Rewarding and Sex Difference Effect of Catha edulis (khat) in Swiss Albino Mice
Received: 03-May-2022 / Manuscript No. jart-22-64781 / Editor assigned: 05-May-2022 / PreQC No. jart-22-64781 (PQ) / Reviewed: 19-May-2022 / QC No. jart-22-64781 / Revised: 21-May-2022 / Manuscript No. jart-22-64781 (R) / Accepted Date: 23-May-2022 / Published Date: 28-May-2022 DOI: 10.4172/2155-6105.100469
Abstract
Background: Burden of substance abuse is becoming a worldwide problem. One of the substances widely consumed in Ethiopia and other East African countries is Catha edulis Forsk (khat). Most of abusers complained that its stimulatory effect is the determinate factor makes them to chew. However, its rewarding and reinforcing potential and variation between sexes has not been investigated. This study was designed to measure the rewarding effects of khat extract in addiction mice model of both sexes.
Materials and Methods: 48 Swiss albino mice of both sexes of age 6-7 weeks having 21-27g body weight were used. The mice were conditioned to khat extract (ke) (100 mg/kg, 200 mg/kg and 300 mg/kg b.w) for 20 days. Control group was conditioned to tween 80 (2%, v /v) in distilled water. The reinforcing effect of khat was evaluated using conditioned place preference (CPP) paradigm. The classical pairing to the extract was made using the place conditioning box. Post-condition tests have been conducted four times and the average values were taken for analysis using SPSS version 21.0.
Results: Time spent in khat paired compartment was higher significantly for mice conditioned to ke 200 mg/kg (p<0.05) and ke 300 mg/kg (p<0.001). The rewarding effects of khat was strong in females at a higher dose when compared with the same sex of mice conditioned to vehicle (p<0.001) or male sex of mice conditioned to the same dose of khat extract (p<0.05). Repeated administration increased khat rewarding sensitization at all dose groups.
Conclusions: Mice showed place conditioning to khat extract and were strong in females. The genes expression alteration induced by khat extract between sexes should be investigated.
Keywords: Addiction, Addiction research, Addiction therapy, Substances use disorders, Conditioned place preference, khat, Place condition score, Time spent in the khat paired compartment
Introduction
Background
Substances use disorders are prevalent and becoming the major public health concerns in global setting [1]. Substance abuse and addiction are enormous public health concerns that affect society and public policy including health care, education, and worker productivity and criminal law [2] . Amphetamines and amphetamine-like substances are currently known to present major drug-abuse concerns [3]. One of the substances reported to have a stimulatory responses related to amphetamines is Catha edulis Forsk [4]. Catha edulis Forsk, commonly called khat, is a flowering evergreen plant under Celastraceae [5]. One of alkaloids found in khat leaves with amphetamine like-structure and function and assumed causes the stimulatory effects f khat is cathinone [6].
Ethiopia, Zimbabwe, Somalia, Kenya, Uganda, Tanzania, South Africa, Madagascar and Djibouti are among East African countries where khat chewing is very common next to Yemen [7]. The magnitude of people chewing khat leaves is increasing from time to time [8]. This is because of the debating whether or not khat can actually cause addiction and physical dependence [9].
Some authors reported its adverse effects and others reported its positive effects [10,11] and others reported its adverse effects and burdens [5,9], indicating that khat is the subject of controversy.
However, no studies have been conducted to measure the potential rewarding effects of khat using In addition, substance use was considered to be primarily a male problem, and many substance abuse studies are conducted with a predominance of male participants [12]. Few researches suggest that males and females differ in their biological and subjective responses to abused drugs. However, studies have not been conducted if differential rewarding response to khat extract could be observed between sexes. The present study was aimed to evaluate the reinforcing effects of khat extract in mice stratified by sex.
Materials and Methods
Chemicals
Diethyl ether and chloroform (Sigma-Aldrich, Germany), tween 80, and 70% ethanol were purchased from the local suppliers in Addis Ababa, Ethiopia.
Plant materials collection
Khat leaves (500 g) were purchased and collected from Hararge in July 2017, natural habitat. The leaves specimen voucher number was given (August 2017, AA001) and stored in natural herbarium, Addis Ababa University. After the edible parts of the leaves were separated and washed with tap water, freeze dried at -20oC [13] for 2 days and crushed using mortar and pistil.
Plant material extraction
700 gm of freeze dried crushed leaves were placed into the conical flask wrapped with aluminum foil [6]. A total of 400ml organic solvents (300ml diethyl ether and 100ml chloroform (3:1v/v ratio) were added into the flask to cover the whole minced leaves. The mixture was shaken under dark condition for 48 hours using a rotary shaker (New Brunswick Scientific Co, USA) at 72g and 20°C. The mixture was filtered initially using cotton gauze followed by grade I Whatman filter paper (Cat No 1001 150). The organic solvents were then removed through evaporation using Rota-vapor under controlled temperature of 360C, rotation of 3g and 240 Pascal negative pressure. The water in the extract was removed through lyophylization to get the dry extract.
Animal preparation
A total of forty eight Swiss albino mice (6-7 weeks) of both sexes from same breeding series weighing between 20-30g were used. Six mice were housed per cages under a natural light and dark (12:12) cycle at room temperature, 21±10C. The pellets and water was supplied with no restriction, ad libitum. Mice were weighed twice a week and the experiment was carried out in accordance with the guidelines for care and use of laboratory animals prepared by the national academies Sciences [14] the research was approved Institutional Review board (IRB) committee, Addis Ababa University with a protocol number of 012/15/Physio.
Grouping of mice and dosing
The mice were randomly assigned into four groups (n=12/group). The first group was conditioned to vehicle (tween 80, 2% v/v in distilled water; T80W).Other groups were conditioned to khat extract (ke) (100 mg/kg, 200 mg/kg and 300 mg/kg). The doses for khat extract were selected based on previous reports [15].
Solution preparation and volume determination
Fresh solution of extract and vehicle were prepared every day and extract was dissolved in T80W. The dose of the extract for each mouse was calculated from selected doses (100 mg/kg, 200 mg/kg and 300 mg/kg) and the total body weight (b.w) of each mouse. The appropriate standard vehicle volume (10 ml/kg b.w of mice) was used to determine how much volume was used to dissolve the calculated dose extract. Each mouse in its respective group received a single daily oral administration of extract from the stock solution or an equivalent volume of vehicle (0.5ml). The final volume for all mice was 0.5ml.
Conditioned place preference (CPP) test
A wooden CPP box with two equal size conditioning (46.5cm×12.7 cm) and one central non-conditioning (7.2cm×12.7cm) compartments were used [16,17]. The box was placed in the sound attenuating neurobehavioral animal study room (2m x 2m). The three compartments of the box had frontal Plexiglas.
Internal and external cues were used constantly throughout the study. The first conditioning compartment had white wall with a rough floor. The other conditioning compartment had black wall and middle non-conditioning compartment had red wall and floor. The compartments were separated by sliding doors. The box was cleaned after each trial with a mild soap solution.
With some modification, the experimental procedure applied in this study was taken from previous study [16]. Briefly, each mice acclimatized the box for four consecutive days for 20 minutes per trial/ day. Each mouse was placed at the non- conditioning compartment and allowed to explore all open compartments freely. The pre-condition test was made 24 hrs after the last acclimatization day. Each mouse received T80 30 min prior to its placements in a non-conditioning compartment to explore the open compartments freely for 10 min. The time spent in each compartment and numbers of entries into conditioning compartments were recorded by video camera.
The Conditioning test was started 24 hrs after the preconditioning phase. Briefly, on day 7, 9, 11, and 13 each mouse was administered with khat extract and placed in least preferred compartment. The same mouse received the vehicle on day 6, 8, 10 and 12 and placed in the preferred compartment. This procedure was repeated for eight consecutive conditioning days and the duration of the pairings was 20 min for each trial with one conditioning session per a day.
The post condition test was evaluated on one day after the last conditioning day (14th). In these phase, each mouse was placed in the non-conditioning compartment with no pairing but in the presence of conditioning stimuli. Each mouse was allowed to explore all open compartments freely for 10min. The total time each mouse spent in the khat paired compartment (KPC), vehicle paired compartment (VPC), number of entries into the conditioning compartments were recorded during a 10min period. The subsequent post-conditioning tests were conducted on day 23, 32 and 41. The post-conditioning tests results were made for analysis (Table 1).
| Phase | Pre-c phase | Conditioning phase | Post-c phase | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treat | T80W | T80W | K | T80W | K | T80W | K | T80W | K | No Pairing |
| Day | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Pre-c = pre-conditioning, post-c = post-conditioning; T80W = Tween 80 in distilled water, K= khat and treat= treatment. | ||||||||||
Table 1: Timeline for Conditioned Place Preference Study for the First Phase.
Statistical analysis
The statistical analysis was done using SPSS version 21.0 and graphs were plotted using Microsoft excel. One-way ANOVA followed by post Hoc Tukey’s test was used to compare the mean difference between the groups. Repeated two ANOVA followed by Bonferroni multiple comparison test was also used to evaluate the potential rewarding effects of the khat extract over a repeated administration. The Dunn’s post hoc comparison test was also used for non-distributed data. The Data were expressed as means ± standard error of mean (SEM). Differences with p < 0.05 were considered statistically significant.
Results
Conditioned place preference effects of khat
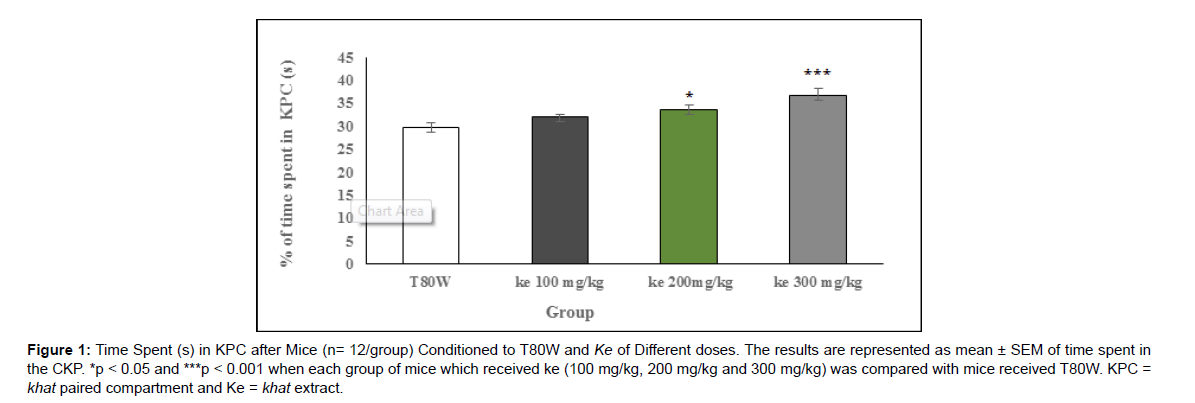
Analysis of one way ANOVA indicated that there was significant difference in time spent in KPC (F (3, 44) = 13.28, p<0.001) between groups. The post hoc Tukey’s test indicated that percentage of time spent in KPC by mice paired with 300 mg/kg and 200 mg/kg khat extract was significantly higher than mice paired with T80W (38.76 ±1.67 vs 29.24±0.88, p<0.001,95% CI [537,13.67] and 33.63±0.99 vs 29.240.088, p <0.05 ,95% CI [0.24,8.54], respectively) (Figure 1).
Figure 1: Time Spent (s) in KPC after Mice (n= 12/group) Conditioned to T80W and Ke of Different doses. The results are represented as mean ± SEM of time spent in the CKP. *p < 0.05 and ***p < 0.001 when each group of mice which received ke (100 mg/kg, 200 mg/kg and 300 mg/kg) was compared with mice received T80W. KPC = khat paired compartment and Ke = khat extract.
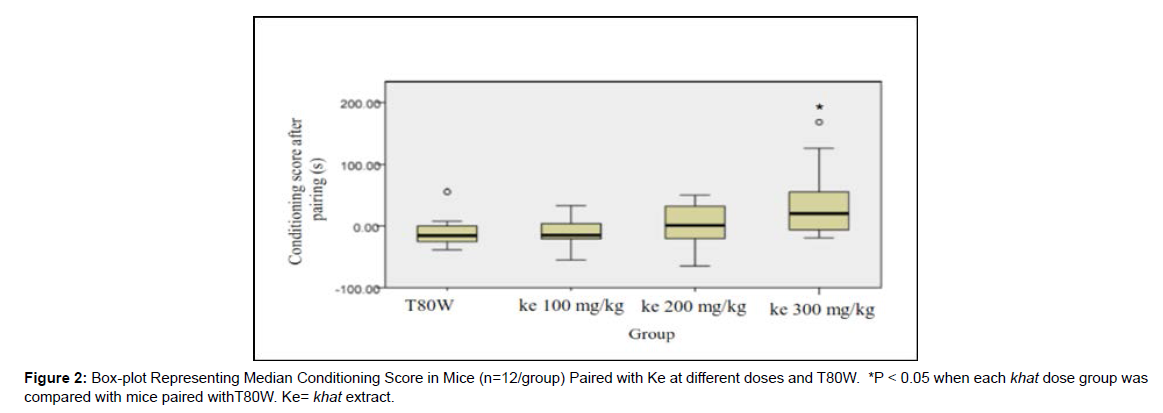
The Dunn’s post hoc comparison showed that mice paired with ke 300 mg/kg had significantly greater median conditioning score than mice paired with T80W after pairing to khat extract and T80W (p<0.05) (Figure 2).
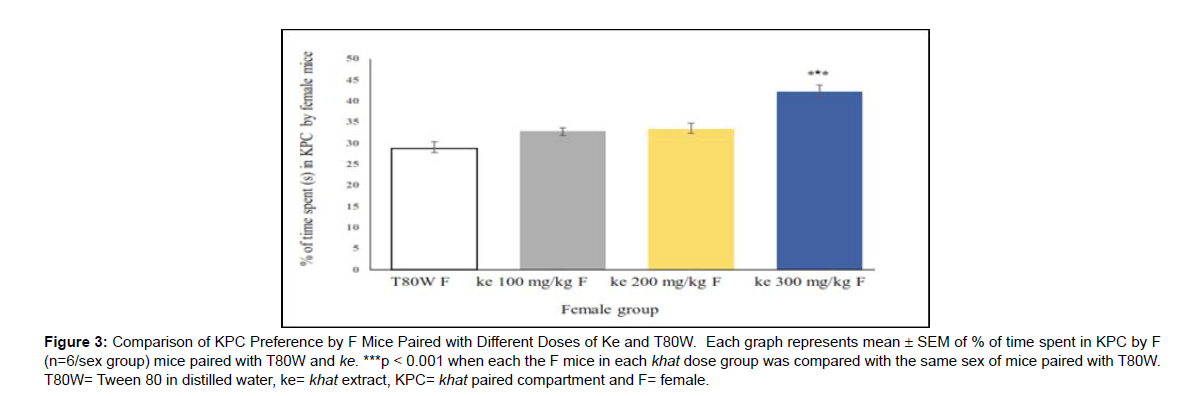
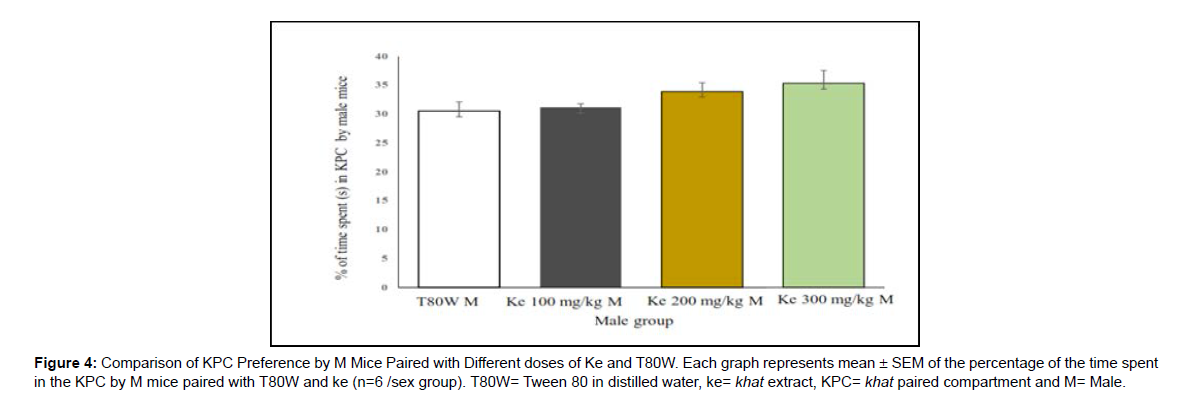
Effect of sex differences on CPP response of khat The place conditioning effect of khat extract in female mice was compared with the same sex of mice paired with T80W or male mice paired with the same dose of extract. Significant difference in time spent in the KPC was observed (F (7, 40) =3.68, p< 0.001) between female groups. The post hoc test showed that time spent in KPC was significantly higher in female mice paired with the higher dose of khat extract (ke 300 mg/kg) compared with the same sex of mice paired with T80W (42.18±1.65 vs 28.75±1.51, p < 0.001, 95% CI [7.00, 19.84]) (Figure 3). However, no significant difference was observed in this parameter between male mice paired with the different doses of khat extract and T80W (Figure 4).
Figure 3: Comparison of KPC Preference by F Mice Paired with Different Doses of Ke and T80W. Each graph represents mean ± SEM of % of time spent in KPC by F (n=6/sex group) mice paired with T80W and ke. ***p < 0.001 when each the F mice in each khat dose group was compared with the same sex of mice paired with T80W. T80W= Tween 80 in distilled water, ke= khat extract, KPC= khat paired compartment and F= female.
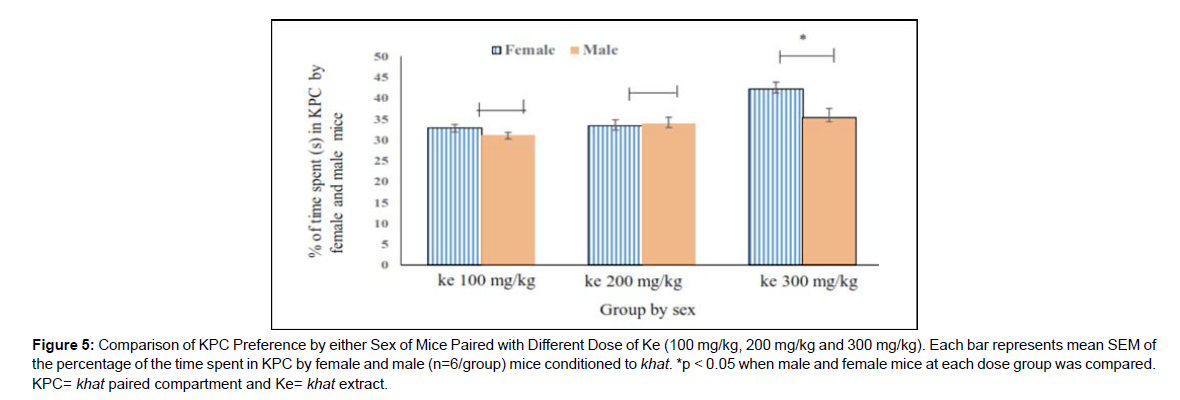
Time spent (s) in KPC by male and female mice in the same khat dose group was compared. Independent t-test result indicated that time spent in KPC by female mice was significantly higher than male at ke 300 mg/ kg (42.18±1.65 vs 35.34±2.19, p<0.05, 95 % CI [0.42, 13.26]) (Figure 5).
Rewarding sensitizing effects of khat
The two ways repeated measure of ANOVA indicated that treatmalest, number of conditioning tests and interaction between treatment and number of tests had significant effect on the time spent in the KPC (F (3,33) = 22.14, p<0.001; F (2.02, 22.01) = 8.61, p <0.01 and F (12,132) = 5.25, p<0.001, respectively) between groups. The post hoc Two ways repeated measure of ANOVA test indicated that the estimated marginal mean of the time spent in KPC was significantly higher in mice paired with k 100 mg/kg (32.09±0.46 vs 28.32±0.53, p<0.01, 95% CI [1.38, 6.18]), k200 mg/kg (34.79±0.96 vs 28.32±0.53, p<0.001, 95% CI [3.05, 9.92]) and k 300 mg/kg (38.69±145 vs 28.32±0.53, p<0.001, 95% CI [5.23,15.53] (Figure 6).
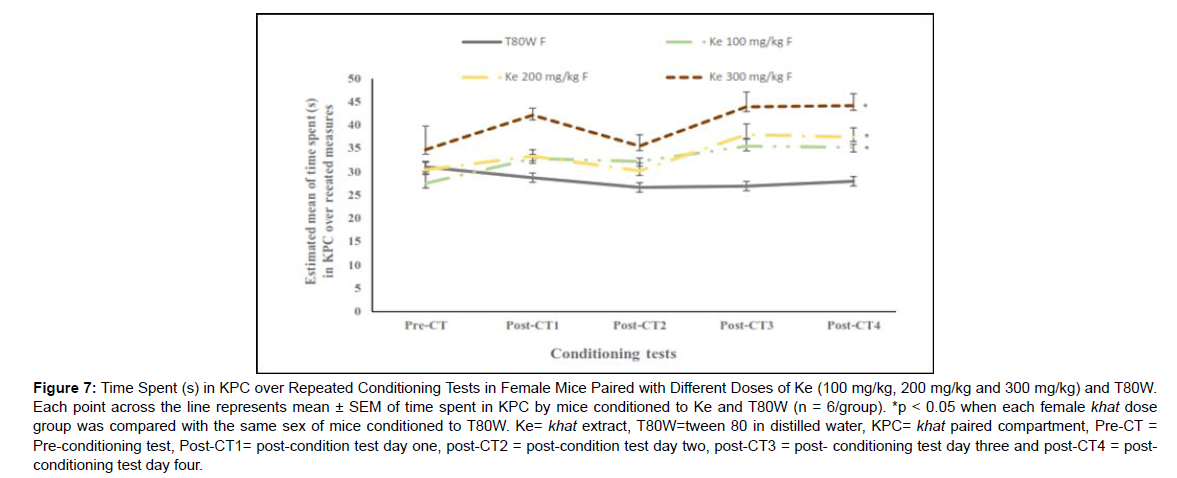
The estimated marginal mean of time spent in KPC in female mice conditioned to ke 100 mg/kg (32.04±0.61 vs 28.36±0.61, p<0.05, 95% CI [0.44, 8.36]), ke 200 mg/kg (32.99±0.76 vs 28.36±0.61, p<0.05, 95% CI [0.39, 10.83]) and ke 300 mg/kg (39.12±2.29 vs 28.36±0.61, p<0.05, 95% CI [1.67, 22.04]) was significantly higher than in the same sex of mice conditioned to T80W (Figure 7).
Discussion
Conditioned place preference
In this study, mice paired with the middle and higher doses of khat extract showed significant rewarding and conditioned place preference (CPP) response dose-dependently (Figure 1). However, taking the conditioning score as a measure of preference, only the higher dose of khat extract contributed to significant place preference in mice (Figure 2). This finding showed that less time was spent by mice paired with khat extract in the initially preferred compartment. This, in turn, revealed that khat extract reversed the initial natural innate place preference in mice.
The previous study indicated that cathinone and amphetamine showed CPP [16]. Similar to the finding observed in this study, other study showed that synthetic cathinone and mephedrone, cathinone in khat like substances, reversed the natural innate preference in mice [18].
The rewarding effect of khat extract observed in this study could be through its action on monoaminergic transmissions. Dopaminergic transmission system in the brain was affected by amphetamine and other stimulants [17]. Previous studies showed that dopamine and GABA level was affected by khat extract [19, 20].
A review conducted by WHO indicated that cathinone enhancing activities were partially blocked by dopamine antagonist, haloperidol, and cathinone increased the release of dopamine from presynaptic terminals [21]. A study also showed that schizophrenic-like syndromes were observed in mice administered with khat extract [22], indicating that khat extract increases the release of dopamine in the dorsal striatum of the brain. Activation of glutamatergic and GABAergic cells in the ventral tegmental area increased the activities of neurons in the nucleus accumbens [23]. Animals showed reinforcing behaviors during this stimulation [23].
Sex difference in CPP effects of khat
In this study, CPP was observed in female mice paired with the higher dose of khat extract compared with the same sex of mice paired with vehicle (Figure 3). However, significant CPP was not observed in male mice when compared with the same sex of mice paired with vehicle (Figure 4). The khat paired compartment preference was significantly higher in female mice compared with males at a higher dose of the extract (Figure 5). This indicated that the rewarding and reinforcing effects of khat, particularly at the higher dose, was stronger in females than in males.
Figure 4: Comparison of KPC Preference by M Mice Paired with Different doses of Ke and T80W. Each graph represents mean ± SEM of the percentage of the time spent in the KPC by M mice paired with T80W and ke (n=6 /sex group). T80W= Tween 80 in distilled water, ke= khat extract, KPC= khat paired compartment and M= Male.
Figure 5: Comparison of KPC Preference by either Sex of Mice Paired with Different Dose of Ke (100 mg/kg, 200 mg/kg and 300 mg/kg). Each bar represents mean SEM of the percentage of the time spent in KPC by female and male (n=6/group) mice conditioned to khat. *p < 0.05 when male and female mice at each dose group was compared. KPC= khat paired compartment and Ke= khat extract.
Though the model in our study was mice, the same finding was reported in the previous study in which female rats showed a greater preference to amphetamine than males [24]. Other report also showed that female rats have been shown to be more sensitive to amphetamine, cocaine and are more vulnerable the different stages of addiction than males [25].
Similar to our study, previous study showed that females and males have different response to nicotine, cocaine and methamphetamine [12,26]. Females got more dependent and suffered more to the adverse effects of methamphetamine than males [26] like females were more khat dependent than males in the current study. Drug escalating was higher in females and get addiction quickly than males after initiation of drug use [27]. Females show greater unpleasant symptoms and stress responses than males during drug withdraw [27].
Other animal study also revealed that female rats moved longer distance, showed more rearing activities, higher and long last stereotypic movement than male received the same amount of methamphetamine [28]. This indicated that differential psychomotor responses are observed in rats administered with methamphetamine between sexes. Animals showed higher psychomotor activities have got addiction easily than showing less psychomotor activities [29], indicating that methamphetamine induced differential response between sexes.
The rewarding and reinforcing differential responses to khat extract between sexes observed in our study could be attributed to dopaminergic response variation to the extract between sexes.
The dopaminergic response to khat extract could be modulated by sex steroids. A previous review indicated that dopaminergic projections from ventral tegmental area and substantia nigra to nucleus accumbens, hippocampus, prefrontal cortex, amygdala and corpus striatum were modulated by sex steroids [30, 31]. Ovarian hormones, estradiol and progesterone, have access to the brain and affected inhibitor control and drug taking behavior [27]. The effects of cocaine and amphetamine tend are more intense during follicular phase, when estradiol level is high in females [27]. Therefore, dopaminergic transmission affected by khat extract [20] is modulated by gonadal hormones [27, 32].
Difference in the pharmacokinetics of khat extract between sexes could also the reason for the differential rewarding response observed between sexes in our study. Previous report indicated that behavioral and neurobiological responses to substances relevant to addiction were affected by their pharmacokinetics [33]. This indicated that the addictive potential effects of substances are depends on their absorption, distribution, metabolism and excretion. These four processes affecting the addictive potential effects of substances might be different between sexes.
Previous study indicated that differences between males and females in drug metabolism affect the drug responses between sexes [12]. A review indicated that mehamphetamine increase the metabolic acitivites more in males then femaoles [26]. How much of the drugs gets to brain, how fast drug level increased in the brain and how often drug level rise and falls in the brain play roles in drug addiction [33]. The faster the drug reach in the brain is the more likely to cause addiction [33]. This shows that the components in khat extract could reach in the brain more quickly in females than do in males.
Thought, the sex differences in the brain are regulated by gonadal hormones and sex chromosome genes, sex chromosome genes are the predetermined factors than gonadal hormones [34]. A previous study indicated that brain sexual dimprhphisom is attributed to the sex chromosome genes [35]. The chromosomal female mice showed faster food-reinforced instrumental habit formation than chromosomal male mice regardless of gonadal phenotype [35]. This indicted that these are chromosome genes determined the goal-directed behaviors in mice than gonads or gonadal hormones.
Other study also showed that sex differences in brain contributed to the variation in addiction-like behaviors between sexes [27]. Therefore, if the sex difference in the brain is regulated by sex chromosome genes influenced the responses to drugs of abuse, progressive changes in the brain after exposure to drugs of abuse, these sex chromosome genes could be affected by khat extract differently between males and females.
Metabolic acitivity and gene expression alteration effects of khat between sexes and brain structural response variation to khat extract between males and females could be attributed to the differential reinforcing effects of khat extract between sexes. The neurochemical and hormonal response differences between sexes could also involve for such differential response between sexes observed in our study. Previous study indicated that female with methamphetamine abusers had larger volumes in the corpus callosum and more hyperperfused regions in the parietal and occipital areas of the brain [26, 27].
Rewarding sensitizing effects of khat extract
In our study, two ways repeated measure of ANOVA showed that number of conditioning tests had a main effect on time spent in khat paired compartment. This indicated that time spent in khat paired compartment was gradually increased with repeated administration of khat extract. Such response revealed that rewarding effect of the same dose of khat extract was increased with time.
Boneferroni confidence interval adjusted post hoc analysis indicated that estimated marginal mean time spent in khat paired compartment was significantly higher in mice paired with all doses of khat extract when compared with mice paired with T80W (Figure 6). Similar to the present findings, previous study indicated that the rewarding response to cocaine was increased through repeated exposures in rodents [36]. Previous study indicated that animals showed sensitization response to amphetamine [37]. The substance showed schizophrenic like locomotor sensitization and hyperfunction of the mesolimbic dopaminergic system, indicating that dopaminergic response to these substances increased gradually [38].
Figure 6: Time Spent (s) in KPC over Repeated Conditioning Tests in Mice Paired with Different Doses of Ke (100 mg/kg, 200 mg/kg and 300 mg/kg) and T80W. Each point across the line represents mean ± SEM of time spent in KPC by mice conditioned to Ke and T80W (n = 12/group). **p < 0.01 and ***p <0.001 when each khat dose group was compared with T80W. Ke= khat extract, T80W=tween 80 in distilled water, KPC= khat paired compartment, Pre-CT = Pre-conditioning test, Post-CT1= post-condition test day one, post-CT2 = post-condition test day two, post-CT3 = post- conditioning test day three and post-CT4 = post-conditioning test day four.
This rewarding and reinforcing sensitization effect of khat extract was also observed in the female mice conditioned to all doses of khat extract when compared with the same sex of mice conditioned to vehicle. However, such sensitization response was not seen in male mice, indicating that the gradual increase response to the same dose of khat extract was strong in females than males. The mechanism for this differential response between sexes could be attributed to factors discussed above in this study. The sex difference in the brain rewarding circuit response to khat extract, sex chromosome genes and the ovarian hormones are among factors contributing to this differential rewarding sensitization response between sexes, discussed above.
Figure 7: Time Spent (s) in KPC over Repeated Conditioning Tests in Female Mice Paired with Different Doses of Ke (100 mg/kg, 200 mg/kg and 300 mg/kg) and T80W. Each point across the line represents mean ± SEM of time spent in KPC by mice conditioned to Ke and T80W (n = 6/group). *p < 0.05 when each female khat dose group was compared with the same sex of mice conditioned to T80W. Ke= khat extract, T80W=tween 80 in distilled water, KPC= khat paired compartment, Pre-CT = Pre-conditioning test, Post-CT1= post-condition test day one, post-CT2 = post-condition test day two, post-CT3 = post- conditioning test day three and post-CT4 = postconditioning test day four.
In conclusion, conditioned place preference and physical dependence has been observed in mice conditioned to khat extract in dose dependent manner. The reinforcing and rewarding sensitization effects of khat extract was higher in females than males, indicating that females were more prone to get khat addiction than males. The genes expression alteration induced by khat extract between sexes should be investigated. At what chromosome and genes level where expression alteration could be induced by khat extract between sexes for such khat induced addiction behavior. Understanding the basic mechanisms mediating the differential reinforcing response induced by khat extract between sexes is important for improving prevention and enhancing treatmalest related to khat addiction and relapse.
Acknowledgment
This research was supported by Addis Ababa University and Department of Physiology. We are am most indebted to Ato Tesfaye Getachew who assisted us during the oral administration of the khat extract and Dr. Nigusie Deyisa who helped us during the statistical analysis. We would like to express our appreciation to Dr. Anne Depping that she read the manuscript and provided important points.
Authors’ Contribution
Abebaye Aragaw Limalesie - Conduct the research, analyze the data, and write the manuscript, Eyasu Mekonnen Eshetu - Show directions regarding to the research, Daniel Seyifu Melka - facilitating research activities , Tesfaye Tolessa Dugul - Follow up the research, facilitating research materials.
References
- Fiona CJ, Sandra D, Crick L, Louisa D, Harvey WA (2014) Mental and Substance Use Disorders in Sub-Saharan Africa: Predictions of Epidemiological Changes and Mental Health Workforce Requirements for the Next 40 Years. PLoS one 9: 1-11.
- Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL (2010) Animal Models of Substance Abuse and Addiction: Implications for Science, Animal Welfare, and Society. Com Med 60: 177-188.
- Raffa RB, Shah S, Tallarida CS, Rawls SM (2013) Amphetamine Conditioned Place Preference in Planarians. J Behav Brain Sci 3: 131-136.
- Dhaifalah I, Santavy J (2004) Khat habit and its health effect: A natural amphetamine. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 148: 11-15.
- Abebe M, Kindie S, Adane K (2015) Adverse Health Effects of Khat: A Review. Fam Med Med Sci Res 4: 2-5.
- Alfaifi H, Ibrahim AS, Syam M, Manal M, Elhassan T, et al. (2017) Catha edulis Forsk (Khat): Evaluation of its Antidepressant-like Activity. Pharmacogn Mag 13: 5354-5357.
- Odenwald M (2007) Chronic Khat use and psychotic disorders: A review of the literature and future prospects. Sucht 53: 9-22.
- Andargachew K, Eskindir L, Atkilt E (2017) Prevalence of Khat chewing and its effect on academic performance in Sidama zone, Southern Ethiopia. African Health Sci 17: 175-185.
- Al-Habori M (2005) The Potential Adverse Effects of Habitual use of Catha edulis (Khat). Expert Opin Drug Saf 4: 1145-1154.
- Thomas S, Williams T (2013) Khat (Catha edulis): A systematic review of evidence and literature pertaining to its harms to UK users and society. Drug Sci Policy Law 1: 1-19.
- Lamina S (2010) Khat (Catha edulis): the herb with officio-legal socio-cultural and economic uncertainty. S Afr J Sci 106:1-4.
- Tuchman E (2010) Women and addiction: the important of gender issue in substance abuse research. J Addict Dis 29(2): 127-138.
- Atlabachew M, Chandravanshi BS, Redi-Abshiro M, Torto N, Chigome S, et al. (2013) Evaluation of the effect of various drying techniques on the composition of the psychoactive phenylpropylamino alkaloids of Khat (Catha edulis forsk) chewing leaves. Bull Chem Soc Ethiop 27: 347-358.
- National academies Sciences (2011) Guide for the care and use of laboratory animals, 8th Edition. The National Academies Press, Washington, DC, USA.
- Shewamene Z, Engidawork E (2014) Sub-acute administration of crude Khat (Catha edulis F.) extract induces mild to moderate nephrotoxicity in rats. BMC Complement Altern Med 14: 66.
- Kitanaka N, Kitanaka J, Hall FS, Uhlgeorge, Watabe K, et al. (2012) Attenuation of Methamphetamine-Induced conditioned place preference in Mice After a Drug-Free period and Facilitation of this effect by exposure to a Running Wheel. J Exp Neurosci 6: 11-19.
- Pourtaqi N, Imenshahidi M, Razavi BM, Hosseinzadeh H (2017) Effect of linalool on the acquisition and reinstatement of morphine induced conditioned place preference in mice. Avicenna J Phytomed 7: 242-249.
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, et al. (2013) Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend 126 : 257-262.
- Shawqi AA, Hussein AK, Mohanad S (2015) Effect of khat-habituation on GABA levels in brain. Asian J Pharmacy Life Sci 3: 74-80.
- El-Sayed MK, Amin HK (2015) Catha edulis chewing effects on treatment of paranoid schizophrenic patients. Neuropsychiatr Dis Treat 11: 1067-1076.
- WHO (2006) Assessment of khat (Catha edulis Forsk). 34th ECDD, USA.
- Bogale T, Engidawork E, Yisma E (2016) Subchronic oral administration of crude Khat extract (Catha edulis Forsk) induces schizophrenic-like symptoms in mice. BMC Complement Altern Med 16: 1-12.
- Yoo JH, Zell V, Gutierrez-Reed N, Wu J, Ressler R, et al. (2016) Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun 7: 1-13.
- Weiss VG (2015) Sex differences in monoamines following amphetamine and social reward in adolescent rats. Exp Clin Psychopharmacol 23: 197-205.
- Anker JJ, Carroll M (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci 8:73-96.
- Dluzen DE, Liu B (2008) Gender Differences in Methamphetamine Use and Responses: A Review. Gend Med 5: 24-35.
- Becker JB, McClellan ML, Reed BG (2017) Sex differences, gender and addiction. J Neurosci Res 95: 136-147.
- Milesi-Halle A, McMillan DE, Laurenzana EM, ByrnesBlake KA, Owens SM (2007) Sex Differences in (+)-Amphetamine- and (+)-Methamphetamine induced Behavioral Response in Male and Female Sprague Dawley Rats. Pharmacol Biochem Behav 86: 140-149.
- Phillips TJ, Pastor R, Scibelli AC, Reed C, Tarragon E (2010) Behavioral Sensitization to Addictive Drugs: Clinical Relevance and Methodological Aspects. Neuro methods 50: 267-305.
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, et al. (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8: 1-14.
- Sinclair D, Tertia D, Purves-Tyson TD, Allen KM, Weickert CS (2014) Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology 231: 1581-1599.
- Yoset KE, Cummings JA, Becker JB (2014) Estradiol, dopamine and motivation. Enteral nervous system agents med chem.2014; 14 (2):83-89.
- Allain F, Minogianis E, Roberts D, Samaha A (2015) How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev 56: 166-179.
- Zagni E, Simoni L, Colombo D (2016) Sex and Gender Differences in Central Nervous System-Related Disorders. Neurosci J 1: 1-13.
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Jane R, et al. (2007) Sex chromosome complement regulates habit formation. Nat Neurosci 10: 1398-1400.
- Riday TT, Kosofsky BE, Malanga CJ (2012) The Rewarding and Locomotor-Sensitizing Effects of Repeated Cocaine Administration are Distinct and Separable in Mice. Neuropharmacology 62: 1858-1866.
- Herrmann AP, Andrejew R, Benvenutti R, Gama CS, Elisabetsky E (2018) Effects of N-acetylcysteine on amphetamine-induced sensitization in mice. Braz J Psychiatry 40:169-173.
- Carlezon WA, Nestler EJ (2002) Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse. Trends Neurosci 25: 610-615.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Limalesie AA, Eshetu EM, Melka DS, Dugul TT (2022) Rewarding and Sex Difference Effect of Catha edulis (khat) in Swiss Albino Mice. J Addict Res Ther 13: 469. DOI: 10.4172/2155-6105.100469
Copyright: © 2022 Limalesie AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1545
- [From(publication date): 0-2022 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 1345
- PDF downloads: 200