITS rDNA, ITS-1 (242 bp) and ITS-2 (215 bp). No length variation was observed in any of the rDNA sequences. The average pairwise sequence percent divergence (the number of non-nucleotide matches between two sequences divided by the length of the sequence) among clones within a single bladed Millepora colony was 0.025% with a range of 0-0.087% (Table 1). The expected Taq polymerase cloning error rate is approximately 0.01% [34]. Within individual rDNA variation was observed across four of five rDNA regions. The lone exception was the partial 18S rDNA sequence, which displayed no variation in the twenty clones. The levels of withinindividual variation were highest for the ITS regions, reaching 0.017% (average of 0.012%) in ITS-1 and 0.172% (average of 0.047%) in ITS-2. Among the three rDNA genes, the variation ranged from 0, for all three rDNA genes, to a high of 0.026% for the partial 28S rDNA fragment.
| Overall Avg | 18s Avg | ITS-1 Avg | 5.8s Avg | ITS-2 | 28s Avg | |
|---|---|---|---|---|---|---|
| (Range) | (Range) | (Range) | (Range) | (Range) | (Range) | |
| Bladed Millepora | 0.025 | 0 | 0.012 | 0.003 | 0.047 | 0.005 |
| (0-0.087) | -- | (0-0.017) | (0-0.013) | (0-0.172) | (0-0.026) | |
| Milleporaexaesa | 0.653 | 0.126 | 0.696 | 0.356 | 0.691 | 0.054 |
| (0.649-0.655) | -- | (0.694-0.703) | (0.354-0.361) | (0.689-0.703) | (0.051-0.077) |
Table 1: Ribosomal DNA variation. Average pairwise sequence divergence (%) an among 20 sequenced bladed Millepora clones showing levels of rDNA variation observed within an individual coral colony and between species. The between species comparison is represented by the 20 sequenced Millepora clones compared to a single M. exaesa sequence [31]. Ranges are also provided.
Average percent interspecific sequence divergence between the bladed Millepora colony and the Mediterranean M. exaesa (Table 1) was 0.653% overall (ranging from 0.649-0.655%), 0.696% in ITS- 1 (ranging from 0.694-0.703%) and 0.691% for ITS-2 (ranging from 0.689-0.703%). As observed for within colony variation, interspecific rDNA variation was highest in the ITS regions and lowest in the rDNA genes.
Phylogenetic reconstructions based on Maximum Parsimony were used to analyze the level and pattern of diversity among sequence variants generated from the 20 bladed Millepora clones and unrooted phylogenies based on ITS-1 and ITS-2 rDNA sequences were constructed (data not shown). All 20 sequences from the bladed Millepora colony were very tightly clustered together and difficult to differentiate. The sequences showed little divergence which supports the percent divergence data. The published outgroup sequence of M. exaesa [33] occupied a distant phylogenetic position in the reconstruction, indicating its divergence from the bladed Millepora colony.
Assessing intragenomic variation using denaturing gradient gel electrophoresis (DGGE)
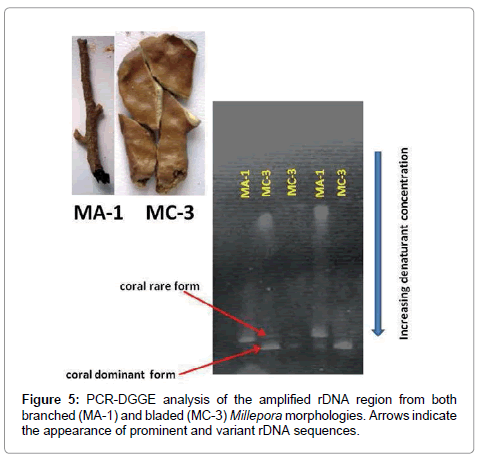
DGGE analysis offers a method for visualizing intragenomic variation when direct (non-plasmid based) sequencing is difficult as in the millepores [1]. We chose to amplified rDNA sequences of a single colony of both bladed and branched Millepora morphologies because these colonies were previously identified to belong to different clades that were separated by five SNPs [1]. Interestingly, the results revealed the different morphologies contained a different dominant and a rare sequence variant (Figure 5). Sequence variation within rDNA is still common despite concerted evolution and is prevalent in ITS regions [23]. A rare rDNA variant can arise in a single generation as a result of a single point mutation. However, there is a low probability that the variant will spread through the rDNA array and become dominant [23].
The DGGE gel results for M. complanata consistently showed the presence of only two variant rDNA forms although only 11 out 20 (55%) rDNA sequences were identical. A comparison of the sequence data showed that of the nine rDNA that did not match, three were identical and the remaining 6 sequences contained single unique nucleotide substitutions. A possible explanation for the appearance of only two rDNA variants observed on the DGGE gels might be the lack of sensitivity of DGGE to detect low copy number single nucleotide rDNA variants. Although all corals examined to date contain some level of rDNA intragenomic variation, LaJeunesse and Pinzón [23] have shown that DGGE can be used to identify dominant sequences which are best suited for phylogenetic reconstructions that are relevant to the evolutionary history of the species. The appearance of only two rDNA variants in the DGGE gels (Figure 5) and the tight clustering of the 20 cloned rDNA fragments observed in the unrooted tree (data not shown) indicate there are only minor nucleotide differences in the rDNA repeats.
Discussion
Eukaryotic genomes typically contain hundreds to thousands of tandemly repeated copies of the 18S, 5.8S and 28S genes, and the two internal transcribed spacers, ITS-1 and ITS-2 [12]. As part of a multigene family, the numerous copies of rDNA are expected to become homogenized through mechanisms of concerted evolution [18]. Homogenization leads to greater similarity among members of a repeated sequence family within a species than between species. This is the reason why rDNA sequences have been extensively used for species-level phylogenetic analysis [13]. However, basing specieslevel phylogenetic reconstructions on ITS regions of rDNA can be problematic because of incomplete homogenization due to the existence of polymorphisms, unequal crossover, gene conversion and interspecific hybridization among repeated units which may cause extensive differentiation even within single individuals [35].
Phylogenetic studies based on rDNA have been used extensively to provide numerous insights into scleractinian coral evolution [25- 27,32,36,37]. However, Vollmer and Palumbi [17] demonstrated that individual coral colonies can contain high levels of intragenomic variation, and rDNA sequences may not be suitable for species-level phylogenetic analysis. Although most of the intragenomic variation was uncovered in the genus Acropora, their results implied that the rate of concerted evolution, and hence homogenization of repeated sequences, is quite variable in corals. This high rate of intragenomic variation may be due to interspecific hybridization as well as incomplete concerted evolution [17].
The level of intragenomic variation present in Millepora is low as demonstrated in the pairwise percent divergence data (Table 1). In addition to the low levels of average genetic divergence in the three conserved rDNA genes, both ITS regions were also extremely low, indicating that the rDNA array may be homogenized in the population of Millepores inhabiting patch reefs surrounding San Salvador, The Bahamas. If enough time has passed for concerted evolution to take place, low levels of intragenomic variation makes sense because the stem-loop secondary structures of both ITS-1 and ITS-2 rRNA play a major role in the excision and maturation of rRNA and reduced tolerance of nucleotide changes are expected as a result of the ITS splicing machinery [38]. Hence, only variants carrying minor changes outside of secondary stem formation in DNA sequence are tolerated. This assumption is supported by the two closely related rDNA variants observed for both bladed and branched forms of Millepora on the DGGE gel (Figure 5). Since DGGE analysis is based on the melting characteristics of the double-stranded PCR products, variant DNA fragments that migrate close together are expected to have small changes in DNA sequence.
In contrast to the low sequence divergence within an individual, the overall pairwise sequence percent divergence average for the bladed form of Millepora compared to M. exaesa is 0.653% (a range of 0.649- 0.655%). When individual rDNA regions are compared, the conserved genes had the lowest sequence divergence (range of 0.051% for the partial 28S gene to 0.361% for the complete 5.8S gene) and the ITS regions had the highest (range of 0.694% for ITS-1 to 0.703% for both ITS-1 and ITS-2; Table 1). The results indicate that the rDNA sequences appear to be homogenized in this population of Millepora. Hence, PCR amplification followed by rDNA cloning and sequencing may be a reliable method for understanding the phylogenetic relationship of the Millepores surrounding San Salvador, The Bahamas.
Our study validates the use of rDNA as a potential phylogenetic tool to determine species-level distinctions in the Millepores and confirms the work of Chen et al. [36] who showed that high intragenomic rDNA sequence diversity seems to be unique to Acropora due to its ability to cross hybridize and then have one of the parent species backcross to the hybrids. Tepper et al. [1] showed that the two clades of Millepores surrounding San Salvador, The Bahamas are not hybridizing because they do not share any SNPs at the five ITS locations and appear to be reproductively isolated. The percent sequence divergence data clearly show that there are low levels of intragenomic rDNA variation, suggesting that the phylogenetic signals are informative for specieslevel comparisons. Similar patterns of low intragenomic variation have been reported for other corals [25-27].
Although the Millepores may be an exception, other lines of evidence indicate that traditional cloning methods overestimate the intragenomic diversity [23]. In order to avoid using phylogenetic markers that can lead to false phylogenies, excising bands from DGGE gels for sequence analysis allows for the detection of prevalent and rare intragenomic variants that may be scored as distinct phylogenetic signals by traditional cloning methods [24].
Most phylogenetic studies use primary DNA sequence information; however, Chen et al. [36] have shown that rRNA secondary structures are useful in phylogenetic reconstructions because they contain characters not found in the primary structure. The transcript folding structure of the ITS-2 region provides the proper orientation for the ribosomal coding regions when they are processed into small and large rRNA. Functional ITS sequences fold themselves to form secondary structures that are conserved and can be used as diagnostic indicators of taxonomic difference. ITS predicted secondary structure has been useful in providing reliable phylogenetic information in many corals including scleractinians (with the exception of Acropora) [36], the hexacoral Zoanthus, [39], and octocorals [24,40]. Grajales et al. [40] reported that predicted ITS-2 secondary structure led to the construction of new phylogenetic relationships for the octocoral species, Eunicea. We are beginning to examine whether predicted rRNA ITS-2 secondary structure can aid in untangling the phylogenetic relationships of the Millepores.
Acknowledgements
This work was conducted in the Bahamas under a permit granted by The Bahamas Environment, Science and Technology (BEST) Commission. We would like to thank Cornell College researchers Aye Mon, Elise Mead, and Helen Pope for their assistance with the molecular data. We would also like to thank Dr. Donald T. Gerace, Chief Executive Officer, and Dr. Tom Rothfus, Executive Director of the Gerace Research Center, San Salvador, Bahamas. Completion of this work was made possible by Cornell College Faculty Development Grants (CST) and a Ringer Endowed Fellowship (CST), McElroy Research Grants (CST), a GRC Student Grant (SCG) and the LI-COR Biosciences Genomics Education Matching Funds Program (CST).
References
- Tepper CS, Squiers L, Hay C, Gorbach D, Friend D, et al. (2012) Cryptic species: A mismatch between genetics and morphology in Millepora. Marine Science 2:57-65.
- Hendry AP (2009) Speciation. Nature 458: 162-164.
- Schluter D (2009) Evidence for ecological speciation and its alternative. Science 323: 737-741.
- Stearn CW, Riding R (197) Forms of the Hydrozoan Millepora on a recent coral reef. Lethaia 6:187-200.
- Harrison PL, Babcock RC, Bull GD, Oliver JK, Wallace CC, et al. (1984) Mass spawning in tropical reef corals. Science 223: 1186-1189.
- Vollmer SV, Palumbi SR (2002) Hybridization and the evolution of coral reef diversity. Science 296: 2023-2025.
- Wallace CC, Willis BL (1994) Systematics of the coral genus Acropora: Implications of new biological findings for species concept. Annual Review of Ecology and Systematics 25: 237-262.
- Barnes DJ, Chalker BE (1990) In: Dubinsky Z (Ed.) Calcification and photosynthesis in reef building corals and algae. Coral Reef Ecosystems of the World. Elsevier, Philadelphia, PA 109-131.
- Barnes DJ, Lough JM (1989) The nature of skeletal density banding in scleractinian corals: Fine banding and seasonal patterns. Journal of Experimental Marine Biology and Ecology 126: 119-134.
- Lewis JB (2006) Biology and ecology of the hydrocoral Millepora on coral reefs.Adv Mar Biol 50: 1-55.
- Ruiz-Ramos DV, Weil E, Schizas NV (2014) Morphological and genetic evaluation of the hydrocoral Millepora species complex in the Caribbean. Zoological Studies 53: 1-15.
- Prokopowich CD, Gregory TR, Crease TJ (2003) The correlation between rDNA copy number and genome size in eukaryotes. Genome 46: 48-50.
- Hillis DM, Dixon MT (1991) Ribosomal DNA: Molecular evolution and phylogenetic inference. Quarterly Review of Biology 66: 411-453.
- Takabayashi M, Carter DA, Loh WKW, Hoegh-Guldberg O (1998) A coral specific primer for PCR amplification of the internal transcribed spacer region in ribosomal DNA. Molecular Ecology 7: 925-931.
- Meroz-Fine E, Brickner I, Loya Y, Ilan M (2003) The hydrozoan coral Milleporadichotoma: Speciation or phenotypic plasticity? Marine Biology 143: 1175-1183.
- Forsman ZH, Barshis DJ, Hunter CL, Toonen RJ (2009) Shape-shifting corals: Molecular markers show morphology is evolutionarily plastic in Porites. BMB Evolutionary Biology 9: 45-53.
- Vollmer SV, Palumbi SR (2004) Testing the utility of internally transcribed spacer sequences in coral phylogeny. Molecular Ecology 13: 2763-2772.
- Elder JF, Turner BJ (1995) Concerted evolution of repetitive DNA sequences in eukaryotes. Quarterly Review of Biology 70: 297-320.
- HarpkeD, Peterson A (2006) Non-concerted ITS evolution in Mammillaria (Cactaceae). Molecular Phylogenetics and Evolution 41: 579-593.
- Harris DJ, Crandall KA (2000) Intragenomic variation within ITS1 and ITS2 of fresh-water crayfishes (Decapoda: Cambaridae): Implications for phylogenetic and microsatellite studies. Molecular Biology and Evolution 17: 284-291.
- Sang T, Crawford D, Stuessy TF (1995) Documentation of reticulate evolution in peonies (Paeonia) using internal transcribed spacer sequences of nuclear ribosomal DNA: Implications for biogeography and concerted evolution. Proceedings of the National Academy of Sciences 92: 6813-6817.
- Zimmer EA, Martin SL, Beverley SM, Kan YW, Wilson AC (1980) Rapid duplication and loss of genes coding for the α chains of hemoglobin. Proceedings of the National Academy of Sciences 77: 2158-2162.
- LaJeunesse TC, Pinzón JH (2007) Screening intragenomicrDNA for dominant variants can provide a consistent retrieval of evolutionary persistent ITS (rDNA) sequences. Molecular Phylogenetics and Evolution 45: 417-422.
- Dorado D, Sánchez JA (2009) Internal transcribed spacer 2 (ITS2) variation in Gorgonian coral Pseudopterogorgiabipinnata in Belize and Panama. Smithsonian Contributions to the Marine Sciences 38: 173-179.
- Forsman ZH, Guzman HM, Chen CA, Fox GE, Wellington GM (2005) An ITS region phylogeny of Siderastrea (Cnidaria: Anthozoa): Is S. glynni endangered or introduced. Coral Reefs 24: 343-347.
- Lam K, Morton B (2003) Morphological and ITS1, 5.8S, and partial ITS2 ribosomal DNA sequence distinctions between two species Playtygyra (Cndaria: Scleractinia) from Hong Kong. Marine Biotechnology 5: 555-567.
- MoothienPillay KRM, Asahida T, Chen CA, Terashima H, Ida H (2005) ITS ribo-somal DNA distinctions and genetic structure of populations in two sympatric species of Pavona (Cnidaria: Scleractinia) from Mauritius. Zoological Studies 45: 132-144.
- http://earthobservatory.nasa.gov/IOTD/view.php?id=76134
- Rowan R, Powers DA (1991) Molecular genetic identification of symbiotic dinoflagelletes (Zooxanthellae). Marine Ecology Progress Series 71: 65-73.
- Lopez JV, Kersanach R, Rehner SA, Knowlton, N (1999) Molecular determination of species boundaries in corals: Genetic analysis of the Montastraeaannularis complex using amplified fragment length polymorphisms and a microsatellite marker. Biology Bulletin 196: 80-93.
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) ClustalW and Clus-talX version 2.0. Bioinformatics 23: 2947-2948.
- Odorico DM, Miller DJ (1997) Internal and external relationships of the Cnidaria: implications of primary and predicted secondary structure of the 5'-end of the 23S-like rDNA. Proceedings of the Royal Society, London B Biological Sciences 264: 77-82.
- Sheffield VC, Cox DR, Lerman LS, Meyers RM (1989) Attachment of a 40-base pair G+C rich sequence (GC-clamp) to genomic fragments by polymerase chain reaction results in improved detection of single-base changes. Proceedings of the National Academy of Sciences 86: 232-236.
- Eckert KA, Kunkel TA (1990) High fidelity DNA-synthesis by the Thermusaquaticus DNA polymerase. Nucleic Acids Res 18: 3739-3744.
- Suh Y, Thien LB, Reeve HE, Zimmer EA (1993) Molecular Evolution and phylogenetic implications of the internal transcribed spacer sequences of ribosomal DNA in Winteraceae. American Journal of Botany 80: 1042-1055.
- Chen CA, Chang C, Wei NV, Chen C, Lein Y, et al. (2004) Secondary structure and phylogenetic utility of the ribosomal internal transcribed spacer 2 (ITS2) in scleractinian corals. Zoological Studies 43: 759-771.
- Lopez JV, Knowlton N (1997) Discrimination of species in the Montastraeaannularis complex using multiple genetic loci. Proceedings of the 8th International Coral Reef Symposium 2: 1613-1618.
- Cote CA, Peculis BA (2001) Role of ITS2-proximal stem and evidence of indirect recognition of processing sites in pre-rRNA processing in yeast. Nucleic Acids Research 29: 2106-2116.
- Aguilar C, Reimer JD (2010) Molecular phylogenetic hypotheses on Zoanthus species (Anthozoa: Hexacorallia) using RNA secondary structure of the internal transcribed spacer 2 (ITS2). Marine Biodiversity 40: 195-204.
- Grajales A, Aguilar C, Sanchez JA (2007) Phylogenetic reconstruction using secondary structures on internal transcribed spacer 2 (ITS2, rDNA): Finding the molecular and morphological gap in the Caribbean gorgonian corals. BMC Evolutionary Biology 7: 90-98.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 11326
- [From(publication date):
February-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10450
- PDF downloads : 876