Rhizoremediation of Hexachlorocyclohexane Through Pesticide Contaminated Soil by Solanum nigrum
Received: 21-Oct-2018 / Accepted Date: 20-Dec-2018 / Published Date: 27-Dec-2018 DOI: 10.4172/2155-6199.1000457
Abstract
Quality and magnitude of World’s food have been improved using pesticides. However, these pesticides, such as Hexachlorocyclohexane, have unfavorably affected the quality of environment and health of human beings. These not only used in Agriculture but in public health activities. Their use has been banned in developed countries, but these are still being used in some developing countries including Pakistan. Present study was aimed at sssessment, of HCHs concentration to be removed by rhizoremediation with Solanum nigrum; Solanum nigrum growth performance and identification of inoculate to be best for rhizo-microbial remediation. Pot experiments at 0, 5 and 10 mg/kg HCHs spiked soil were conducted for 90 days. Soil physicochemical properties (pH, TOC, OM, EC, MBC) were measured. Residual HCHs concentration in spiked soil was 1.73, 2.33, 3.9 and 6.1 mg/kg for 5% HCHs +Solanum nigrum+Inoculate; 5% HCHs+Inoculate; 10% HCHs Solanum nigrum+Inoculate; 10%HCHs+Inoculate respectively. While HCHs accumulation in Solanum nigrum in different treatments was 1.233, 2.133, 2.6667 mg/kg for 5% HCHs+Solanum nigrum+Inoculate; 10% HCHs+Solanum nigrum; 10% HCHs+Solanum nigrum+inoculate respectively. Strain which effectively improved the plant root and biomass was ST47 which improved root length almost 6.9 cm. Results elucidated the use of Solanum nigrum along with ST47 strain as the effective and promising remediation technique for HCHs degradation.
Keywords: HCHs; Solanum nigrum; Pesticide; Rhizoremediation; Contamination
Introduction
As the population of world is growing day by day, sustenance of a country requires the use of techniques that not only benefit the country economically, industrially and in food industry but should support quality of environment. To support the food requirement of large population pesticides have become the common source. If the use of pesticides exceeds the safe limit they pollute the environment, degrading its ecological aspects and hampering its natural processes to sustain life [1]. However, some pesticides itself are dangerous in their low limit, that are persistent in nature and are called persistent organic pollutants [2].
Hexachlorocyclohexane (HCH) isomers namely the alpha HCH, beta-HCH, and gamma-HCH (commercially known as lindane) are considered as new POPs and included in POPs list in 2009 [3] and will therefore be addressed at global level because of their dumped waste remaining from the historic utilization and manufacture [4]. Hexachlorocyclohexane is a type of organochlorine pesticide. Benzene molecule is chlorinated in presence of ultraviolet light to manufacture HCHs [5]. HCHs began to be produced in 1940s since then they have resulted in many serious environmental issues [6]. These are toxic not only to humans and animals but also cause harm to the soil and water [7]. Production of commercial HCHs also generates large number of byproducts (alpha, beta and partial isomers of HCHs), considered as waste called “HCH Muck”, e.g., 8 to 12 tons of other isomers of HCHs are generated along with 1 tons of commercial gamma isomer of HCHs manufacture [8]. These wastes are notorious in nature and are more problematic than commercial HCHs itself, and results in higher contamination at point source. Main problem is management of HCHs Muck [3]. Pakistan is one of those developing countries which is having problem of management of pesticide residual. Agriculture has largest share (21%) in Pakistan’s economy, 44% of labour force is employed in this sector (economic survey, 2009-2010). Pests and diseases are main cause of crops destruction in each year, so they should be managed in a proper manner to improve the food production. In this way growing population can be feed [9].
Eqani et al. [10] did a study in river Chenab in Pakistan to check the level and distribution of selected organochlorine insecticides. Results revealed that concentrations of gamma HCH, heptachlor endoepoxide, dieldrin, and DDTs (isomers and metabolites) in all sediment samples were well above interim sediment quality guidelines (ISQGs) and probable effect limits (PEL) given by Canadian Sediment Quality Guidelines (CSQGs).
The 1998 Food and Agriculture Organization (FAO) inventory of obsolete, unwanted, and/or banned pesticides also found unused stockpiles of both technical HCH and lindane (2,785 tons of technical HCH, 304 tons of lindane, and 45 tons of unspecified HCH material) scattered in dump sites in Africa and the Near East (http://www.fao.org). Weber et al. estimated that four to six million tons of various HCH materials have been dumped worldwide, which is similar in scale to the combined totals of dumped materials for all other persistent organic pollutants (POPs) defined by the Stockholm Convention [8].
To protect the environment and secure the public health different cleaning methods have been projected to remove HCHs from contaminated sites. One of those methods is remediation which returns contaminated environment to its natural condition. Among different remediation techniques rhizoremediation is considered as best, which is dissipation of contaminant by microbe in the rhizosphere of plant. Present study was conducted to degrade HCHs in rhizosphere of Solanum nigrum by microbe isolated from roots of plant grown on HCHs contaminated soil. This study was aimed at Isolation of inoculate to be best for combination with Solanum nigrum plant species for rhizo-remediation of HCHs and Assessment of HCHs concentration to be removed with rhizo-microbial remediation. Finally, assessment of the growth performance of Solanum nigrum grown in HCHs contaminated soil.
Research Methodology
Collection of Rhizospheric soil
Plants grown on contaminated soil were uprooted and their roots were gently shaken to get non rhizospheric soil, same procedure was repeated to get rhizospheric soil.
Isolation of HCHs degrading bacteria
Rhizospheric soil suspension was used to prepare series of dilution and from each dilution 200 μl suspension was plated on HCHs containing agar medium. Then it was incubated at 30°C for 48 hours. Isolates were further tested on agar medium containing HCHs (50 mg/l) and the most competent rhizobacteria were screened on the basis of their efficiency to consume HCHs as the only source of carbon. The most efficient rhizobacteria were further evaluated for their ability to support the development of Solanum nigrum and potential to degrade HCHs under controlled conditions. Enrichment technique was used for preparation of bacterial culture. In this method mineral salt medium was added into 250 ml conical flasks then these flasks were inoculated with 50 mg/l HCHs as a carbon source. Same process was repeated for preparation of suspension for each sample. Incubation of these covered flasks was done at 35°C at 180 rpm for seven days. Centrifugation at 10,000 rpm for fifteen minutes to eradicate cells was used for measuring degradation rate of contaminant. 1 ml culture suspension was spread onto agar plate by dilution plate technique after incubation period. Bacterial colonies which showed prolific growth in the medium were selected. Selected colonies were spread onto fresh agar medium and streaking was done to isolate pure culture. In order to get accuracy process was repeated twice. The initial streaks were stretch out diagonally the plate in successive cycles of sterilization and cross streaking. All selected isolates were sub cultured in nutrient agar slants and finally, all the purified rhizobacteria were maintained at 4°C till further used [11].
Sample collection
Uncontaminated soil was collected from NARC agricultural field and was ground, sieved with 2 mm pore size and air dried. Soil texture was measured by using Bouyoucos hydrometer method. Seeds and plants of Solanum nigrum were also collected from NARC.
Soil treatments
Soils samples were divided in nine treatments. Firstly, control soil with Solanum nigrum was used. Second treatment was comprised of control soil with Solanum nigrum and Inoculate. Third was consist of control soil with inoculate. Fourth treatment was done by using Solanum nigrum in 5% HCHs contaminated soil. In fifth treatment inoculate was injected into 5% HCHs spiked soil along with Solanum nigrum . 5% HCHs spiked soil was treated with inoculate in sixth treatment. Solanum nigrum were used in 10% HCHs spiked soil for seventh treatment. Solanum nigrum was used in presence of inoculate in soil spiked with 10% HCHs in eighth treatment. And last treatment was executed on 10% HCHs spiked soil along with inoculate. The samples were stored in polythene bags for 15 days for aging process [12].
Pot experiment
Pot experiments were carried out to assess the remediation of HCHs from contaminated soil by plant. One plant species Solanum nigrum and inoculate isolated from plant grown on HCHs contaminated soil was selected. Pot trials were carried out in Environmental Sciences department of International Islamic University Islamabad. Small plants of Solanum nigrum were grown in pots. Each pot was filled with 1.5 kg sieved soil.
For pot trials the plants of Solanum nigrum was transplanted into pots after 15 days of spiking with HCH. In each pot, Solanum nigrum were grown and inoculated with prepared inocula and irrigation was provided when needed. Three pot trials were used in each condition. The pot trails were performed with three replications using completely randomized design (CRD). Ambient light and temperature was provided to pots in the green house. The plants were harvested after three (03) month and plant growth parameters e.g., fresh and dry biomass and root, shoot length were collected and analysed statistically.
Soil physico-chemical analysis
Physical and chemical properties like soil moisture, pH, EC, TOC, MBC, organic matter of collected samples were analyzed. For soil moisture 10 g soil was reweighed then it was dried at 105°C for 24 hours at digital oven then this formula was used to calculate soil moisture.
Soil Moisture (%)=loss of weight in soil samples/weight of oven dried soil × 100
pH was measured by pH meter (Model: BMS pH-200L), EC was analyzed by EC meter in micro semen, Walkly 1997 titration method was used to calculate total organic carbon, following formula was used to calculate organic matter:
% Organic Matter (w/w)=1.724 *% Total Organic Matter
Rapid microwave irradiation and extraction technique was used to calculate Microbial Biomass Carbon [13].
Plant growth parameters
Plant root, shoot length was measured with meter rod and fresh and dry weight was measured with electrical weight balance before and at the end of experiment.
Hexachlorocyclohexane analysis
HCHs extraction from soil: 1 g soil samples were collected from each pot. Soil probe was pushed into soil up to depth of 6 inch and the collected soil was weighed in a weigh balance. Soil samples were then transferred into 100 ml Teflon tubes then mixed with 5 ml of dichloromethane and each sample was extracted for 2 hr in water bath at temperature of 38°C. Then samples were centrifuged at 4000 r min-1 for 5 min to separate the supernatant from soil. The extract was eluted with 1 and 2 ml mix of n-hexane: Dichloromethane (v/v 50:50) and the supernatant will the extract for HCH and then dried by sparging with N2; Solid residues were re dissolved in 1 ml of acetonitrile.
HCHs extraction from plant material: Plant material (root and shoot) were put through to water bath extractions for 20 min in ethyl acetate. Membrane filter was used to remove insoluble materials. The extract was conceded through silica gel packed column and the compounds on the silica gel column eluted with 250 ml of benzene. Elution was concentrated under reduced pressure, dried under nitrogen stream and then elute was re-dissolved in 1 ml of acetonitrile for GC-MS analysis [14].
Gas Chromatography - Mass Spectrometry (GC-MS)
The Gas chromatography-Mass spectrometry (GC-MS) was used for the analysis of hexachlorocyclohexane. For HCH, the temperature was start at 70°C for 3 min; it was then ramped at 5°C/min to 250°C and held for 1 min, was ramped to 300°C at 6 C/min and was held for 6 min and finally was ramped to 325°C at 10 C/min and was held for 5 min [14]. The standards of HCH mixture was run on GC-MS and retention times were optimized with individual HCH then samples were run on GC-MS.
Statistical analysis
The data obtained were evaluated on excel sheets. The statistical analysis was carried out with SPSS. Other analysis including, comparison of means, was performed.
Results and Discussion
The results that have been obtained after conducting a thorough study, on evaluating the potential of Solanum nigrum to degrade HCHs in the presence and absence of Inoculate, through series of experiment are described in the following section.
Soil physicochemical analysis
The result on soil properties as influenced by planting Solanum nigrum on spiked soil and inoculating it with bacterial strains in comparison to planting Solanum nigrum without bacterial strains on control soil for 90 days are presented in given Table 1.
| Soil type | Sandy clay loam |
|---|---|
| Silt (%) | 16 |
| Clay (%) | 19.8 |
| Sand (%) | 64.2 |
| Color | Brown |
| Moisture (%) | 35.89% |
Table 1: Soil physical properties.
Soil chemical properties: pH of soil was measured before and at the end of experiment and it was cleared that it showed a decreasing trend as the treatment time swap. Figure 1a showing that pH of the soil before experiment was slightly alkaline having range of 7.9 to 8.2 while its range at the end of research was 6.5 to 7.8. Treatment contaminated with 10% HCHs and Solanum nigrum along with inoculate showed highest decreasing trend while control soil with inoculate represented lowest decreasing trend. As the experiment begins T8 had 7.9 pH and after 90 days it was 6.5 on the other hand T3 had 7.8 pH values at the end of treatment. Decrease in pH value can be attributed to Solanum nigrum which releases specific organic acids thus decreasing the soil pH value. This study result coincides with another previous study in which pH of soil grown by Solanum nigrum was compared with S. lycopersicum and it was concluded that high organic acid secretion from S. nigrum lowers the pH value. Fernandez et al. [15] resulted from a study based on HCHs effects on soil microbiological properties that the drop off in pH and the raise in amount of Cl2 indicates that HCHs have been dissipated in spiked soil. Introduction of inoculate also resulted in lowering pH. The present study result is comparable to a study in which effect of legume inoculation was determined on soil pH by Kopittk et al. [16].
Figure 1a: pH. T1: control soil+solanum nigrum ; T2: control soil +Solanum nigrum +inoculate; T3: control soil+inoculate; T4: 5% HCHs+Solanum nigrum ; T5: 5% HCHs+Solanum nigrum +inoculate; T6: 5% HCHs+inoculate; T7: 10% HCHs+Solanum nigrum ; T8: 10%HCHS+Solanum nigrum +inoculate. T9: 10% HCHs+inoculate.
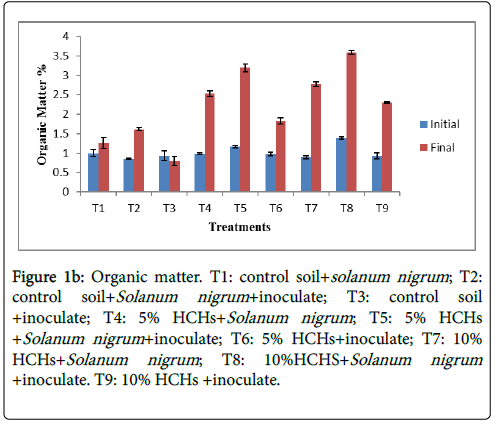
Organic matter of different treatments showed an increasing trend with the passage of experimental time Figure 1b. 10% HCHs spiked soil with Solanum nigrum in the presence of microbes (T8) represented higher organic matter 3.597%, at the start of experiment it was 1.39% while inoculated treatment on control soil (T3) showed decreased value from 0.93% to 0.79% in organic matter. Some treatments showed reasonable organic matter contents such as T4, T5, T7 and T9 had 2.53%, 3.19%, 2.77% and 2.29% organic matter respectively (Figure 1b).
Figure 1b: Organic matter. T1: control soil+solanum nigrum ; T2: control soil+Solanum nigrum +inoculate; T3: control soil +inoculate; T4: 5% HCHs+Solanum nigrum ; T5: 5% HCHs +Solanum nigrum +inoculate; T6: 5% HCHs+inoculate; T7: 10% HCHs+Solanum nigrum ; T8: 10%HCHS+Solanum nigrum +inoculate. T9: 10% HCHs +inoculate.
Variation rate was different for all treatments. Higher concentration of organic matter may be endorsed to the presence of inoculated Solanum nigrum and contamination with HCHs. Decomposition of Plant roots and mineralization of HCHs by microbes increases the organic content of soil. Present work is in consistence with Leal et al. [17] work, in which it was depicted that organic matter increased in crop cover soil as compared to bare soil. Treatments without contamination of HCHs depicted lower organic matter as compared to those having contamination and inoculated Solanum nigrum . This may be due to less mineralization and less microbial activity. HCHs mineralization hence improved the carbon content of soil [18]. Comparable results have been obtained by McMurry and Clayden et al. [19,20].
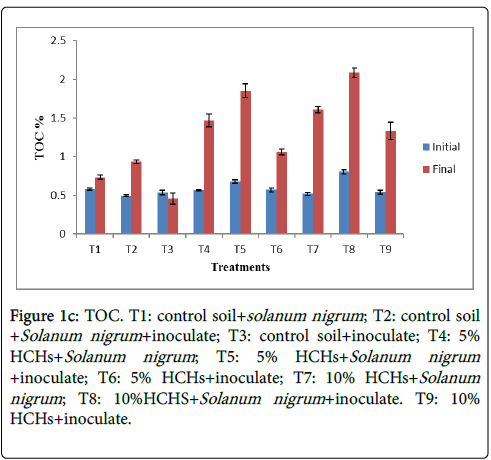
Results of the study showed that total organic carbon of treatments having HCHs and inoculated Solanum nigrum showed an increasing value from the start of experiment till the end (Figure 1c). Higher value 2.086% was observed at T8 in which 10% HCHs contamination was associated with Solanum nigrum and inoculate. It increased from 0.806% to 2.086%. While lowest 0.458% (initial concentration was 0.539%) was observed at T3 in which control soil amended with inoculate. Higher value of TOC is linked to higher organic matter and presence of HCHs which releases carbon into the soil by decomposition. Bacterial strains increase the rate of this decomposition resulting in higher concentration of TOC in the soil. Various previous studies confirmed the result of present study [21]. Mishra, K. and his colleagues found in a study that total organic carbon was positively associated with HCH of soil. Control soil amended with inoculate had lowest TOC because this had lowest organic matter and not contaminated with HCHs so little carbon is present in it that was used by microbes as their energy source.
Figure 1c: TOC. T1: control soil+solanum nigrum ; T2: control soil +Solanum nigrum +inoculate; T3: control soil+inoculate; T4: 5% HCHs+Solanum nigrum ; T5: 5% HCHs+Solanum nigrum +inoculate; T6: 5% HCHs+inoculate; T7: 10% HCHs+Solanum nigrum ; T8: 10%HCHS+Solanum nigrum +inoculate. T9: 10% HCHs+inoculate.
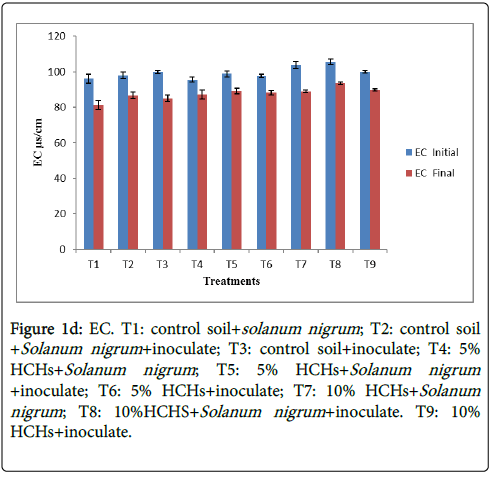
At the beginning value of EC was higher while with the passage of time it goes on decreasing. Electrical conductivity values ranged from 100.86 μs/cm to 85.95 μs/cm represented in Figure 1d. Higher (87.77 μs/cm, initially it was 100.86 μs/cm) being observed at T8 (Inoculated Solanum nigum+10% HCH) and lowest (88.33 μs/cm, initially it was 100.17 μs/cm) being observed at T1 (Control soil+Solanum nigrum ). Other treatments T2, T3, T4, T5, T6, T7 and T9 showed EC values as 88.7 μs/cm, 85.95 μs/cm, 86.23 μs/cm, 89.11 μs/cm, 88.2 μs/cm, 87.95 μs/cm and 87.77 μs/cm respectively. This reduction might be due to taking up of ions, produced after mineralization, by plants. Similar contamination was evaluated on physic chemical characteristics of soil and growth of vetiver grass. Inoculated treatments showed comparatively higher electrical conductivity as compared to uninoculated. Those treatments in which only Solanum nigrum was planted in control soil represented lowest electrical conductivity due to less microbial activity and low organic matter.
Figure 1d: 1d: EC. T1: control soil+solanum nigrum ; T2: control soil +Solanum nigrum +inoculate; T3: control soil+inoculate; T4: 5% HCHs+Solanum nigrum ; T5: 5% HCHs+Solanum nigrum +inoculate; T6: 5% HCHs+inoculate; T7: 10% HCHs+Solanum nigrum ; T8: 10%HCHS+Solanum nigrum +inoculate. T9: 10% HCHs+inoculate.
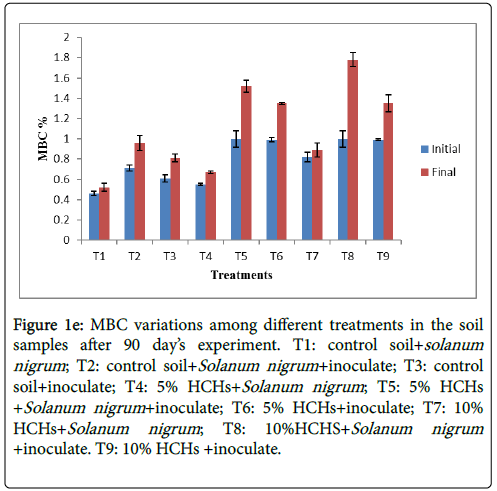
The overall parameters of experiment indicated the microbial development and metabolic activity in the soil, such as microbial biomass (Figure 1e). trend was observed by Nisa et al. [22] in which effect of diesel
Figure 1e: MBC variations among different treatments in the soil samples after 90 day’s experiment. T1: control soil+solanum nigrum ; T2: control soil+Solanum nigrum +inoculate; T3: control soil+inoculate; T4: 5% HCHs+Solanum nigrum ; T5: 5% HCHs +Solanum nigrum +inoculate; T6: 5% HCHs+inoculate; T7: 10% HCHs+Solanum nigrum ; T8: 10%HCHS+Solanum nigrum +inoculate. T9: 10% HCHs +inoculate.
The treatment in which inoculated Solanum nigrum was planted in 10% HCHs contamination (T8) showed the peak value (1.78%) of microbial biomass while control soil with Solanum nigrum (T1) had lowest value (0.52%). T2, T5, T6 and T9 had 0.96%, 1.52%, 1.35%, and 1.32% significant MBC respectively (Figure 1e). Promotion of microbial biomass in treatments with HCHs contamination and inoculated Solanum nigrum was due to availability of HCHs which serve as energy source for microbes and presence of plant which provide essential nutrients (photosynthesis based organic compounds) to microbes thus improved their number. Some microbes are sensitive, cannot comply with contaminant and dies as exposed to it while other shows an increasing trend in their number as exposed to contaminant. The reason is they uses contaminant as their food source, secondly organic components released from dead microorganisms are also used as their power source and thirdly because of dead of some microbe’s competition reduces that results in boost up of compatible MBC [23]. In general microbial community flourished with introduction of Pesticides [24,25]. Perucci et al. [26] reported that introduction of gemma hexachlorocyclohexane improved the microbial biomass carbon of soil. The microbes that combat contaminant can degrade that contaminant [27]. The present study resulted some microbial strains that can sustained with HCHs, similar results were obtained by Hussain et al. [27] for different contaminants.
All soil chemical properties of soil along with their mean values and standard deviation are represented in Table 2.
| Treatments | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | Mean | STDEV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial pH | 8.1 | 7.8 | 7.9 | 7.8 | 7.7 | 8.1 | 7.8 | 8.2 | 7.7 | 7.9 | 0.187083 |
| Final pH | 7.5 | 7.3 | 7.8 | 6.9 | 7.3 | 7.2 | 6.8 | 6.5 | 7.1 | 7.155556 | 0.387657 |
| Initial Organic Matter | 0.996667 | 0.851 | 0.93 | 0.9773 | 1.166667 | 0.983333 | 0.891 | 1.39 | 0.933333 | 1.013256 | 0.166781 |
| Final Organic Matter | 1.26408 | 1.609933 | 0.79 | 2.533333 | 3.193333 | 1.833333 | 2.776667 | 3.597 | 2.299923 | 2.210845 | 0.917713 |
| Intial TOC | 0.5777 | 0.4936 | 0.539 | 0.567 | 0.677 | 0.57 | 0.517 | 0.806 | 0.541 | 0.587589 | 0.096745 |
| Final TOC | 0.733 | 0.9338 | 0.458 | 1.469 | 1.852 | 1.063 | 1.61 | 2.086 | 1.333 | 1.281978 | 0.532218 |
| Initial EC | 100.17 | 99.97 | 99.77 | 99.56 | 100.8 | 100.6 | 99.73 | 100.6 | 100 | 100.1333 | 0.440057 |
| Final EC | 88.33 | 88.7 | 85.95 | 86.23 | 89.11 | 88.2 | 87.95 | 88.53 | 87.77 | 87.86333 | 1.082509 |
| Intial MBC | 0.461 | 0.71 | 0.61 | 0.55 | 1 | 0.99 | 0.82 | 1 | 0.99 | 0.792333 | 0.216128 |
| Final MBC | 0.52 | 0.96 | 0.81 | 0.67 | 1.52 | 1.35 | 0.89 | 1.78 | 1.35 | 1.094444 | 0.423176 |
Table 2: Soil’s chemical properties.
Isolation of rhizospheric bacteria
Hexachlorocyclohexane (HCH) degrading bacteria were isolated from the rhizosphere of plants growing on HCH contaminated soil. The most competent rhizobacteria were screened based on their efficiency to consume HCH as the only source of carbon. The most efficient rhizobacteria were further evaluated for their ability to support the development of Solanum nigrum under greenhouse circumstances. The results of these experiments are discussed below.
Isolation of HCHs degrading bacteria: Various strains of bacteria were secluded from the rhizospheric soil based on their ability to use HCHs as the major source of carbon. 107 rhizobacterial isolates were chosen at the start, 82 isolates manifested productive growth on the agar medium containing 50 mg HCH. Of those 23% of the rhizobacteria were competent of mounting energetically on ACC medium.
Screening rhizobacteria for plant growth promoting activity under controlled conditions: Laboratory experiments were conducted in petri plates for selection of the bacterial isolates/strains that efficiently improved the growth activities of Solanum nigrum.
Petri plate experiment: Petri plate experiment was conducted in controlled conditions to evaluate the effect of rhizobacteria on growth performance of Solanum nigrum . The outcome discloses that development of seedlings of inoculated Solanum nigrum appreciably improved as compared to uninoculated Solanum nigrum . Effect of different strains on seedling of Solanum nigrum has been shown in Table 2. Observation of outcome shows that seedling of Solanum nigrum respond differently to different strains. No improvement was observed in some isolates while others improved the root length of Solanum nigrum in effective manner, highest (6.9 cm) being observed with SN47.
Effect of inoculation on Solanum nigrum under soil condition
Selected PGPR were tested for their efficiencies in improving Solanum nigrum biomass in HCHs contaminated soil, by Pot experiments under greenhouse conditions (Table 3).
| Codes assigned to bacterial isolates | Root Length (cm) | Dry Root Weight (g) |
|---|---|---|
| Mean ± SEa | Mean ± SE | |
| SN4 | 6.1 ± 0.93 | 0.038 ± 0.018 |
| SN9 | 6.8 ± 0.41 | 0.017 ± 0.001 |
| SN13 | 6.4 ± 0.51 | 0.014 ± 0.001 |
| SN27 | 4.81 ± 0.34 | 0.037 ± 0.014 |
| SN33 | 5.84 ± 0.67 | 0.026 ± 0.001 |
| SN39 | 5.58 ± 0.91 | 0.021 ± 0.008 |
| SN47 | 6.9 ± 0.88 | 0.048 ± 0.019 |
| SN54 | 5.85 ± 0.64 | 0.016 ± 0.001 |
| SN62 | 5.52 ± 0.98 | 0.017 ± 0.002 |
| SN66 | 5.06 ± 0.97 | 0.020 ± 0.002 |
| SN71 | 5.69 ± 0.22 | 0.026 ± 0.001 |
| SN80 | 6.18 ± 1.03 | 0.017± 0.004 |
Table 3: Effect of rhizobacterial inoculation on root growth of makoi (Solanum nigrum ) in petri plate experiment under axenic conditions (Average of four replications) (a*standard error).
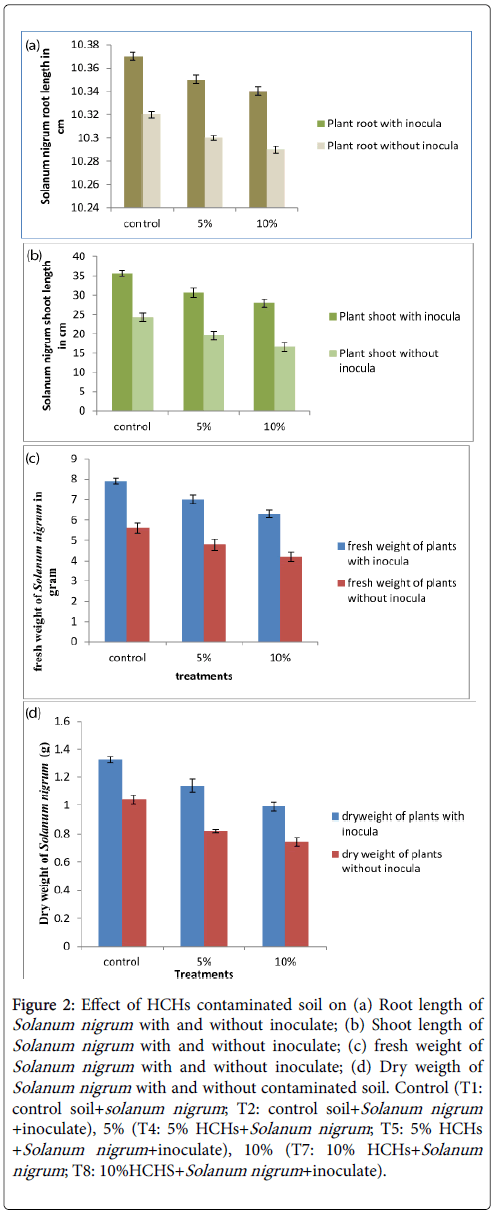
Biomass and root shoot length: Our data indicated that plant biomass improved significantly with the passage of time, but its trend was different for different treatments. Solanum nigrum grown on contaminated soil improved biomass and root, shoot length but at a slow rate while that grown on control soil resulted at high biomass and root, shoot length.
T7 (10% HCHs with Solanum nigrum ) showed very slow growth rate while T2 (control soil with SN47 inoculated Solanum nigrum ) had highest growth. Presence of inoculate increased biomass by increasing root length and improving plant growth. Initially average root length of all plants was 5 cm while average shoot length was 11 cm. After harvesting their root and shoot length and biomass increased at a very fast rate. Average root length of all treatments was 10 cm, their shoot lengths were maximum for T2, T5 and T8 35.66 cm, 30.66 cm, and 28 cm; their fresh weights were 7.9 g, 7.0 g, and 6.3 g and their dry weights were 1.327 g, 1.14 g, and 0.99 g respectively revealed in Figures 2a-2d.
Figure 2: Effect of HCHs contaminated soil on (a) Root length of Solanum nigrum with and without inoculate; (b) Shoot length of Solanum nigrum with and without inoculate; (c) fresh weight of Solanum nigrum with and without inoculate; (d) Dry weigth of Solanum nigrum with and without contaminated soil. Control (T1: control soil+solanum nigrum ; T2: control soil+Solanum nigrum +inoculate), 5% (T4: 5% HCHs+Solanum nigrum ; T5: 5% HCHs +Solanum nigrum +inoculate), 10% (T7: 10% HCHs+Solanum nigrum ; T8: 10%HCHS+Solanum nigrum +inoculate).
Results demonstrated that inoculation with rhizobacterial strains SN47 significantly improved the plant growth. Because symbiosis relationship between plant and rhizobacterial strains can amplify the antioxidant activities to relieve oxidative damage and enhance the growth of plant under stress. Result like present study was reported by Long et al. [29] who used rhizobacteria (Pseudomonas brassicacearum ) to inoculate Solanum nigrum and compared its biomass with Solanum nigrum grown in the absence of rhizobacteria and concluded that inoculation helps in plant growth. Abhilash et al. [4] assessed the growth of W. somnifera in control soil, and lindane spiked soil with and without microbial inoculation and concluded that W. somnifera enhanced the growth rate in the presence of microbial strain. The above result had cleared that application of pesticide had a negative effect on plant growth because all plants that were grown on contaminated soil showed lower growth as compared to those on control soil, but the effect was not that much higher illustrating that Solanum nigrum can tolerate HCHs. The result agreed with Chuluun et al. [30] in that reduction in plant biomass was observed after spiking the soil with organochlorine pesticide as compared to control soil. Previous study conducted by Abhilash et al. [31] revealed that there was no considerable discrepancy in growth of Jatropha plant when exposed to low level of HCHs; however, the development was compacted to some extent as the HCHs application and contact time prolonged.
HCHs analysis
HCHs concentration was deliberated in soil and Solanum nigrum before and after harvesting the plants. The samples were analysed through GC-MS. Results obtained are discussed below:
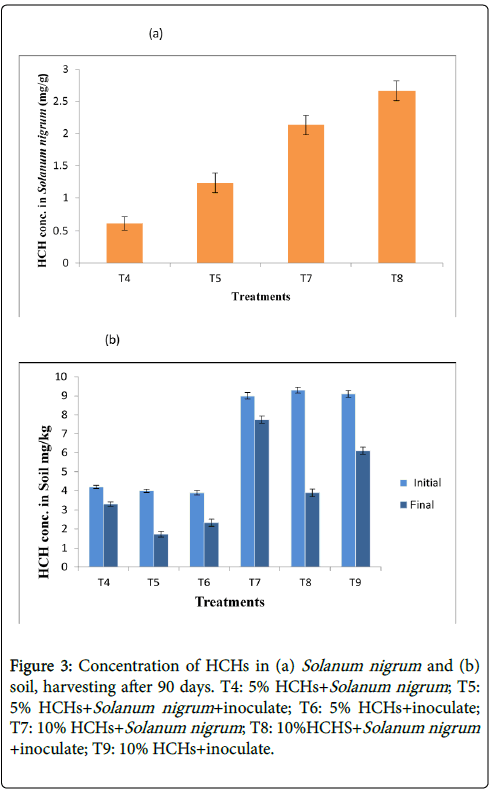
HCHs extraction from soil and Solanum nigrum: When the Solanum nigrum and bacterial isolates were grown on contaminated soil there was noticeable effect on HCHs degradation in all treatments. A drastic decrease in HCHs concentration in soil was observed in T8. In which Solanum nigrum was grown along with inoculate on 10% HCHs contaminated soil.
T4 (5% HCHs+Solanum nigrum ) represented lowest degradation. Detection limit of the HCHs in soil after harvesting plants ranged from 0.0013 to 7.733 for different concentration studied. T4, T5, T6, T7, T8, T9 had 4.2, 4, 3.9, 9, 9.3 and 9.1 mg/kg HCHs concentration respectively in soil before the experiment while at the end of 90 days this amount reduced to 3.3, 1.733, 2.533, 7.733, 3.9 and 6.1 mg/kg respectively. Figure 3b representing the residual HCHs for all treatments in spiked soil. HCHs concentration in Solanum nigrum at T5, T7 and T8 was 1.233, 2.1333 and 2.6667 respectively, shown in Figures 3a and 3b. Maximum was accumulated in Solanum nigrum grown in 10% HCHs contaminated soil along with SN47 strain. HCHs accumulation in plants grown in control soil represented no significant difference even in presence of inoculate but on contaminated soil Solanum nigrum showed higher HCHs in its tissues in presence of inoculate.
The reduction in the HCHs concentration in soil after harvesting the Solanum nigrum could be attributed to secretions by Solanum nigrum and bacterial strain SN47 which improved the plant growth, helped in reducing oxidative stress in plant and used HCHs as food source. The physico-chemical properties of the soil have also been found to have a significant effect on the degradation of contaminant. pH of the soil was moderate for degradation of HCHs. Previous studies reported maximum degradation at approximately neutral pH. Gosh and Singh, 2005 reported that contaminant degradation in soils is principally controlled by pH, CEP, OC, TOC of soil.
Hyper accumulation of S. nigrum without showing toxicity symptoms possibly ascribed to sound extend detoxification system based on abstraction of contaminant in vacuole, by fastening them on suitable functional groups i.e., organic acids, proteins and peptides in the presence of enzymes that work efficiently even in presence of higher concentration of toxic substances. S. nigrum secretes alkaloidal contents especially solanine, which is a neurotoxic glycoalkaloid. Recently it is reported by Ravi et al. that the berry of S. nigrum contains four steroidal alkaloid glycosides namely, solamargine, solasonine, α and β solanigrine. The accumulated alkaloids are involved in defensive mechanism against stress in the plant [32]. Puhui et al. [33] represented Solanum nigrum as hyperaccumulator for Cd.
HCHs degradation by SN47 was comparatively higher to Solanum nigrum . Dissipation rate by microbes may be explained through growth profile. MBC content in different HCHs contaminated treatments tend to increase. This resulted reduction in HCHs concentration. Previous studies confirmed the present study’s result. Abhilash et al. [4] who used isolated microbial strains from HCHs contaminated soil to degrade 5, 50 and 100 mg/kg concentration of HCHs. The resulted residual HCHs amount in contaminated soil treatments were condensed to 0, 41 and 33 percent respectively. A study was carried out by Benimeli et al. in which soil was spiked with HCHs in concentration of 100, 150, 200 and 300 g/kg, was treated with streptomyces M7 specie. The result showed residual concentration of HCHs in soil as 29.1%, 78.03%, 38.81% and 14.42% respectively. That ensures high potential of this species for HCH’s degradation.
Presence of microbes improves the plant growth and reduces the oxidative damage (due to presence of contaminant) of plant by increasing antioxidant acitivities. In addition to this, microbes utilize the HCHs as their food source, and secrete enzymes that mineralize the HCHs resulting further decrease in residual HCHs in spiked soil. In reaction plants provides biological active soil sites which improves microbial activity and enhances contaminant bioavailability [34]. Previous study confirmed present result as a research addressed by Marques et al. [35] explained the higher detoxification level of S. nigrum in presence of G. claroideum or G. intraradices . The study result demonstrated S. nigrum as extractor and accumulator, with no apparent sign of toxicity, to higher amount of Zn that was concentrated in its parts as it was revealed to site polluted by Zinc. Addition of inoculate results in manufacturing of thiols (gulathione), by triggering the plant’s natural ability to fight against any attack [36]. Theses thiols are produced by phytochelatin synthase enzyme. This removes contaminant from the soil by sticking it and making it available for plant uptake [37]. Khan et al. [38] did a study in which Solanum nigrum was used alone and inoculated with RSC-14 in Cd contaminated soil. RSC-14 secreted plant growth-promoting phytohormones such as indole-3-acetic acid, which improved the Plant roots, shoots and biomass significantly. Result of present study also represented significant accumulation of HCHs in Solanum nigrum that were inoculated with bacterial strain SN47 proving this combination to be effective for remediating HCHs from environment. Anova of all values of HCHs in soil and plant was calculated, it showed a positive significant value of p<0.05 and it is represented in Table 4.
| R square and p value of HCHs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Regression Statistics | ||||||||
| Multiple R | 0.83250498 | |||||||
| R square | 0.693064541 | |||||||
| Adjusted R Square | 0.693064541 | |||||||
| standard error | 1.621997337 | |||||||
| Observations | 9 | |||||||
| ANOVA | ||||||||
| df | SS | MS | F | Significans F | ||||
| Regression | 1 | 41.58387246 | 41.58387 | 15.806 | 0.005353274 | |||
| Residual | 7 | 18.41612754 | 2.630875 | |||||
| Total | 8 | 60 | ||||||
| Coefficients | Standard Error | t Stat | P-value | Lower 95% | Upper 95% | Lower 95.0% | Upper 95.0% | |
| Intercept | 2.707169201 | 0.790517092 | 3.424555 | 0.0111 | 0.837893313 | 4.576445089 | 0.837893313 | 4.576445089 |
| X Variable 1 | 0.822010637 | 0.206759327 | 3.975688 | 0.0054 | 0.333102519 | 1.310918755 | 0.333102519 | 1.310918755 |
Table 4: ANOVA of HCHs concentration.
Conclusion
The food requirements of present generation need improved and advanced technologies in agriculture. But these technologies should not be at the cost of environment. Human should adapt to earth environment, which he depends to survive, and abide by the native laws, in this regard he can lead nourishing and viable life. On a concluding note outcome of study include that Solanum nigrum is best for phytoremediation of HCHs but its inoculation with SN47 microbes presents it to be promising and effective remover of HCHs from contaminated soil releasing no adverse impact on environment. HCHs accumulation in Solanum nigrum didn’t show any toxic symptoms showing higher tolerance of this plant for HCHs contamination. Inoculation to Solanum nigrum with SN47 showed highest dissipation rate of HCHs as compared to only Solanum nigrum . Degradation rate was in the order of Solanum nigrum +SN47 strains>SN47 Strain>Solanum nigrum . Physicochemical properties of soil also contributed in degradation potential of HCHs by Solanum nigrum and SN47 strains.
References
- Anwar T, Ahmad I, Tahir S (2012) Determination of pesticide residues in soil of Nawabshah District, Sindh, Pakistan. Pakistan J Zool 44: 87-93.
- Girish K, Kunhi MA (2013) Microbial degradation of gamma-hexachlorocyclohexane (lindane). African Journal of Microbiology Research 7: 1635-1643.
- Vijgen J, Yi LF, Forter M, Lal R, Weber R (2006) The legacy of lindane and technical HCH production. Organohalog Comp 68: 899-904.
- Vijgen J, Abhilash PC, Li YF, Lal R, Forter M, et al. (2011) Hexachlorocyclohexane (HCH) as new Stockholm Convention POPs—a global perspective on the management of Lindane and its waste isomers. Environmental Science and Pollution Research 18: 152-162.
- Nagpal V, Srinivasan MC, Paknikar KM (2008) Biodegradation of γ-hexachlorocyclohexane (Lindane) by a non-white rot fungus conidiobolus 03-1-56 isolated from litter. Indian Journal of Microbiology 48: 134.
- Turnbull A (1996) Chlorinated pesticides. In: Chlorinated Organic Micropollutants. Environmental Science and Technology. The Royal Society of Chemistry, Cambridge, UK pp: 113-135.
- Rochika P, Dharmender K (2014) Biodegradation study of gemma HCH using selected bacteria isolated from agricultural soil. African Journal of Microbiology Research 8: 3335-3346.
- Weber R, Gaus C, Tysklind M, Johnston P, Forter M, et al. (2008) Dioxin-and POP-contaminated sites—contemporary and future relevance and challenges. Environ Sci Pollut Res 15: 363-393.
- Aftab T, Ahmed D, Saud H, Yusaf S, Ahmad MA (2007) Evaluation suspected chronic pesticide poisoning among residents near agriculture fields. Biomedica 23: 1553-1560.
- Eqani SAMAS, Malik RN, Mohammad A (2011) The level and distribution of selected organochlorine pesticides in sediments from River Chenab, Pakistan. Environ Geochem Health 33: 33-47.
- Wollum AG (1982) Cultural methods for soil microorganisms. ASA and SSSA Pub, Madison, Wisconsin, USA 2: 719-802.
- Park S, Kim K S, Kim JT, Kang D, Sung K (2011) Effects of humic acid on phytodegradation of petroleum hydrocarbons in soil simultaneously contaminated with heavy metals. Journal of Environmental Sciences 23: 2034-2041.
- Islam KR, Weil RR (1998) Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biology and Fertility of Soils 27: 408-416.
- Rhind SM, Kyle CE, Kerr C, Osprey M, Zhang ZL, et al. (2013) Concentrations and geographic distribution of selected organic pollutants in Scottish surface soils. Environmental pollution 182: 15-27
- Fernandez P, Poisa-Beiro A, Acea MJ (2008) Effect of hexachlorocyclohexane isomers on some soil microbiological properties. EDAFOLO gÃa 15: 155-168.
- Kopittke PM, Menzies NW, Soa HB, Fulton I (2004) Effect of Mn deficiency and legume inoculation on rhizosphere pH in highly alkaline bauxite residue. International Soil Conservation Organisation Conference 13: 122-134.
- Leal ODA, Castilhos RMV, Pauletto EA, Pinto LFS, Pillon CN, et al. (2015) Organic matter fractions and quality of the surface layer of a constructed and vegetated soil after coal mining. II-Physical compartments and carbon management index. Revista Brasileira de Ciência do Solo 39: 895-902.
- Spark KM, Swift RS (2002) Effect of soil composition and dissolved organic matter on pesticide sorption. Science of the Total Environment 298: 147-161.
- Clayden J, Greeves N (2001) Organic Chemistry. Oxford University Press, UK, pp: 524-525.
- Malik MA, Khan KS, Marschner P (2013) Microbial biomass, nutrient availability and nutrient uptake by wheat in two soils with organic amendments. Journal of Soil Science and Plant Nutrition 13: 955-966.
- Nisa WU, Bangash N, Saleem AR, Rashid A, Dawson L (2016) Effect of diesel contamination on the physico-chemical characteristics of soil and growth of vetiver grass. Soil Environ 35: 91-98.
- Das AC, Mukherjee D (2000) Soil application of insecticides influences microorganisms and plant nutrients. Appl Soil Ecol 14: 55-62.
- Kidd PS, Prieto-Fernández A, Monterroso C, Acea MJ (2008) Rhizosphere microbial community and hexachlorocyclohexane degradative potential in contrasting plant species. Plant and soil 302: 233-247.
- Wang MC, Liu YH, Wang Q, Gong M, Hua XM, et al. (2008) Impacts of methamidophos on the biochemical, catabolic, and genetic characteristics of soil microbial communities. Soil Biology and Biochemistry 40: 778-788.
- Perucci P, Scarponi L (1994) Effects of the herbicide imazethapyr on soil microbial biomass and various soil enzyme activities. Biology and Fertility of Soils 17: 237-240.
- Hernandez ML, Sanchez-Salinas E (2010) Biodegradation of the organophosphate pesticide tetrachlorvinphos by bacteria isolated from agricultural soils in México. Rev Int Contam Ambient 26: 27-38.
- Hussain S, Siddique T, Saleem M, Arshad M, Khalid A (2009) Impact of pesticides on soil microbial diversity, enzymes, and biochemical reactions. Advances in Agronomy 102: 159-200.
- Long HH, Schmidt DD, Baldwin IT (2008) Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS One 3: 270-279.
- Chuluun B, Iamchaturapatr J, Rhee JS (2009) Phytoremediation of organophosphorus and organochlorine pesticides by acorus gramineus. Environmental Engineering Research 14: 226-236.
- Abhilash PC, Singh B, Srivastava P, Schaeffer A, Singh N (2013) Remediation of lindane by Jatropha curcas L: utilization of multipurpose species for rhizoremediation. Biomass and bioenergy 51: 189-193.
- Gogoi P, Islam M (2012) Phytochemical screening of Solanum nigrum L and S. myriacanthus Dunal from districts of upper Assam, India. IOSR Journal of Pharmacy 2: 455-459.
- Ji P, Song Y, Jiang Y, Tang X, Tong YA, et al. (2016) A two-year field study of phytoremediation using Solanum nigrum L. in China. International Journal of Phytoremediation 18: 924-928.
- Gerhardt KE, Huang XD, Glick BR, Greenberg BM (2009) Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci 176: 20-30.
- Marques AP, Oliveira RS, Samardjieva KA, Pissarra J, Rangel AO, et al. (2007) Solanum nigrum grown in contaminated soil: effect of arbuscular mycorrhizal fungi on zinc accumulation and histolocalisation. Environmental Pollution 145: 691-699.
- Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metalâ€induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany 53: 1351-1365.
- Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology 54: 159-182.
- Khan AR, Ullah I, Khan AL, Park GS, Waqas M, et al. (2015) Improvement in phytoremediation potential of Solanum nigrum under cadmium contamination through endophytic-assisted Serratia sp. RSC-14 inoculation. Environmental Science and Pollution Research 22: 14032-14042.
Citation: Bibi A, Un-Nisa W, Qasim A, Malik TH (2019) Rhizoremediation of Hexachlorocyclohexane Through Pesticide Contaminated Soil by Solanum nigrum. J Bioremediat Biodegrad 10: 457. DOI: 10.4172/2155-6199.1000457
Copyright: © 2018 Bibi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4304
- [From(publication date): 0-2019 - Nov 20, 2025]
- Breakdown by view type
- HTML page views: 3361
- PDF downloads: 943