Research Article Open Access
Rheological Properties of Polyethylene Glycol Solutions and Gels
AV Brikov1*, AN Markin2 and SV Sukhoverkhov2
1Sakhalin Energy Investment Company, Ltd., Yuzhno-Sakhalinsk Branch 35, Dzerzhinskogo, Yuzhno-Sakhalinsk, Russian Federation
2Institute of Chemistry, FEB of Russian Academy of Science 159, 100-letiya Vladivostok, Vladivostok, Russian Federation
- *Corresponding Author:
- A. V. Brikov
Sakhalin Energy Investment Company
Ltd., Yuzhno-Sakhalinsk Branch 35
Dzerzhinskogo, Yuzhno-Sakhalinsk
Russian Federation
Tel: +7 4242 66 2000
E-mail: Alexander.Brikov@sakhalinenergy.ru
Received date: September 11, 2015; Accepted date: September 19, 2015; Published date: September 26, 2015
Citation: Brikov AV, Markin AN, Sukhoverkhov SV (2015) Rheological Properties of Polyethylene Glycol Solutions and Gels. Ind Chem Open Access 1:102. doi: 10.4172/2469-9764.1000102
Copyright: © 2015 Brikov AV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Industrial Chemistry
Abstract
It is shown that under defined conditions gel is formed in the glycol from the glycol regeneration plants, which created significant operational problems. The results of laboratory research on the production of gels from polyethylene glycol water and water-glycol solutions as well as study of rheological properties of these gels and polyethylene glycol solutions are provided. It is shown that upon exceeding the certain shear rate value gels demonstrate abnormal rheological property (instant flow cessation).
Keywords
Oil and gas production; Glycol regeneration; Polyethylene glycol (PEG); Gel; Shear stress
Introduction
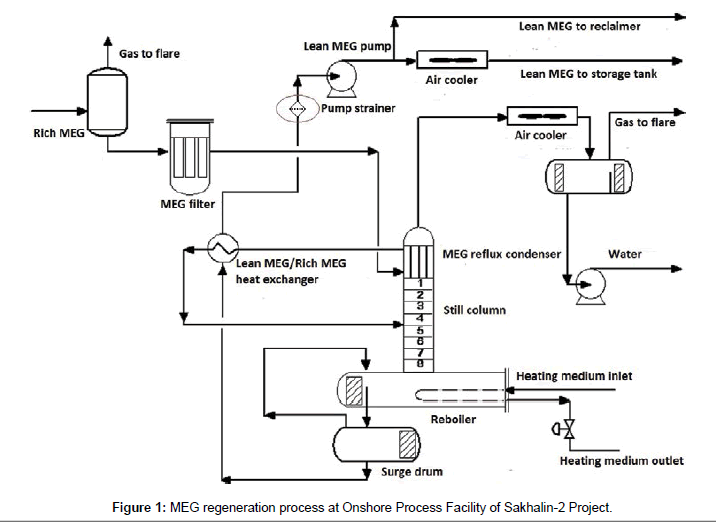
Monoethylene glycol (MEG) is widely applied in oil and gas production. One of applications is prevention of hydrates [1,2]. There is possibility of hydrates buildup in multiphase pipeline during gas and condensate transportation from LUN-A offshore platform to Onshore Process Facility (OPF) of Sakhalin-2 Project. To prevent hydrates the platform injects MEG water solution into pipeline at concentration of 85 mass%. OPF separates and regenerates MEG. Regenerated MEG returns to platform via dedicated pipeline. OPF MEG regeneration process schematic is shown on Figure 1.
Prior to regeneration rich MEG is fed into two-phase separator to remove gas and remove solids on MEG filter. Then MEG is warmed up in the reflux condenser by vapor to 75°С and in lean MEG /rich MEG heat exchanger to 100°С. Then MEG solution comes to reboiler where water in glycol is evaporated to the required concentration of glycol. MEG solution in reboiler is heated up to 126°С at atmospheric pressure. Working temperature of heating element surface in reboiler is 170°С, maximum temperature (without cooling down by MEG solution) - 210°С. Regenerated MEG is cooled down using air coolers and pumped to storage tank (not shown on Figure 1) for subsequent supply to offshore platform.
In April 2011, during routine operation of OPF MEG regeneration unit rapid buildup of pressure differential across lean MEG pump suction strainer was observed. Cleaning of strainer showed that it was completely covered with green gel deposits. Strainer clogging lasted for several days (strainer had to be cleaned every 1.5-3 hours), then suddenly stopped [3].
Investigations of possible reasons of gel formation identified:
1. gel formation started after well from Lunskoye oil rim section was put in operation
2. salinity of produced water from this well was ~ 16 g/dm3, ions concentration (mg/dm3): HCO3 - - 1600-2600; Са2+ - 115-1 40; Mg2+ - 22-34; Fe2+ - less than 1; concentration of carboxylic acids as of acetic acid - 1000-1 200
3. over the production period of the well sharp increase of concentration of dissolved salts in MEG solution (from 750 mg/dm3 to ~ 1350 mg/dm3) and concentration of calcium ions were observed
4. green color of gel means inclusion of Fe2+ ions into gel composition, concentration of Fe2+ ions in MEG solution has not changed being ~ 10 mg/dm3
5. it was found that MEG solution at OPF MEG regeneration system contains polyethylene glycol (PEG) at concentrations up to 480 mg/ dm3 at average molar weight of 5500 Da
6. gel formation stopped after shutdown of the oil rim well
Worth to mention that gel in glycol regeneration systems was also observed at other fields - in particular NAM L9 platforms in the North Sea (MEG regeneration system), Bacton Gas Terminal in the North Sea (MEG regeneration system) and Menza Project in Gulf of Mexico (TEG regeneration system) [3,4]. Earlier authors showed that gel formation in glycol regeneration systems occurs due to PEG generation in such systems [5-7] with subsequent crosslinking with calcium or iron ions [3,4]. It is known [8-12] that PEG in small concentrations (less than 0.1 mass %) has no significant effect on viscosity of glycol solution. Thus, PEG generation can not be a reasonable cause of described above process failures. Obviously under certain conditions PEG gel formation occurs resulting in abrupt increase of MEG viscosity.
To confirm this hypothesis laboratory experiments were performed to prepare PEG gels and to study their rheological properties.
Experimental
To prepare gel from MEG water solution 20.0 g of PEG of 4000 Da molar weight was dissolved in 50 vol. % MEG solution, 14.6 g of CaCl2.2H2O was added and diluted to 100 cm3. Concentration of PEG constituted 0.05 mol/dm3, calcium concentration - 1.00 mol/dm3. Solution in a sealed glass bottle was heated in drying oven at 120°С. Gel was observed after 1 hour of heating (Gel 1).
To prepare gel from water solution 20 g of PEG of molar weight 4000 Da was dissolved in distilled water, 21.9 g of CaCl2.2H2O was added and diluted to 100 cm3. Concentration of PEG constituted 0.05 mol/dm3, calcium- 1.5 mol/dm3. Solution in a sealed glass bottle was heated in drying oven at 120°С. Gel was observed after 1 hour of heating (Gel 2).
Rheological properties of PEG solutions and gels were studied using HAAKE VT-550 viscosimeter with MV-1 sensor. Dependency of shear stress and dynamic viscosity on shear rate (γ) was explored in experiment.
Results and Discussion
We have established that PEG gels below certain values of γ behave as Newtonian fluid. However, when shear rate exceeds a certain value, which depends on gel viscosity, gels show abnormal property - instant flow cessation (full stop of spindle rotor) that is inherent to dilatant liquids during sharp increase of viscosity.
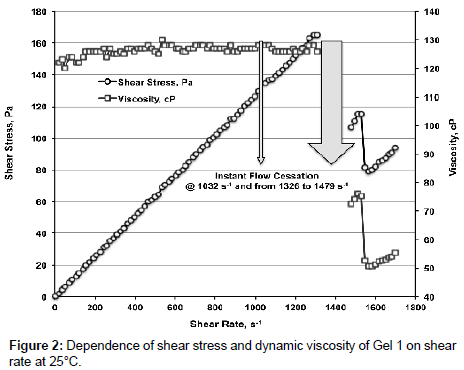
Figure 2 shows dependence of shear stress and dynamic viscosity of Gel 1 on γ at 25°С. Below γ ≈ 1000 c-1 gel behaves as Newtonian fluid - shear stress is linearly increasing with increasing of γ, viscosity is constant being 126.2 ± 1.8 mPa�??s. At γ=1032 s-1 and in the interval of 1326 ≤ γ ≤ 1479 s-1 instant flow cessation and total stop of spindle rotor occurs. With further increasing of γ shear stress and viscosity fall notably.
We assume that at certain shear rate the structure of gel partially breaks causing sharp reduction of gel viscosity. Nonetheless further tests of the same gel showed that dependence of shear stress and viscosity on γ is not changing and the flow cessation occurs at the same values of γ; i.e., if gel structure is broken it is restoring over a short period of time after shear stress removal.
When temperature increased to 60°С viscosity of Gel 1 drops to 31.91 ± 0.75 mPa�??s, cessation of flow does not occur till γ=1872 s-1.
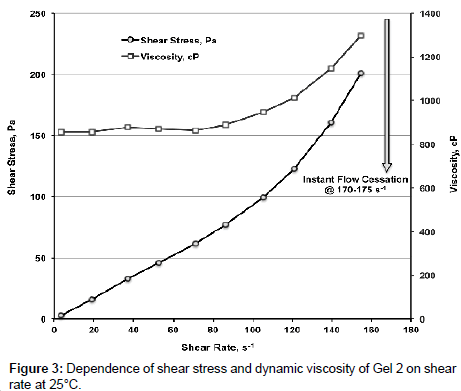
Figure 3 shows dependence of shear stress and dynamic viscosity of Gel 2 on γ. Below γ ≈ 70 s-1 Gel 2 behaves as Newtonian fluid, viscosity is 865.0 ± 9.0 mPa�??s. With γ increasing viscosity increases sharply and at γ ≥ 170-1 75 s-1 instant flow cessation occurs.
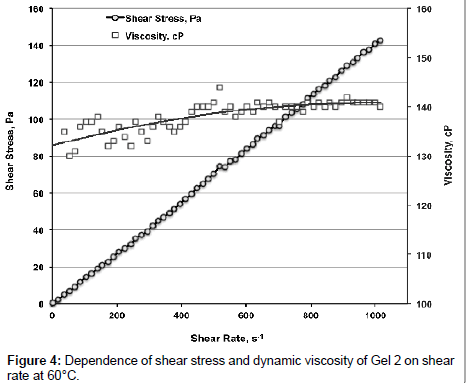
Data from Figure 3 allow concluding that PEG gels are dilatant liquids when γ reaches certain values. At 60°С viscosity of Gel 2 is slightly increasing (by ~ 7%) with increasing of γ being (average value in the interval of 0<γ<1000 s-1) 138.2 ± 3.1 mPa�??s (Figure 4). At γ=1034 s-1 and in the interval of 1350<γ<1490 s-1 instant flow cessation occurs.
Abnormal property of PEG gels, namely instant flow cessation when shear rate exceeds a certain value is of practical importance for operation of glycol regeneration units. At low shear rates (below critical γ values) observed in pipelines and vessels viscosity of gel is not high and is not affecting operation of regeneration system. With sharp increase of shear rate (e.g., when gel passing through fine pores of filters) sharp increase of viscosity occurs resulting in clogging of filters. Slowdown or stoppage of flow removes shear rate and viscosity of gel returns to initial value thus resuming the flow. However, when shear rate increases again described above reoccurs. Thus, presence of gel with abnormal rheological property in the glycol regeneration systems may cause filters clogging if critical shear rate would be reached.
Water solutions of PEG are also dilatant liquids. Figure 5 shows dependence of shear stress and dynamic viscosity of 15 mass % PEG solution with molar weight 4000 Da on γ at 25-28°С.
When γ changes from 0 to 1700 s-1 viscosity increases from 4.6 to 8.8 mPa�??s. Similar values were obtained for other PEG solutions. Thus, viscosity of 1 mass % of PEG with molar weight 6000 Da water solution at same conditions increases from 2.4 to 3.9 mPa�??s.
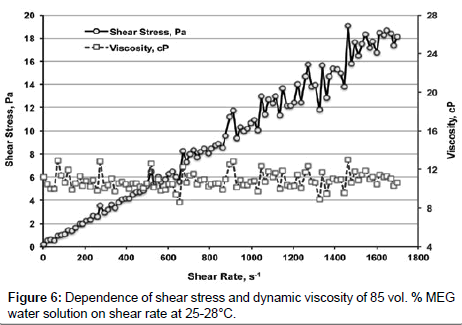
MEG water solutions behave as Newtonian liquids when γ ranges from 0 to 1872 s-1. Figure 6 shows dependence of shear stress and dynamic viscosity of 85 vol. % MEG water solution on γ at 25-28°С.
Shear stress is linearly increasing with increasing of γ, viscosity is constant being 10.8 ± 0.9 mPa�??s.
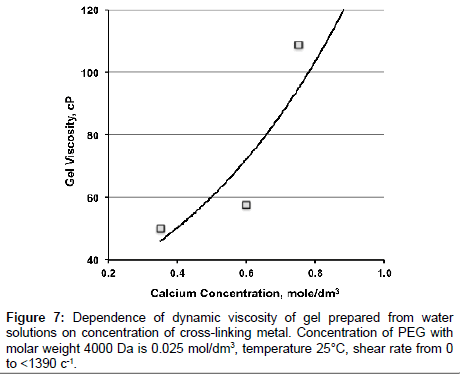
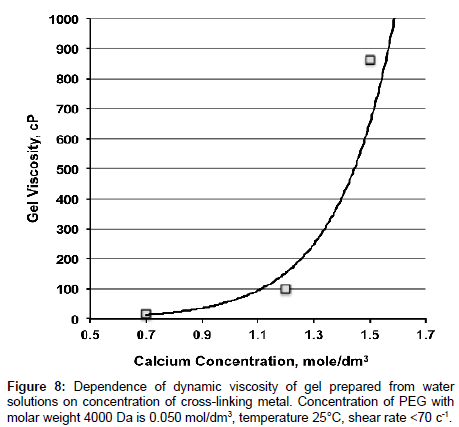
Also as a part of experiments we studied dependence of viscosities of gels formed from PEG solutions on concentration of PEG in the solution and on PEG/cross-linking metal ratio. It is found that viscosity of gels exponentially increasing with increasing of concentration of cross-linking metal at constant concentration of PEG (Table 1; Figures 7 and 8). Conditions of gel preparation are similar to Gel 2.
| Concentration of PEG with molar weight 4000 Da, mol/dm3 | Concentration of calcium ions,mol/dm3 | Calcium/PEG ratio | Viscosity of gel,mPa�??s |
|---|---|---|---|
| 0.025 | 0.35 | 14 | 50.0 ± 1.1 |
| 0.60 | 24 | 57.7 ± 1.1 | |
| 0.75 | 30 | 108.9 ± 1.0 | |
| 0.050 | 0.70 | 14 | 16.4 ± 1.2 |
| 1.20 | 24 | 97.9 ± 1.1 | |
| 1.50 | 30 | 865.0 ± 9.0 |
Table 1: Dependence of gels viscosity on concentration of PEG and on PEG/crosslinking metal ratio at 25°С.
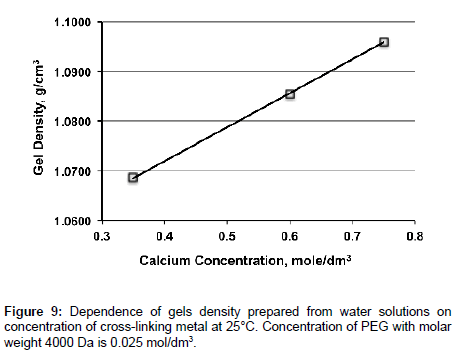
Unlike viscosity density of gels linearly increases with increasing of concentration of cross-linking metal at constant concentration of PEG (Figure 9).
Similar results were obtained for gels prepared from water-glycol PEG solutions.
It should be noted that PEG gels are soluble in water-glycol solutions and significantly increase viscosity of these solutions. For instance adding of 15 vol. % of Gel 1 to 85 vol. % MEG water solution increases viscosity (25-28°С, 0<γ<1479 s-1) from 10.81 ± 0.86 mPa�??s to 15.75 ± 0.96 mPa�??s (by 1.46 times). This property of gels also shall be considered when operating of glycol regeneration units.
Summary and Conclusion
PEG gels up to certain shear rates behave as Newtonian liquids. However, on exceeding certain shear rate value that is dependent on the gel viscosity instant flow cessation occurs.
PEG gels viscosity is significantly reduced with temperature increase.
Viscosity of gel increase exponentially with increasing of concentration of cross-linking metal at constant concentration of PEG. Therefore to reduce viscosity of gel it is necessary to control concentration of calcium and iron ions in water-glycol solutions of glycol regeneration units.
Density of gels is linearly increasing with increasing of concentration of cross-linking metal at constant concentration of PEG.
PEG gels are soluble in water-glycol solutions significantly increasing their viscosity.
Abnormal rheological property of PEG gels (instant flow cessation on exceeding certain shear rate value) may cause problems in operation of glycol regeneration units.
To prevent gel formation in the glycol regeneration systems operators shall develop and implement a glycol quality control procedure with focus on monitoring of concentration of PEG, calcium, and iron ions. Recommended glycol quality control parameters are developed in ref. [7].
References
- Berlin MA, Gorechenkov VG, Volkov NP (1981) Processing of petroleum and natural gases. Moscow: Chemistry. p: 472.
- Drachevsky SV, Karatun ON (2008) Specific methods of treatment of hydrocarbon gas containing sulfur compounds. Herald of Astrakhan State Technical University 47:158-160.
- Markin AN, Brikov AV, Sukhoverkhov SV(2015)Formation of Gel in Glycol Regeneration Systems.Scientific-technical journal Oilfield Engineering 9 (In press).
- Sukhoverkhov SV, Brikov AV, Markin AN (2015) Scale in Monoethylene Glycol Regeneration System of Onshore Processing Facility of "Sakhalin-2" Project. Scientific-technical journal Oilfield Engineering 2: 40-43.
- Sukhoverkhov SV, Brikov AV, Markin AN (2015) Quality Parameters of Monoethylene Glycol Used in Glycol Regeneration Systems at Offshore Oil and Gas Production Platforms. Scientific-technical journal Oilfield Engineering 3: 36-42.
- Shulyak IV, Grushova EI, Paskova AN (2013) Viscosimetric studies of water and water-salt solution of polyethylene glycol. Proceedings of BSTU. Chemistry, Organic Substances Technology and Biotechnology 4: 12-15.
- Le-He Mei, Dong-Qiang Lin, Zi-Qiang Zhu, Zhao-Xiong Han (1995) Densities and Viscosities of Polyethylene Glycol + Salt + Water Systems at 20°C. J ChemEng Data 40: 1168-1171.
- Syal K, Chauhan A, Chauhan S (2005) Ultrasonic velocity, viscosity and density studies of poly (ethylene glycol) (REG-8000, PEG-20000) in acetonitrile (AN) and water (H2O) mixtures at 25°C. J Pure ApplUltrason 27: 61-69.
- Goncalves CB, Trevisan N, Meirelles AJA (2005) Kinematic Viscosity of Systems Containing Polyethylene Glycol + Salt + Water at 298.2 K. J ChemEng Data50: 177-181.
- Fang Han K, Zhang J, Chen G, We X (2008) Density, viscosity and excess properties for aqueous polyethylene glycol solutions from (298.15 to 323.15) K. J ChemEng Data 53: 2598-2601.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 20512
- [From(publication date):
October-2015 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 15613
- PDF downloads : 4899