Research Article Open Access
Rewarding Effects of N-Methyl-1-(4-Methoxyphenyl)-2-Aminopropane (PMMA) in Mice: Roleof Modifications of Dopamine System Mediated through its Monoamine Oxidase Inhibition
Masahiko Funada*, NaoyaAoo and Kiyoshi Wada
Department of Drug Dependence Research, National Institute of Mental Health, NationalCenter of Neurology and Psychiatry, Tokyo, Japan
- *Corresponding Author:
- Masahiko Funada, Ph.D
Section Chief, Section of Addictive Drugs Research
Department of Drug Dependence Research
National Institute of Mental Health
National Center of Neurology and Psychiatry 4-1-1 Ogawa-higashi
Kodaira, Tokyo 187-8553, Japan
Tel: +81-42-346-1896
Fax: +81-42-346-1954
E-mail: mfunada@ncnp.go.jp
Received date: December 19, 2013; Accepted date: January 27, 2014; Published date: February 11, 2014
Citation: Funada M, Aoo N, Wada K (2014) Rewarding Effects of N-Methyl-1-(4-Methoxyphenyl)-2-Aminopropane (PMMA) in Mice: Roleof Modifications of Dopamine System Mediated through its Monoamine Oxidase Inhibition. J Addict Res Ther 5:172. doi:10.4172/2155-6105.1000172
Copyright: © 2014 Funada M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Background: N-methyl-1-(4-methoxyphenyl)-2-aminopropane (para-methoxymethamphetamine, PMMA) is a structurally abbreviated congener of methamphetamine that is abused as a “designer drug”. The aim of the present study was to investigate the behavioral and neurochemical properties of PMMA in mice.
Methods: Using conditioned place preference paradigm, the rewarding effect of PMMA were examined in the ICR mice. As neurochemical study, we examined the effect of PMMA on monoamine transmission and monoamine oxidase (MAO) activity in the mouse limbic forebrain tissue (containing the nucleus accumbens).
Results: PMMA (1-30 mg/kg) produced a significant heperlocomotion and conditioned place preference. The hyper locomotion and rewarding effects of PMMA were completely suppressed by the dopamine D1 receptor antagonist SCH23390, but were not modified by the dopamine D2 receptor antagonist sulpiride or the 5-HT2 receptor antagonist ketanserin.
PMMA also significantly increased the dopamine and 3-methoxytyramine (3MT) contents in the limbic forebrain. On the other hand, the levels of the dopamine metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and Homovanillic acid (HVA) were markedly reduced by PMMA in a dose-dependent manner. Monoamine oxidase (MAO) activities in the limbic forebrain were suppressed by PMMA treatment (0.04-4mM).
Conclusion: The present findings demonstrated that the dopamine D1 receptors might be involved in the expression of PMMA-induced heperlocomotion and the rewarding effects of the drug. Furthermore, the MAO-inhibitory effects of PMMA may play an important role in the PMMA-induced elevation of dopamine transmission. These behavioral and neurochemical data indicate that PMMA has a psychic dependence liability.
Keywords
Conditioned Place Preference; Monoamine Oxidase; PMMA; Reward
Introduction
The phenylisopropylamine N-methyl-1-(4-methoxyphenyl)- 2-aminopropane (para-methoxymethamphetamine, PMMA) is a structural congener of psycho stimulant methamphetamine (see Figure 1). PMMA is sometimes sold on the clandestine market as a substitute for MDMA and induces fatalities by overdose [1,2]. Previous drug discrimination studies have found that N-methyl- 1-(3,4-methylenedioxyphenyl)-2-aminopropane (methylene dioxy methamphetamine, MDMA) and PMMA substitute for one another, suggesting that they produce similar discriminative stimulus effects in rodents [3,4]. Furthermore, it was shown that MDMA induced rewarding effects in the conditioned place-preference procedure and reinforcing effects in self-administration studies indicating that MDMA has abuse liability [5,6]. On the other hand, the rewarding effects of PMMA have not yet been clearly characterized.
It has been shown that PMMA modifies the functions of the catecholaminergic system in the brain. In an in vivo microdialysis study, PMMA produced an increase of dopamine and serotonin (5-HT) in the striatum [7]. PMMA is a structural hybrid of two phenyl isopropyl amine chemicals: methamphetamine and Para-methoxyamphetamine (PMA, Figure 1). PMA is structurally and pharmacologically similar to PMMA [4]. It has been shown that PMA potently inhibits Mono Monoamine oxidase (MAO) activity [8]. It appears that PMA-induced 5-HT release and behavioral changes may be partially mediated through its inhibition of MAO [9]. Thus, these previous reports indicate that the central dopamine and/or the 5-HT system may be involved in the expression of PMMA-induced behavioral changes. However, the exact role that the dopamine system plays in PMMA-induced behavioral changes has yet to be clearly elucidated. It is also unclear what influence PMMA might have on the activities of MAO.
In the present behavioral analysis study, we investigated the effects of PMMA on locomotor activity and other rewarding effects in mice and whether any of the associated effects discovered might be involved with the dopamine receptors. Furthermore, we focused both on the ability of PMMA to inhibit MAO activities and on changes in the concentration of dopamine and dopamine metabolites in the mouse limbic forebrain (containing the nucleus accumbens and olfactory tubercle). Thus, the present studies were designed to evaluate the abuse liability of PMMA in the relationship between induced behavioral changes and the monoamine system.
Materials and Methods
Animals
Male ICR mice (20-25 g) were obtained from Clea Japan, Inc. (Tokyo, Japan). The mice were maintained on a 12-h light/dark schedule (lights on at 0800h), and laboratory mouse chow and water were provided ad libitum. The present study was conducted in accordance with the Guidelines of the Ethics Review Committee for Animal Experimentation of the National Center of Neurology and Psychiatry. Mice were acclimatized to daily handling for about 1 week prior to the experiment. All efforts were made to minimize the number of animals used and their suffering.
Locomotor activity
To measure the locomotor activity in the mice, we utilized an animal movement analyzing system (Actimo-100 system, Shintechno Ltd., Fukuoka, Japan), which consisted of a rectangular enclosure (30 × 20 cm), with a side wall equipped with photosensors located at intervals of 2 cm. This photosensor system was interfaced with a microprocessor, which automatically recorded the total number of photocell beam breaks. Each pair of photosensors scanned animal movement at 0.5 s intervals. In all of the experiments, mice were habituated to plastic cages (TPX, 18 × 26 cm, Clea Japan, Inc.) for 3 h. For all tests, activity counts were recorded every 10 min during the 120-min period following the administration of the PMMA (5 - 30 mg/ kg, i.p.). For the antagonist study, SCH23390 (0.03 mg/kg s.c.), sulpiride (50 mg/kg s.c.) or ketanserin (0.3 mg/kg s.c.) was administered 10 min before the administration of the PMMA (30 mg/kg, i.p.). In the control group, vehicle was administered 5 min before the administration of the PMMA. All experiments were conducted between 9:00 and 16:00 hours.
Conditioned place preference paradigm
The experimental apparatus consisted of a shuttle box (15 × 30 × 15 cm: width × depth × height) made of an acrylic resin board and divided into two equal-sized compartments (ATI, Neuroscience Co., Tokyo, Japan). One compartment was white with a textured floor, while the other was black with a smooth floor. Place conditioning was conducted as described previously [10,11]. Conditioning sessions (3 for the drug and 3 for the vehicle) were conducted once a day for 6 days. The treatment compartments of the shuttle box along with the order of the administration of the drug and the vehicle were counterbalanced [10,11]. The mice were injected with drug or vehicle and immediately confined to either the black or white compartment of the apparatus for 40 min. During the conditioning sessions, PMMA (1 - 30 mg/kg, i.p.) or vehicle was administered daily for 6 days. For the antagonist study, SCH23390 (0.03 mg/kg s.c.), sulpiride (50 mg/kg s.c.) or ketanserin (0.3 mg/kg s.c.) was administered 10 min before every PMMA treatment during the conditioning sessions. In the controls, vehicle was administered 5 min before every vehicle treatment. Test sessions were carried out 1 day after the final training session with mice in a drugfree state. The time a mouse spent in each compartment during the 900-s session was measured automatically in a blind fashion by using an infrared beam sensor (Neuroscience Co., Ltd., Tokyo, Japan), as has been previously described [10,11]. All sessions were conducted under the conditions of dim illumination (18 lx) and white noise.
Determination of monoamine contents
The concentrations of dopamine, 3,4-dihydroxyphenylacetic acid Acid (DOPAC), Homovanillic acid (HVA), 3-methoxytyramine (3MT) (3-MT), serotonin (5-HT) and 5-hydroxyindoleacetic acid (5HIAA) were determined by using high-performance liquid chromatography (HPLC) with Electro Chemical Detection (HPLC-ECD), as has been previously described [11,12]. Mice were sacrificed by cervical dislocation 30 min after administration of PMMA (5, 30 mg/kg, i.p.) or vehicle. The whole brain was quickly removed, with the limbic forebrain then dissected on an ice-cold glass plate. The tissues were homogenized in 250 μl of 0.2 M perchloric acid containing 100 μM EDTA (2Na) and 100 ng isoproterenol as the internal standard. The homogenates were then centrifuged at 15,000 × g for 60 min at 4ºC, with the supernatants then maintained at pH 3.0 by using 1 M sodium acetate. Samples were analyzed by HPLC-ECD. The HPLC system consisted of a delivery system (EP-10, Eicom Co., Kyoto, Japan), an analytical column (Eicompac, MA-5ODS, Eicom Co.), and a guard column (Eicom Co.). The column used to separate the dopamine and its metabolites used a mobile phase containing sodium acetate (0.1 M), citric acid monohydrate (0.1 M), sodium 1-octane sulfonate (170 mg/l), EDTA (2Na) (10 mg/l), and 15% methanol. The mobile phase was delivered at a flow rate of 0.23 ml/min. Identification of dopamine and its metabolites was determined according to the retention times of these standards, and the amounts were quantified by calculating the peak area.
Monoamine oxidase activity
Mice were sacrificed by cervical dislocation. The whole brain was immediately removed, with the limbic forebrain then dissected on an ice-cold glass plate. The tissues were homogenized in 10 volumes of ice-cold 50 mM Tris-buffered saline (pH 7.5). These homogenates were centrifuged at 1,000 × g for 10 min. Subsequently, the supernatants were centrifuged at 10,000 × g for 10 min with the pellets then resuspended in Tris-buffered saline (pH 7.5). The protein concentrations of the tissue homogenates were measured using the Bradford protein assay [13]. Monoamine oxidase (MAO) activity was determined with the Amplex Red Monoamine Oxidase Assay Kit (Molecular Probes, OR, USA). The tissue homogenates were mixed in equal amounts with a working solution composed of Amplex Red (200 μM), horseradish peroxidase (1 U/ml) and MAO substrate (p-tyramine or benzylamine; 1 mM), and incubated at 37ºC for 1 h. Assays for total MAO (MAO-A and MAO-B) activities were conducted using p-tyramine as the substrate for both MAO-A and MAO-B. MAO-B activity was detected using benzylamine for the MAO-B substrate. The fluorescence intensity was measured using a fluorescence plate reader (Infinite F200, Tecan, Salzburg, Austria) at an excitation wavelength of 535 nm and an emission wavelength of 590 nm.
Drugs
PMMA was synthesized by Drs. Hanajiri and Goda (Department of Pharmacognosy, Phytochemistry and Narcotics, National Institute of Health Sciences, Tokyo, Japan). R-(+)-7-chloro-8-hydroxy-3- methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390), (-)-sulpiride and ketanserin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). SCH23390, sulpiride and ketanserin were initially dissolved in a minimum volume of 0.1 N HCl and were then diluted with distilled water (the pH of each solution was adjusted to about 4 with NaHCO3).
Statistical Analysis
For all data, statistical analyses were performed using the software Graph Pad Prism 4.0 (San Diego, CA, USA). Behavioral and neurochemical data are presented as means ± S.E.M. In the conditioned place-preference study, conditioning scores represent the time spent in the drug-paired compartment minus the time spent in the vehiclepaired compartment, with these scores presented as means ± S.E.M. The ED50 values with confidence intervals (CIs) for substitution were calculated by a nonlinear regression analysis (variable slope) of the dose-response curves using the software Graph Pad Prism 4.0.
The statistical significance of the differences between the groups was determined by using one-way ANOVA followed by a post hoc Bonferroni’s multiple comparison test. Significance was ascribed for results with a P< 0.05.
Results
Locomotor activity
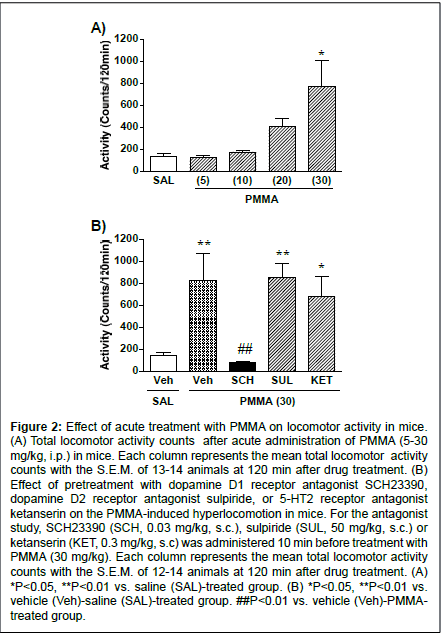
Figure 2: Effect of acute treatment with PMMA on locomotor activity in mice. (A) Total locomotor activity counts after acute administration of PMMA (5-30 mg/kg, i.p.) in mice. Each column represents the mean total locomotor activity counts with the S.E.M. of 13-14 animals at 120 min after drug treatment. (B) Effect of pretreatment with dopamine D1 receptor antagonist SCH23390, dopamine D2 receptor antagonist sulpiride, or 5-HT2 receptor antagonist ketanserin on the PMMA-induced hyperlocomotion in mice. For the antagonist study, SCH23390 (SCH, 0.03 mg/kg, s.c.), sulpiride (SUL, 50 mg/kg, s.c.) or ketanserin (KET, 0.3 mg/kg, s.c) was administered 10 min before treatment with PMMA (30 mg/kg). Each column represents the mean total locomotor activity counts with the S.E.M. of 12-14 animals at 120 min after drug treatment. (A) *P<0.05, **P<0.01 vs. saline (SAL)-treated group. (B) *P<0.05, **P<0.01 vs. vehicle (Veh)-saline (SAL)-treated group. ##P<0.01 vs. vehicle (Veh)-PMMAtreated group.
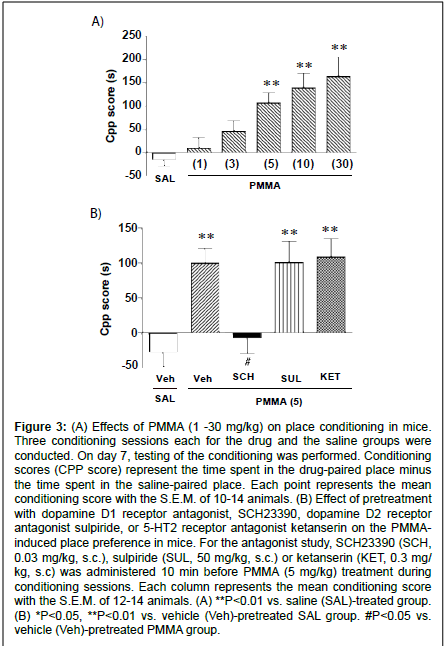
Figure 3: (A) Effects of PMMA (1 -30 mg/kg) on place conditioning in mice. Three conditioning sessions each for the drug and the saline groups were conducted. On day 7, testing of the conditioning was performed. Conditioning scores (CPP score) represent the time spent in the drug-paired place minus the time spent in the saline-paired place. Each point represents the mean conditioning score with the S.E.M. of 10-14 animals. (B) Effect of pretreatment with dopamine D1 receptor antagonist, SCH23390, dopamine D2 receptor antagonist sulpiride, or 5-HT2 receptor antagonist ketanserin on the PMMAinduced place preference in mice. For the antagonist study, SCH23390 (SCH, 0.03 mg/kg, s.c.), sulpiride (SUL, 50 mg/kg, s.c.) or ketanserin (KET, 0.3 mg/ kg, s.c) was administered 10 min before PMMA (5 mg/kg) treatment during conditioning sessions. Each column represents the mean conditioning score with the S.E.M. of 12-14 animals. (A) **P<0.01 vs. saline (SAL)-treated group. (B) *P<0.05, **P<0.01 vs. vehicle (Veh)-pretreated SAL group. #P<0.05 vs. vehicle (Veh)-pretreated PMMA group.
Figure 2A shows the total activity counts for 120 min following the PMMA treatment in mice. Administration of PMMA produced a significant increase in the locomotor activity in a dose-dependent manner (F(4,62)=6.39, P<0.0001). PMMA at the dose of 30 mg/kg produced a significant increase in locomotor activity compared to the control (P<0.01). The influence of the dopamine receptor antagonists on PMMA-induced hyperlocomotion is shown in Figure 2B.
The mean total activity counts for PMMA (30 mg/kg) for 120 min were significantly reduced when mice were pretreated with the dopamine D1 receptor antagonist SCH23390 (F(4,63)=6.23, P<0.0003). The significant hyper locomotive effects of PMMA (30 mg/kg) compared to saline treatment were not, however, suppressed by pretreatment with the dopamine D2 receptor antagonist sulpiride or the 5-HT2 receptor antagonist ketanserin.
Place conditioning
During the conditioning sessions and at the test sessions, there were no significant differences observed in the PMMA-treated group for gross behavior or body weight as compared to the saline-treated group. In addition, the saline-treated group exhibited no preference for either compartment. The mean conditioning score was -14.6 ± 13.3 s (n=14). Place conditioning by PMMA (1 - 30 mg/kg, i.p.) is shown in Fig. 3A. Administration of PMMA produced a dose-dependent preference for the drug-associated compartment (F(5,69)=7.70; P<0.0001, Fig. 3A). Significant conditioning scores were observed at doses of 5, 10 and 30 mg/kg.
To determine whether the PMMA-induced place preference is mediated by the dopamine system, we examined the effect of pretreatment with the dopamine D1 receptor antagonist SCH23390 or the dopamine D2 receptor antagonist sulpiride on the place conditioning by PMMA. As shown in fig. 3B, the place preference produced by PMMA (5 mg/kg) was significantly suppressed by pretreatment with SCH23390 (mean conditioning score of -7.47 ± 21.1 s, n=12). On the other hand, PMMA (5 mg/kg)-induced place preference was not suppressed by pretreatment with sulpiride (mean conditioning score of 100.8 ± 30.0 s, n=11) or ketanserin (mean conditioning score of 109.0 ± 26.0 s, n=12). The SCH23390 (0.03 mg/kg, i.p.), sulpiride (50 mg/kg, i.p.) or ketanserin (0.3 mg/kg) pretreated saline group exhibited neither place preference nor place aversion [The mean conditioning score: SCH23390 (0.03 mg/kg) = -22.4 ± 23.7 s (n=14), sulpiride (50 mg/kg) = -1.9 ± 19.9 s (n=14) and ketanserin (0.3 mg/kg) = -2.7 ± 41.3 s (n=12)].
Monoamine levels
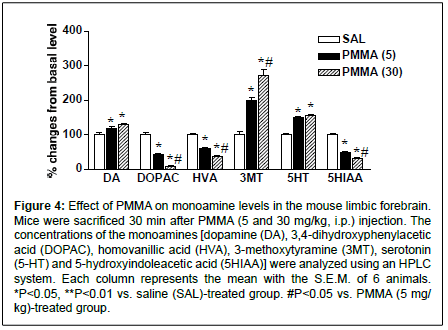
To determine the involvement of the monoaminergic system on PMMA-induced behavioral changes, we examined the levels of dopamine, 5-HT and its metabolites in the limbic forebrain. In salinetreated mice, monoamine concentrations (ng/mg of tissue) in the limbic forebrain were as follows: dopamine, 8.24 ± 0.46; DOPAC, 7.94 ± 0.35; HVA, 3.16 ± 0.16; 3MT, 0.52 ± 0.05; 5HT, 2.57 ± 0.22; and 5HIAA, 2.59 ± 0.13. As shown in Figure 4, the administration of PMMA (5 and 30 mg/kg) significantly elevated the levels of dopamine (F(2,15)=17.8, P=0.0047) and its metabolite, 3MT (F(2,15)=51.0, P<0.0001). Levels of 3MT were significantly more elevated by PMMA at dose of 30 mg/kg than PMMA at dose of 5 mg/kg. On the other hand, the levels of the dopamine metabolites DOPAC and HVA were markedly reduced by PMMA (DOPAC, F(2,15)=143.1, P<0.0001, and HVA, F(2,15)=223.1, P<0.0001). Levels of DOPAC and HVA were significantly lower in the 30-mg/kg PMMA group than the 5-mg/kg group. Similarly, the administration of PMMA significantly increased the level of 5-HT (F(2,15)=53.3, P<0.0001), accompanied with significant reductions of the level of the 5-HT metabolite 5-HIAA (F(2,15)=316.2, P<0.0001). The level of 5HIAA was significantly lower in the 30-mg/kg PMMA group than in the 5-mg/kg group.
MAO activity
Figure 4: Effect of PMMA on monoamine levels in the mouse limbic forebrain. Mice were sacrificed 30 min after PMMA (5 and 30 mg/kg, i.p.) injection. The concentrations of the monoamines [dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 3-methoxytyramine (3MT), serotonin (5-HT) and 5-hydroxyindoleacetic acid (5HIAA)] were analyzed using an HPLC system. Each column represents the mean with the S.E.M. of 6 animals. *P<0.05, **P<0.01 vs. saline (SAL)-treated group. #P<0.05 vs. PMMA (5 mg/ kg)-treated group.
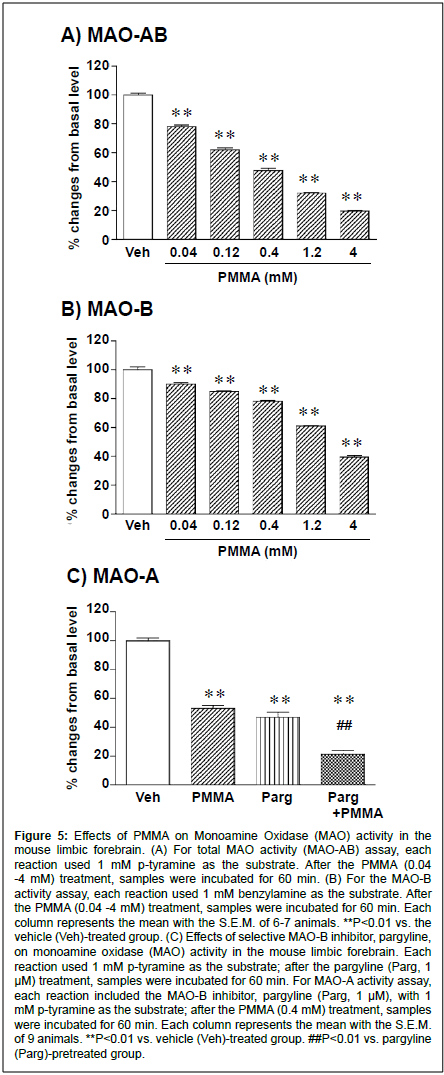
Figure 5:Effects of PMMA on Monoamine Oxidase (MAO) activity in the mouse limbic forebrain. (A) For total MAO activity (MAO-AB) assay, each reaction used 1 mM p-tyramine as the substrate. After the PMMA (0.04 -4 mM) treatment, samples were incubated for 60 min. (B) For the MAO-B activity assay, each reaction used 1 mM benzylamine as the substrate. After the PMMA (0.04 -4 mM) treatment, samples were incubated for 60 min. Each column represents the mean with the S.E.M. of 6-7 animals. **P<0.01 vs. the vehicle (Veh)-treated group. (C) Effects of selective MAO-B inhibitor, pargyline, on monoamine oxidase (MAO) activity in the mouse limbic forebrain. Each reaction used 1 mM p-tyramine as the substrate; after the pargyline (Parg, 1 μM) treatment, samples were incubated for 60 min. For MAO-A activity assay, each reaction included the MAO-B inhibitor, pargyline (Parg, 1 μM), with 1 mM p-tyramine as the substrate; after the PMMA (0.4 mM) treatment, samples were incubated for 60 min. Each column represents the mean with the S.E.M. of 9 animals. **P<0.01 vs. vehicle (Veh)-treated group. ##P<0.01 vs. pargyline (Parg)-pretreated group.
Administration of PMMA markedly reduced the level of the dopamine metabolites DOPAC and HVA in the limbic forebrain. Therefore, we investigated the effects of PMMA on basal MAO activity in the mouse limbic forebrain. As shown in Figure 5A, the total MAO (MAO-AB) activity was significantly decreased by PMMA in a concentration-dependent manner (F(5,30)=623, P<0.0001). The MAO MAOAB inhibitory effects of PMMA were significant at concentrations of 0.04 -4 mM. The EC50 value of the MAO-AB inhibitory effect of PMMA was 0.96 (0.63 -1.31) mM (y=-29.6 X log(x)+35.7, R2=0.998). Similarly, the MAO-B activity was significantly decreased by PMMA in a concentration-dependent manner (F(5,30)=421.8, P<0.0001) (Fig. 5B). The MAO-B inhibitory effects of PMMA were significant at concentrations of 0.04 -4 mM. The EC50 value of the MAO-B inhibitory effect of PMMA was 2.89 (1.97 -5.20) mM (y=-25.1 X log(x)+60.6, R2=0.921). To determine whether PMMA has an inhibitory effect on MAO-A, the influences of a selective MAO-B inhibitor, pargyline, were examined. As shown in Figure 5C, the total MAO (MAO-AB) activity in the mouse limbic forebrain was markedly inhibited by the selective MAO-B inhibitor pargyline (1μM, 100+2.1% to 45.8+5.2%, F(1,12)=98.9, P=0.001). Although the basal MAO activity was markedly decreased following treatment by the MAO-B inhibitor pargyline (1μM), the inhibitory effect of PMMA (0.4 mM) was still observed, indicating that PMMA has MAO-A inhibitory effects.
Discussion
The major objective of the present study was to analyze the behavioral and neurochemical properties of PMMA. We constructed an experimental system in animals that could be used to clarify the physiological mechanisms of the action for methamphetamine derivative PMMA and their risks.
The present findings demonstrate that the administration of PMMA induced hyperlocomotion and rewarding effects in a conditioned place-preference paradigm. A great deal of evidence has suggested that psychostimulant-induced hyperlocomotion and rewarding effects may be mediated by the enhancement of the central dopaminergic system [14,15].Thus, the search for the substrates of the prototypic psychostimulant amphetamine, which induces hyperlocomotion and place preference, have centered on the mesolimbic dopamine system. For example, amphetamine-induced hyperlocomotion is inhibited by intra-accumbens administration of dopamine antagonists or 6-hydroxydopamine (6-OHDA) [16-20]. Similarly, the rewarding effects of amphetamine are inhibited by either D1 or D2 receptor antagonists or by 6-OHDA lesions of the nucleus accumbens [20-23]. Furthermore, administration of amphetamine elevates extracellular levels of dopamine in the nucleus accumbens [24]. In the present study, we examined both the role of dopamine receptors in PMMA-induced hyperlocomotion and rewarding effects and the effect of PMMA on the level of monoamines in the limbic forebrain, a terminal region of the ventral tegmental area (VTA).
The present results demonstrate that PMMA produced hyperlocomotion in mice. These findings are consistent with those of a previous report [25]. The expression of PMMA-induced hyperlocomotion was completely abolished by pretreatment with the dopamine D1 receptor antagonist SCH23390. On the other hand, PMMA-induced hyperlocomotion was not suppressed by the dopamine D2 receptor antagonist sulpiride. A previous study showed that the dopamine D1 receptor antagonist SCH23390 completely reduced the hyper locomotion produced by psycho stimulants such as amphetamine and cocaine, whereas the dopamine D2 receptor antagonist raclopride only partially attenuated the effect of these psycho stimulants [26]. It has been shown that SCH23390 is a highly potent and selective dopamine D1-like receptor antagonist, and binds to the 5-HT2 receptor subtypes in vitro [27]. However, the doses required to induce a similar response in vivo are greater than 10-fold higher than those required to induce a D1-mediated response [28]. Indeed, the present results indicated that PMMA-induced hyper locomotion was not modified by pretreatment with the 5-HT2 receptor antagonist ketanserin (0.3 mg/ kg) in mice, even though we used an adequate dose to block the 5-HT2 receptor [29-31]. Our present results suggest that the activation of the dopamine receptors, especially the D1 receptors, may be involved in the expression of PMMA-induced hyper locomotion.
Using the conditioned place-preference paradigm, the present results also demonstrated that PMMA produced rewarding effects in mice. The mesolimbic dopamine system has been identified as one of the important structures with regard to rewards that are associated with psycho stimulants such as amphetamine, methamphetamine and cocaine [20,22,23,32]. In the present investigation, pretreatment with the dopamine D1 receptor antagonist SCH23390 completely abolished the expression of place preference produced by PMMA. On the other hand, the PMMA-induced place preference was not suppressed by the dopamine D2 receptor antagonist sulpiride, even though we used an adequate dose to block central D2 receptors [33]. Furthermore, the present results indicated that PMMA-induced rewarding effects were not modified by pretreatment with the 5-HT2 receptor antagonist ketanserin (0.3 mg/kg) in mice. The present findings suggest that activation of dopamine receptors, especially D1 receptors, may be involved in the expression of the rewarding effects of PMMA.
In our neurochemical study, we examined the influences of PMMA on the contents of the monoamines in the limbic forebrain, a terminal region of the VTA. After the administration of PMMA, there was an increase in the levels of dopamine and 3MT in the limbic forebrain. Especially, after a high dose of PMMA (30 mg/kg) treatment, there was an approximate 2.5-fold increase in the levels of 3MT. It has been previously suggested that changes of 3MT in brain tissue may be a specific reference for dopamine transmission. In fact, one study has proposed that changes in 3MT levels of the brain tissue are an indicator of dopamine release [34]. In a previous micro dialysis study, PMMA also increased the extracellular levels of dopamine in the striatum [7]. When taken in conjunction with this previous report, our present results seem to indicate that PMMA indeed enhances the dopamine transmission in the limbic forebrain. On the other hand, PMMA decreased the levels of dopamine metabolites (DOPAC and HVA) and the 5-HT metabolite (5-HIAA). It is well known that MAO-A preferentially oxidizes 5-HT and norepinephrine, whereas MAO-B preferentially oxidizes phenyl ethylamine and benzyl amine [35]. Furthermore, both MAO-A and MAO-B metabolize dopamine to DOPAC and HVA in human and rodents [36]. PMMA is a structural hybrid of two phenyl isopropyl amine chemicals: methamphetamine and para-methoxy amphetamine (PMA).
PMA is structurally and pharmacologically similar to PMMA [4]. It has been shown that PMA, which is structurally similar to PMMA, potently inhibits MAO activity [8]. Our results indicated that PMMA has the ability to inhibit the activities of both MAO-A and MAO-B in the limbic forebrain. Thus, enhancement of the catecholaminergic activity produced by PMMA may occur via the inhibitory effect that PMMA has on both MAO-A and MAO-B activities. It should be noted that the selective MAO-A inhibitor clorgyline increased striatal dopamine release and decreased striatal DOPAC and HVA levels in MAO-B knockout mice, actions that were not seen when using the selective MAO-B inhibitor l-deprenyl [37]. This appears to indicate that both MAO-A and MAO-B metabolize dopamine in the mouse brain [37,38]. Since our present findings suggest that inhibition of MAO-A and MAO-B activities by PMMA may be involved in the enhancement of the dopamine transmission in the limbic forebrain, the elevation of dopaminergic neuron activity produced by PMMA may play an important role in the expression of its hyper locomotion and rewarding effects as well.
In conclusion, the present findings show that PMMA produces hyper locomotion and rewarding effects. The monoamine system, which is mainly the dopamine system, may play an important role in the expression of the PMMA-induced psycho stimulant-like effects. These hyper locomotive and rewarding effects of PMMA may occur via the dopamine D1 receptors. Furthermore, the enhancement of the catecholaminergic activity produced by PMMA may also be due to PMMA’s inhibitory effect on MAO activity. These behavioral and neurochemical data indicate that PMMA has a psychic dependence liability.
Acknowledgements
This work was supported by a Research Grant for Regulatory Science of Pharmaceuticals and Medical Devices, Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan (to M.F.). Authors thank Yoshiko Shoji and Kayo Funada for their assistance.
References
- Dal Cason TA (2001) A re-examination of the mono-methoxy positional ring isomers of amphetamine, methamphetamine and phenyl-2-propanone. Forensic SciInt 119: 168-194.
- Johansen SS, Hansen AC, Müller IB, Lundemose JB, Franzmann MB (2003) Three fatal cases of PMA and PMMA poisoning in Denmark. J Anal Toxicol 27: 253-256.
- Glennon RA, Higgs R (1992) Investigation of MDMA-related agents in rats trained to discriminate MDMA from saline. PharmacolBiochemBehav 43: 759-763.
- Glennon RA, Young R, Dukat M, Cheng Y (1997) Initial characterization of PMMA as a discriminative stimulus. PharmacolBiochemBehav 57: 151-158.
- Bilsky EJ, Reid LD (1991) MDL72222, a serotonin 5-HT3 receptor antagonist, blocks MDMA's ability to establish a conditioned place preference. PharmacolBiochemBehav 39: 509-512.
- Bubar MJ, Pack KM, Frankel PS, Cunningham KA (2004) Effects of dopamine D1- or D2-like receptor antagonists on the hypermotive and discriminative stimulus effects of (+)-MDMA. Psychopharmacology (Berl) 173: 326-336.
- Romero CA, Bustamante DA, Zapata-Torres G, Goiny M, Cassels B, et al. (2006) Neurochemical and behaviouralcharacterisation of alkoxyamphetamine derivatives in rats. Neurotox Res 10: 11-22.
- Scorza MC, Carrau C, Silveira R, Zapata-Torres G, Cassels BK, et al. (1997) Monoamine oxidase inhibitory properties of some methoxylated and alkylthio amphetamine derivatives: structure-activity relationships. BiochemPharmacol 54: 1361-1369.
- Freezer A, Salem A, Irvine RJ (2005) Effects of 3,4-methylenedioxymethamphetamine (MDMA, 'Ecstasy') and para-methoxyamphetamine on striatal 5-HT when co-administered with moclobemide. Brain Res 1041: 48-55.
- Funada M, Sato M, Makino Y, Wada K (2002) Evaluation of rewarding effect of toluene by the conditioned place preference procedure in mice. Brain Res Brain Res Protoc 10: 47-54.
- Sato M, Wada K, Funada M (2005) Barium potentiates the conditioned aversion to, but not the somatic signs of, morphine withdrawal in mice. Eur J Pharmacol 519: 215-222.
- Funada M, Suzuki T, Narita M, Misawa M, Nagase H (1993) Blockade of morphine reward through the activation of kappa-opioid receptors in mice. Neuropharmacology 32: 1315-1323.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25: 192-216.
- Koob GF, Sanna PP, Bloom FE (1998) Neuroscience of addiction. Neuron 21: 467-476.
- Pijnenburg AJ, Honig WM, Van Rossum JM (1975) Inhibition of d-amphetamine-induced locomotor activity by injection of haloperidol into the nucleus accumbens of the rat. Psychopharmacologia 41: 87-95.
- Roberts DC, Zis AP, Fibiger HC (1975) Ascending catecholamine pathways and amphetamine-induced locomotor activity: importance of dopamine and apparent non-involvement of norepinephrine. Brain Res 93: 441-454.
- Joyce EM, Stinus L, Iversen SD (1983) Effect of injections of 6-OHDA into either nucleus accumbenssepti or frontal cortex on spontaneous and drug-induced activity. Neuropharmacology 22: 1141-1145.
- Clarke PB, Jakubovic A, Fibiger HC (1988) Anatomical analysis of the involvement of mesolimbocortical dopamine in the locomotor stimulant actions of d-amphetamine and apomorphine. Psychopharmacology (Berl) 96: 511-520.
- Sellings LH, Clarke PB (2003) Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci 23: 6295-6303.
- Leone P, Di Chiara G (1987) Blockade of D-1 receptors by SCH 23390 antagonizes morphine- and amphetamine-induced place preference conditioning. Eur J Pharmacol 135: 251-254.
- Hiroi N, White NM (1991) The amphetamine conditioned place preference: differential involvement of dopamine receptor subtypes and two dopaminergic terminal areas. Brain Res 552: 141-152.
- Spyraki C, Fibiger HC, Phillips AG (1982) Dopaminergic substrates of amphetamine-induced place preference conditioning. Brain Res 253: 185-193.
- Carboni E, Imperato A, Perezzani L, Di Chiara G (1989) Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience 28: 653-661.
- Glennon RA, Ismaiel AE, Martin B, Poff D, Sutton M (1988) A preliminary behavioral investigation of PMMA, the 4-methoxy analog of methamphetamine. PharmacolBiochemBehav 31: 9-13.
- O'Neill MF, Shaw G (1999) Comparison of dopamine receptor antagonists on hyperlocomotion induced by cocaine, amphetamine, MK-801 and the dopamine D1 agonist C-APB in mice. Psychopharmacology (Berl) 145: 237-250.
- Bischoff S, Heinrich M, Sonntag JM, Krauss J (1986) The D-1 dopamine receptor antagonist SCH 23390 also interacts potently with brain serotonin (5-HT2) receptors. Eur J Pharmacol 129: 367-370.
- Bourne JA (2001) SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS Drug Rev 7: 399-414.
- Gleason SD, Shannon HE (1997) Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl) 129: 79-84.
- Indra B, Tadano T, Nakagawasai O, Arai Y, Yasuhara H, et al. (2002) Suppressive effect of nantenine, isolated from Nandinadomestica Thunberg, on the 5-hydroxy-L-tryptophan plus clorgyline-induced head-twitch response in mice. Life Sci 70: 2647-2656.
- Dalton GL, Lee MD, Kennett GA, Dourish CT, Clifton PG (2004) mCPP-induced hyperactivity in 5-HT2C receptor mutant mice is mediated by activation of multiple 5-HT receptor subtypes. Neuropharmacology 46: 663-671.
- McBride WJ, Murphy JM, Ikemoto S (1999) Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res 101: 129-152.
- Noda Y, Miyamoto Y, Mamiya T, Kamei H, Furukawa H, et al. (1998) Involvement of dopaminergic system in phencyclidine-induced place preference in mice pretreated with phencyclidine repeatedly. J PharmacolExpTher 286: 44-51.
- Kehr W (1976) 3-Methoxytyramine as an indicator of impulse-induced dopamine release in rat brain in vivo. NaunynSchmiedebergs Arch Pharmacol 293: 209-215.
- Fowler CJ, Mantle TJ, Tipton KF (1982) The nature of the inhibition of rat liver monoamine oxidase types A and B by the acetylenic inhibitors clorgyline, l-deprenyl and pargyline. BiochemPharmacol 31: 3555-3561.
- O'Carroll AM, Fowler CJ, Phillips JP, Tobbia I, Tipton KF (1983) The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. NaunynSchmiedebergs Arch Pharmacol 322: 198-202.
- Fornai F, Chen K, Giorgi FS, Gesi M, Alessandri MG, et al. (1999) Striatal dopamine metabolism in monoamine oxidase B-deficient mice: a brain dialysis study. J Neurochem 73: 2434-2440.
- Shih JC, Chen K, Ridd MJ (1999) Monoamine oxidase: from genes to behavior. Annu Rev Neurosci 22: 197-217.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 13751
- [From(publication date):
February-2014 - Jul 17, 2024] - Breakdown by view type
- HTML page views : 9380
- PDF downloads : 4371