Research Article Open Access
Revisiting Sulphide Mineral (Bio) Processing: A Few Priorities and Directions
Hanumantha Rao K*1, Javadi A1, Karlkvist T1, Patra A1, Vilinska A2 and Chernyshova IV2
1Mineral Processing Group, Division of Sustainable Process Engineering, Department of Civil, Environmental and Natural Resources Engineering, Luleå University of Technology, SE-971 87 LULEÅ, Sweden
2Department of Earth and Environmental Engineering, Henry Krumb School of Mines, Columbia University, New York, NY 10027, USA
- *Corresponding Author:
- K. Hanumantha Rao
Mineral Processing Group
Division of Sustainable Process Engineering
Department of Civil, Environmental and Natural Resources Engineering
Luleå University of Technology, SE-971 87 LULEÅ, Sweden
E-mail: hanumantha.rao@ltu.se
Received Date: September 19, 2013; Accepted Date: October 10, 2013; Published Date: October 17, 2013
Citation: Hanumantha Rao K, Javadi A, Karlkvist T, Patra A, Vilinska A, et al. (2013) Revisiting Sulphide Mineral (Bio) Processing: A Few Priorities and Directions. J Powder Metall Min 2:116. doi: 10.4172/2168-9806.1000116
Copyright: © 2013 Hanumantha Rao K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
Large efforts are being made to streamline the conventional (chemical and physical) technological schemes of ore processing, remediation and environmental protection towards reducing overall costs, limiting the use of dangerous substances, decreasing waste streams and improving waste disposal and recycling practice. Hitherto, search for such innovations has been performed mainly empirically and there is an urgent need to shift these technologies to be more innovative and effective. Alternative biotechnological solutions and solutions mimicking natural processes are also being proposed. However, except for bioleaching, practical exploitation of the biotechnological potential in extractive industries and accompanying environmental protection measures remains far from feasibility. Understanding of the fundamental concepts of aquatic chemistry of minerals–selective adsorption and selective redox reactions at mineral– bacteria–solution interfaces, impact innovating conventional and bio-flotation, as well as (bio) remediation/detoxification of mineral and chemical wastes are necessary. Molecular-level knowledge and coherent understanding of minerals contacted with aqueous solutions is required that underlie great opportunities in controlling abiotic and biotic mineral– solution interfaces towards the grand challenge of tomorrow’s science and mineral processing technology
Keywords
Sulfide mineral; Flotation; Adsorption; Bioprocessing; Surface reactivity; FTIR Spectroscopy
Introduction
Processes on minerals in aquatic media give the world as we know it. Understanding these processes is important for mineral resources sustainability and to develop technologies as diverse as ore processing, heterogeneous catalysis in solution, (bio)remediation of contaminants, radioactive waste storage and disposal, biochemical engineering, development of novel surfactants, sorbents, sensors, synthesis of novel materials and coatings, and especially in emerging nanotechnologies, where stringent control of materials and surfaces is crucial. Despite the obvious importance and the long history of chemistry of aquatic heterogeneous processes [1], many fundamental issues, especially related to biotic interfaces, are still unresolved or poorly understood.
Froth flotation is the main separation process used for collecting selectively valuable minerals from a mined ore, and is based on differences in hydrophobicity of particles. Particles with size below 10 microns are a common constituent of a flotation pulp, originating from non-sulfide gangue, grinding in steel mills, and oxidation of sulfides. Iron oxide/sulfide fines are the primary component of acid mine drainage that control scavenging of toxic cations. Such fine particles are produced during grinding of iron ores pose a serious problem for flotation and degrade the quality of the concentrate. Generally, attempts to remove and discard fines before beneficiation operations result in significant economic losses. On the other hand, the continual reduction in the ore grade is forcing miners to produce ultrafines in order to liberate valuable minerals. Hence, improvements in both flotation of fines and flotation of coarse particles in the presence of fines will increase the sustainability of the mineral resources, while also reducing the potential environmental impact of the discarded minerals. In addition, there are persistent problem in sulfide flotation of nonferrous metal sulfides selectivity against ferrous pyrite, inadvertent activation of silicate gangue by metal ions, hetero-coagulation between sulfide and gangue minerals, and fine particle flotation. The solutions to these problems are highly rewarded: even small technological improvements would provide high economical and ecological return on investments, with regard to the tonnages of material treated and the multi-dimensional impact of ore processing.
Recent developments in biotechnology have given promise of not only aiding mineral processing–hydrometallurgy operations but also providing means for bioremediation of environmental problems generated by the mineral industries. Many other uses of microorganisms and their derivatives are potentially possible. These include the use of microorganisms in flocculation and flotation of minerals [2]. The biomodification of mineral surfaces involves the complex action of microorganism on the mineral surface. There are three different mechanisms by means of which the biomodification can occur: i) attachment of microbial cells to the solid substrate, ii) oxidation reactions and iii) adsorption and/or chemical reaction with the metabolite products (EPS). Several types of autotrophic and heterotrophic bacteria, fungi, yeasts and algae are implicated in minerals biobeneficiation.
Aqueous redox chemistry of sulfides
The sulfide minerals are the most common on the Earth, most important, most diverse, and richest in terms of physical, chemical, and structural properties. Such diversity originates from the more complex crystal and electronic structures compared to other classes of materials [3]. The main reasons are found in the variety of oxidation states, coordination numbers, symmetry, crystal field stabilization, density, stoichiometry, and acid-base surface properties that metal sulfides exhibit. The combination of variety of properties and applications of sulfides makes redox reactions catalyzed by these minerals in aqueous solutions a very important subject of research from both fundamental and industrial standpoints.
Quantifying and predicting redox chemistry of sulfides is separately motivated within geochemical, electrochemical, and technological communities. These minerals significantly impact bio-environment geochemistry of near surface systems by altering pH and redox conditions, thereby increasing or decreasing mineral bioavailability [4]. Charge transfer processes on semiconducting sulfide electrodes are today under intense investigation towards novel techniques for conversion of solar energy and environmentally clean fuels such as hydrogen [5]. Within a few years well over a thousand publications about this topic appeared (see ref. 5 for a list). Therefore, increased knowledge of electronic properties of the mineral surfaces, the detailed mechanisms of charge transfer across their interfaces, and driving forces in the interactions with species from solution will help in improving the research in this area of electrochemistry.
Despite significant steps have been taken theoretically and experimentally to obtain a molecular-level and coherent understanding of aquatic reactivity of sulfides [5-8], a general theory of redox chemistry of these solids in waters still does not exist. In the case of oxides, the chemical mechanism is commonly considered [9,10], which implies one of the following charge transfer reactions: 1) From the solid to the adsorbate, 2) Between sorbates, or 3) From the adsorbate to the solid. The main idea behind this mechanism is that catalytic effect of the surface is caused by a decrease in the energy needed to modify the solvent structure, the standard redox potential of the reductant, and steric hindrance of the oxidant-reductant interaction [11]. However, the chemical model does not explain different modes and different rates of the oxidation film growth on different minerals [12-14] as well as heterogeneous processes on sulfides [15]. A general disadvantage of this model is in its explanatory character and low ability to predict.
An alternative is the electrochemical mechanism, in which the anodic and cathodic semi-reactions of the sum redox reaction are spatially separated. This mechanism has been invoked for interpreting abiotic chemistry of semiconducting sulfides [15-18] and photochemical reactions on both oxides and sulfides [5,9]. Apart from the energy level redistribution among species in the (inner and outer) Helmholtz layer, this model takes into account semiconducting properties of the solid [19]. Provided the position of the Fermi level relative to edges of the conduction and valence bands as well as specification, relative density and position of the surface states are known, the electron-accepting and electron-donating abilities of different solids can be compared and correlated with the direction and rates of a particular heterogeneous redox reaction, including biotic reductive/oxidative degradation of oxides and sulfides.
Oxidation of sulfides
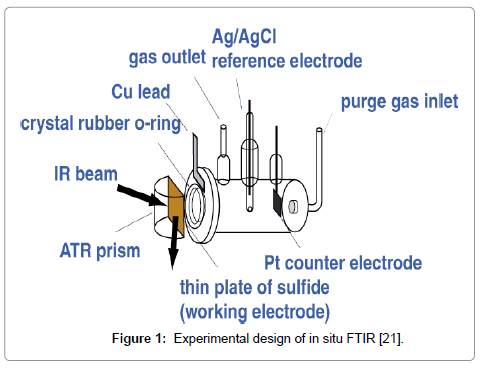
Since reactivity of semiconducting sulfides under flotation conditions is mainly electrochemical, spectro-electrochemical studies that allows characterizing the sulfide surface (its composition and structure) as a function of the mineral potential and the electrolyte composition (including deoxygenation and/or carbonization) are expected to be most advanced. Easily manipulating the interfacial potential difference by a potentiostat, one can determine all states of the mineral surface reactivity, which is of substantial importance for modeling the flotation process. FTIR spectroscopy has become the dominant tool for in situ studies of reactions at the electrode/electolyte or mineral/water interface over the last decade due to a large body of information provided, simplicity of the application in situ, and a short-time scale of the measurements, which can be performed simultaneously with a voltammogram. Three FTIR techniques have so far been applied to strongly absorbing IR radiation natural sulfides. Based on the comparitive analysis performed [20], the authentic ATR technique that uses a glued mineral plate [21] has more advantages as it is freer from kinetic limitations and technical artifacts (Figure 1). In addition to in situ FTIR measurements, complimentary data by XPS for the atomic surface composition and the valence state of the surface atoms will provide surface structures of adsorbed species.
The mechanisms suggested for the oxidation of galena in previous indirect studies at alkaline pH are:
a) Initial stage:
PbS + 2H2O + 2xh+ = Pb1-xS + xPb(OH)2
PbS + 2xh+ = Pb1-xS + xPb2+
PbS + 2H2O + 2h+ = Pb(OH)2 + S + 2H+
PbS = Pb2+ + S2-
b) Bulk oxidation:
2PbS + 5H2O = PbS2O3 + Pb(OH)2 + 8H+ + 8e-
2PbS + 7H2O = 2Pb(OH)2 + S2O32- + 10H+ + 8e-
2PbS + 2CO32- + 3H2O = 2PbCO3 + S2O32- + 6H+ + 8e-
Where h+ stands for hole [16,17], and has been initially suggested [22,23] to explain the prepeak in voltammograms (at -0.05 V (SHE) in 0.001 M n-butyl xanthate solution [24]).
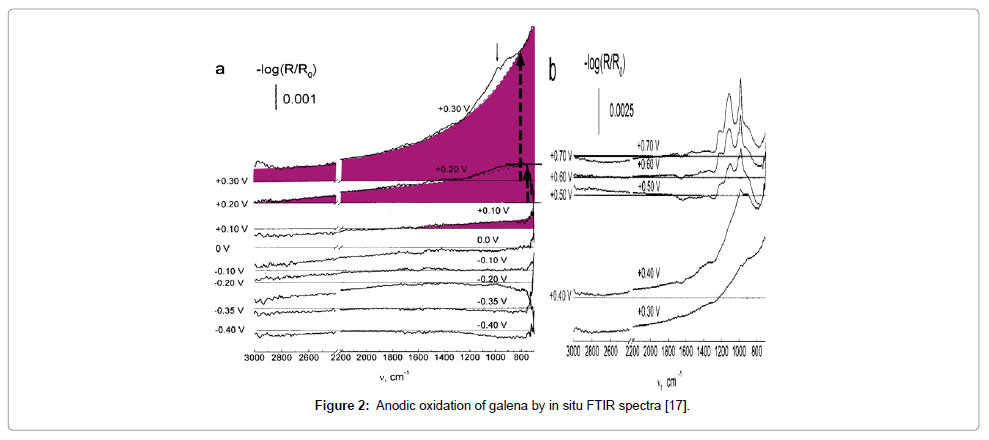
The products of anodic oxidation determined by in situ FTIR spectra [16,17] (Figure 2) with increasing potential in deaerated 0.01 M borate buffer of pH 9.2 at 0.1 V: Pb1-xS; 0.2 V: Pb(OH)2 and PbSO3; 0.3 V: PbS2O3; and 0.4 V: SnO62-.s
Based on the spectral results (Figure 2), the first stage produces Pb1-xS and then S0:
PbS + 2xh+ = Pb1-xS + xPb2+
PbS + 2h+ = Pb2+ + S0 (E0 = 0.354 V)
Then, Pb(OH)2 is formed by a precipitation mechanism followed by lead sulfite and thiosulfate from the elemental sulfur produced in the first stage:
S0 + 3H2O + 4h+ = SO32- + 6H+ (E0 = +0.59 V)
SO32- + Pb2+ = PbSO3
SO32- + S0 = S2O32- (log K = 8.14)
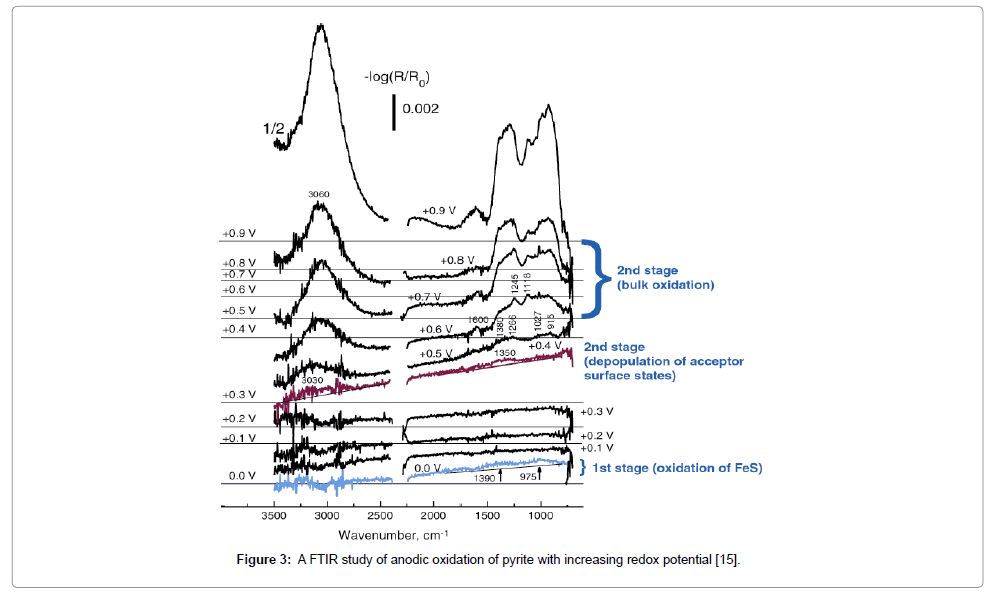
Oxygen not only provides the cathodic half-reaction of the galena oxidation but also react with the anodic areas, accelerating the incongruent dissolution. Similarly, the anodic oxidation of pyrite by in situ FTIR spectra (Figure 3) Revealed that the surface consist at 0 V: bulk hydrated ferric hydroxide gel and bulk FeSO3 in the amount less than one monoloayer; 0.4 V: less than one monolayer of a surfce ferric iron-hydroxyl complex; 0.5 V: bulk oxidation produced ferric hydroxide, ferrous sulfite and polythionates [15]; and at 0.6 V: SO42-. Anodic reactions on pyrite are: 1) FeS = Fe2+ + S2-, followed by hydrolysis, oxidation of (bi)sulfide ion (SS) and hydrolyzed iron(II) either chemically by dissolved oxygen or electrochemically, and precipitation of the products (elemental sulfur, sulfite, and ferric hydroxide); 2) SSred+ h+ + OH- = SSoxOH; and 3) thiosulfate pathway: FeS2 + 3H2O + 6h+ = Fe2+ + S2O32- + 6H+, thiosulfate and ferrous ions are further oxidized both electrochemically and chemically (by ferric hydroxide) to sulfate at the outer side of the pyriote surface, while elemental sulfur can be formed from polythionates. On some surfaces a pseudo-electrochemical (PE) mechanism is expected, when the redox process is activated at the edge of the surface heterogeneity domain and proceeds through enlarging the initial spot, not starting new ones. Relative contribution of the different pathways obviously depends on the electronic properties of the mineral bulk and surface. Assuming that bacteria recognize favorable materials for colonization through redox sensing [25], it is reasonable to suggest that there can exist a “redox” source of enhanced and selective reactivity of minerals with redox-active solutes.
For redox modification of mineral surfaces as a tool for providing adsorption selectivity, it is imperative to investigate and answer the following questions:
• Do the mineral surfaces have intrinsic oxidizing and reducing sites? In situ FTIR-ATR study of the CO, CN-, Fe(CN)63-, and Fe(CN)64- adsorption. Discrimination and characterization of mono- and clustered surface defects based on the effect of dynamic coupling of the adsorbates on the vibrational spectra and, where possible, on the STM/AFM data
• What are adsorption forms of the redox-active anions ubiquitous in geochemical systems (sulfite/sulfate, selenate/ selenite, arsenate/arsenite, chromate, etc.)? How does introduction of S2-, CN-, O2, and H2O2 before/after the anion adsorption influence this picture? Complex in situ and ex situ spectroscopic study
• How does the partitioning between adsorbed and adsorbedand- reacted anions change with time? Evaluation of the kinetic parameters of the redox reactions catalyzed by the minerals. Inferring the reaction mechanisms
• What are adsorption forms of redox-active surfactants (xanthates and dithiophosphates) in the absence and presence of the pre-adsorbed anions? How this picture is influenced by solvated S2-, CN-, O2, and H2O2? In situ and ex situ complex spectroscopic measurements
• What is the difference between the anodic semi-reaction paths on (semi)conducting oxides and sulfides in the redox process controlled “chemically” and electrochemically with a potentiostat? FTIR-ATR spectro-electrochemical study
• Analysis including surface complexation modeling of the whole data. Development of a predictive general model of redox catalytic activity of wide band-gap metal oxides and sulfides
Mechanisms of collector adsorption
Molecule-level knowledge of the mechanisms of the interfacial processes will certainly boost technological innovations in ore processing and (bio) remediation of organic and inorganic contaminants. For example, despite a more than one-century history of flotation – the major industrial process for mineral separation – suitable reagents and reagent regimes are still being developed mainly empirically and scientists are just in the beginning of understanding of basic principles of selective interactions of minerals with hydrophobizing reagents (collectors). One of the possible ways to selectively control adsorption of a collector is conducting redox reactions before or after its adsorption at the mineral surface, as used, e.g., in sulfide flotation with xanthates [25]. It is well known that the toxicity, solubility, sorption, bioavailability, and transport of elements in soil and aquatic systems are strongly dependent on the oxidation state [10].
Interaction of galena with xanthate by in situ FTIR spectroscopy is shown [16,18] in Figure 4. The se spectral results confirmed that i) At the first stage xanthate is adsorbed with charge transfer (chemisorbed), ii) dixanthogen is formed at the reversible potential for the xanthate/ dixanthogen pair, independently of the xanthate concentration. The appearance of dixanthogen makes the surface most hydrophobic, which can be followed from the changes in the water spectrum, and iii) the mechanisms of the xanthate adsorption at high and low concentrations have differences. At a concentration of 10-3 M, oxidative decomposition of galena and adsorbed xanthate is inhibited by the xanthate chemisorption, which is followed by the formation of lead xanthate, Pb(X)2, and (X)2. At 8.10-5 M, bulk Pb(X)2 is formed by the precipitation mechanism. At higher applied potentials first Pb(X)2 transforms into Pb(OH)2. Afterward (X)2 decomposes into a dimer of monothiocarbonate,
(ROCS2)2 + 2OH- + 2h+ = (ROCSO)2 + 2H+
while galena decomposes into lead sulfite and lead thiosulfate.
Thus, surface speciation of sulfides in the absence and presence of collectors as a function of the sulfide potential controlled electrochemically using a potentiostat or chemically by changing the redox potential of the pulp needs to be carried out by in situ spectroelectrochemistry. The results may lead to surface speciation with significant inference of hydrophobic or hydrophilic nature of the surface for different redox conditions.
Complex sulfide ore flotation
The use of NaHSO3 as a flotation depressant [26] is being practiced in selective flotation of sulfide minerals. The depressing effect generally increases from copper sulfides to galena, pyrite and sphalerite. However, there is no common agreement about the depression mechanisms. In particular, the following three effects have previously been proposed for depression of pyrite flotation by SO32-: 1) stripping/decomposition of xanthate [27]; 2) reaction with the pyrite surface to form hydrophilic iron oxides [28]; and 3) a decrease of redox potential of the pulp below a level at which binding/oxidation of a collector (electron donor) becomes energetically unfavorable [29]. In the presence of copper, sulfite was shown [30] to promote the oxidation of copper on the pyrite surface, preventing the adsorption of xanthate and thus leading to the mineral depression, but has no effect on sphalerite. At the same time, in the case of chalcopyrite, it was postulated [31] that sulfite removes both the sulfur-rich phase and iron oxides from the surface, leaving a sulfur-rich sulfide layer, which in turn promotes collector adsorption. Also, it was found [32] that the depressing effect of sulfite on chalcopyrite flotation depends on the presence of Fe3+ ions released from grinding media. Apart from the decomposition of xanthate/dixanthogen and the decrease in xanthate adsorption following a decrease of the redox potential of the pulp, several additional mechanisms have been put forward to explain the depression of the flotation of sphalerite [33]. These include: the formation of a zinc sulfite hydrophilic layer at the mineral surface; the reduction of copper-activation as a result of consumption of copper in solution as copper sulfite; and the consumption of dissolved oxygen. Sulfite ions are also known to react with polysulfide or elemental sulfur and form thiosulfate ions; a decrease in surface hydrophobicity is therefore expected from this reaction. Finally, compared to sulfate, this reduced sulfoxyanion has higher adsorption affinity due to lower S–O bond order [34]. Therefore, we expect that sulfate anions produced upon catalytic oxidation of sulfite species will much more strongly be bound to the sulfide surface compared with the sulfate anions that are directly adsorbed through ion exchange/outer-sphere complexation, thus competing more efficiently with collectors for the adsorption sites on sulfides, which may strengthen the depressing effect of sulfite. This effect, if properly understood, can open a new cost-effective approach to selectively regulate surface properties of sulfides.
Recently it was revealed [35] that ferric defects on ground pyrite surfaces can generate OH• radicals upon interaction with water. It may be the existence and reactivity of OH• that plays a crucial role in catalytic degradation of organic pollutants by pyrite. However, participation of these species, if any, in non-selective oxidation of the pulp components and hence in deteriorating the concentrate grade has not still been explored yet. To fill the gap, it is important to build correlation between percentage of pyrite in the concentrate, grinding conditions and concentration of OH•/H2O2 in the pulp as well as to study possible ways of flexibly controlling the formation of these species through known chemical means. One of such ways can be addition of chloride ions, which are known to inhibit the deposition of elemental sulfur on the pyrite electrode surface by promoting the oxidation of an adsorbed intermediate, believed to be the thiosulfate ion, to soluble tetrathionate ions. In the absence of chloride ions, the thiosulfate intermediate undergoes acid decomposition on the pyrite surface to yield elemental sulfur [36].
For the problem of low selectivity between ferrous and non-ferrous sulfides, and among sulfides, the effect of production of H2O2 by metal sulfides needs to be examined. To pinpoint the dominant contribution (natural hydrophobicity, formation of elemental sulfur on the surface under the flotation conditions, and/or activation) to low selectivity of sulfide flotation against pyrite, surfaces of pyrite particles from both concentrate and tailings need spectroscopy characterization for surface speciation.
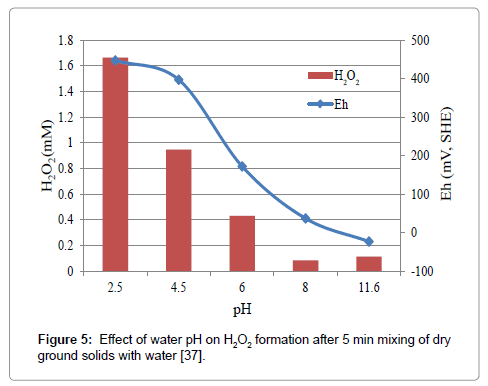
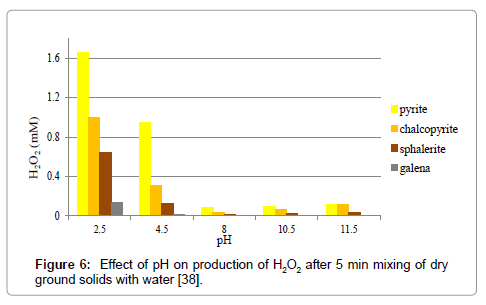
Our recent results of dry-ground pyrite when mixed with water leading to the formation of H2O2 are shown in Figure 5. The water pH in which the ground solids are placed has a significant influence [37] on H2O2 formation of its increase with decreasing pH below 8. Figure 5 shows that the formation of H2O2 is lower at pH 8 and above. This observation found to be valid with other sulfide minerals [38] as well (Figure 6).
Fine particle flotation
The floatability of fine particles has slow kinetics due to low momentum through the pulp resulting by their small mass, which leads to a lower probability of collision with passing air bubbles. Furthermore, slimes have a significant depressant effect on polysulfide ores and their depressant effect is three-fold: 1) fines of non-sulfide gangue report to concentrate; 2) valuable sulfides are lost due to low floatability of the fine particles and 3) non-sulfide gangue slimes covering the originallyhydrophobic sulfide particles through hetero-coagulation mechanism rendering the particles hydrophilic.
However, recent data showed that there are intrinsic differences in the surface and bulk stoichiometry and crystal structure of very fine particles from those of the corresponding large bulk crystals [39]. Atoms exposed on nano-sized particles experience an anisotropic environment. To lower free energy per unit surface, the crystal structure of the near surface region is distorted, which in real conditions is accompanied by adsorption of water/hydroxyls. It is expected that with decreasing particle size the redox potential of the sulfide decreases, along with sorption capacity per nm2. These effects can be balanced by using the more easily oxidizable homologues of xanthate and carbamate with longer chain lengths and/or employing sterically appropriate chelating legands with flexible distance between the reactive groups. Thus to shed light on solution and solid state chemistry of submicron particles and to get a possibility to control their reactivity, which is important for flotation, it is necessary to perform a systematic study of how and why reactivity of sulfides changes with particle sizes.
Biobeneficiation of metal sulfides
The biobeneficiation includes the processes of bio-flotation and bio-flocculation where selective removal of undesirable mineral constituents from an ore is accomplished. Several studies showed that the microorganisms could function similarly as that of traditional reagents in flotation and flocculation processes [2,40]. Adhesion of microbes to particle surfaces can alter the hydrophobicity of minerals markedly. Such surface modification to impart hydrophobicity or hydrophilicity is used in flotation of sulfide and non-sulfide minerals. In some systems they also cause excellent flocculation of fine particles. Since the bacteria adhere to mineral surfaces and alter the surface properties that are essential in mineral beneficiation techniques, the microorganisms have formidable applications in flotation and flocculation processes. The adhesion of microorganisms results an alteration of the surface chemistry of minerals due to a consequence of the formation of a biofilm on the surface or surface-active chemicals and their adsorption, accumulation and precipitation of ions and compounds on the surface.
Microorganisms adhere to mineral surfaces for various reasons. S. oneidensis utilize minerals as the terminal electron acceptor in the respiratory cycle. Bacteria of Thiobacilli group recover energy from minerals by the enzymatic oxidation. Common to both these microbiologic processes is the bacterial need to access, adhere to, and react with the mineral-water interface. Previous studies have shown that under most physiological conditions the bacterial cell surface carries a net negative charge, while, along with electrostatic forces, hydrophobic, entropic, acid-base, and Van der Waals interactions and H-bonding are important in the bacterial adhesion [2].
The information on the mechanisms of both bacterium adsorption and reagent (collector) adsorption in the presence and absence of the adsorbed bacteria is necessary. However, this area is a dark spot at the moment. The tremendously complex problem of gaining insight into the mechanisms of adsorption of living organisms is met by a notless- complex surface chemistry in a pulp as far as even in the absence of adsorbed bacteria the sulfide surface species are instable. We have showed that adapted bacteria of the Thiobacilli group (Thiobacillus ferrooxidans, Thibacillus thiooxidans and Leptospirilum ferrooxidans), which are associated of sulfide ores and mine water, and Paenibacillus polymyxa, can be selectively attached to sulfides, thereby essentially modifying the following adsorption of flotation reagents [41-43] (these systems are among few that have been extensively reported in the literature). This phenomenon can be put into the basis of elaboration of principally novel flotation schemes providing both ecological and economical gain.
Though the mechanisms of adsorption of the microbe’s extracellular polymeric substance (EPS) components onto solid surfaces are generally known [44], they are system specific. The interaction of the biopolymers produced by bacteria grown in the presence of specific minerals with surfactants requires investigation focusing on the applicability to bioflotation. More specifically, the EPS produced by bacteria of Acidithiobacilli group during the growth in 1) culture media, 2) in the presence of either chalcopyrite, pyrite, galena or sphalerite, and 3) in the presence of both mineral and flotation reagent (xanthate and dithiophosphate). The interaction of the biopolymers produced by bacteria grown in the presence of specific minerals and minerals with xanthate and dithiophosphate require special attention.
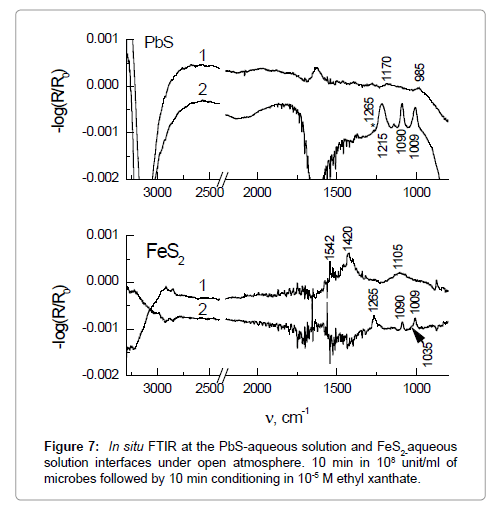
Our initial in situ FTIR spectra of Paenibacius polymixa interaction with galena and pyrite are shown in Figure 7. Spectra (1) on the top and bottom panels show the sulfide–water interface after conditioning with a suspension of Paenibacius polymixa adapted to FeS2. The microbe concentration was 10–8 ml–1 (pH 6.5). One can see the appearance of weak bands of PbSO4 at 1170 and 985 cm–1 in the case of galena. The spectral picture is somewhat different in the case of pyrite: First, except for bands of a Fe(III)–SO4 complex at 1105 and 980 cm–1, prominent bands at 1420 and 1542 cm–1 assignable to Fe(III) carbonate are observed. Second, intensity of these bands is noticeably higher than for galena, implying that interaction with the microbe results in a higher degree of corrosion for pyrite. The presence of the carbonate species implies the formation of amorphous ferric (hydr)oxide, the absorption of which is masked by the water absorption below 800 cm–1.
The subsequent addition of a 10–5 М aqueous solution of ethyl xanthate results in the formation of both lead xanthate (1215, 1090, and 1009 cm–1) and dixanthogen (a component at 1265 cm–1 marked by an asterisk) on galena. This process is accompanied by appearance of a strong negative band (δH2O) of water at 1630 cm–1, which indicates that water is pushed off the interface, consistent with experimentally observed hydrophobicity of the mineral under similar conditions. Though some dixanthogen is formed upon the xanthate adsorption on pyrite (bands at 1265, 1090, and 1009), the presence of a band at 1035 cm–1 testifies to the concomitant formation of ferric xanthate-hydroxide species. Positive intensity of the water band at 1630 cm–1 shows that density of the interfacial water increases, in agreement with the observed depression of pyrite. Inspection with an optical microscope of the sulfide minerals after the experiments described revealed that about 10 microbes had adhered to the polished pyrite surface of 10 mm2, while none to galena. Based on these observations and since no typical bands of biopolymers (proteins, polysachharides, etc.) are present in the in situ FTIR spectra, one can make preliminary conclusion that the main mechanism of bioregulation of the surface properties relates to biooxidation of the sulfides and/or to change in the sulfide potential (in that particular experiment we did not measure potential of the sulfide electrode but this can be done) rather than to surface properties of the adsorbed microbes themselves or expressed biopolymers.
To provide a new insight into the phenomena, the whole problem of the mineral-bacterium interaction can be conveniently divided into two problems: biochemical and geochemical. The former is related to the bacterium side of the interface (the bacterium envelope) in terms of particular mechanisms of sensing/recognition and response to extracellular minerals, molecule-specific pathways of charge transfer, and genomic mechanisms of regulation of these processes. The geochemical problem concerns the mineral response to the bacterium presence, which is essentially interplay between microorganism and the physicochemical properties of the mineral surface, such as the atomic and electronic structure, the net charge/potential, acid-base properties, and wettability of the surface. Consequently, the biocatalyzed electrontransfer reactions on metal oxides and sulfides can be controlled through variation of these parameters, which require a systematic study in order to find answers to the following questions:
• Are the redox mechanisms established for abiotic interfaces are valid in the case of bacterium-mediated reduction/oxidation of the mineral?
• Is direct bacteria-mineral contact necessary for oxidative or reductive dissolution of sulfides, iron oxides, or manganese oxides?
• Do bacteria exploit the energetic perturbations present in the surface defects to adhere and oxidize/reduce the mineral? If yes, defects of which type (point vs. clustered) are more preferable by bacteria?
• Which type of surface defects bacteria generate by their own? What is the mechanism of generation of these defects?
• What are the roles of different kinds of the initial and bacteriuminduced mineral heterogeneity in the overall biogeochemical interaction?
• How size of the mineral particle influences the microbe adhesion and the rate of the biotic oxidation/reduction of the mineral?
• How the mineral potential (regulated either by pH or potentiostatically) influences the attachment of the whole bacteria and the EPS components and the heterogeneous charge transfer reaction?
• How the mineral potential affects forms of collector adsorption and surface equilibrium of model redox couples in the presence of the bacteria?
Though the mechanisms of adsorption of the microbe’s extracellular polymeric substance (EPS) components onto solid surfaces are generally known, they are system specific. The mechanisms of the interaction of the biopolymers produced by bacteria grown in the presence of i) culture media, ii) specific minerals and iii) both mineral and surfactant (flotation collector) need special attention focusing on the applicability to bioflotation. Another environmentally sound and cost-effective biochemical innovation in sulfide flotation could be replacement of Na2S and reduced sulfur compounds, which are widely used in flotation practice as regulator (reductants), by sulfate reducing bacteria. Clearly, to develop a biochemical control of sulfide flotation, it is necessary first of all to understand difference in floatability of the sulfide surfaces reduced chemically and biochemically, which require intensified effort.
Summary
The molecule-level understanding of the aquatic solids interfaces is the key to innovate many society-formative technologies including ore processing, waste recycling and the environmental protection that are based on stringent control of interfacial processes. There are no general concepts of selective interactions of minerals with solutes, which imply that fundamental additions to aquatic chemistry of minerals are one of the major demands of the day. The interface between biological and geological materials, as well as a means to design and manipulate that interface, is currently virtually completely unexplored. The lack of knowledge could be recognized to the complexity and technical constraints of the problem. Progress in this area is hampered by a deficit of direct, not to mention in situ, spectroscopic molecular/atomic-level data about the mineral–bacterium interfaces. What is exactly happening at the mineral-bacteria-solution interfaces at the molecular level and in real time controls mineral (bio) processing technologies and there is an urgent need to understand the interfacial charge transfer and adsorption mechanisms to shift the technologies to be more innovative and effective.
Acknowledgement
The financial support from the Centre for Advanced Mining and Metallurgy (CAMM), Luleå University of Technology, Luleå, Luossavaara-Kiirunavaara AB (LKAB), Kiruna and Boliden Mineral AB, Boliden, all in Sweden, are gratefully acknowledged.
References
- Hochella MFJ, White AF (1990) Mineral-water interface geochemistry. Washington DC: Mineralogical Society of America.
- Hanumantha Rao K, Subramanian S (2007) Microbial processing of metal sulfides. Springer 267: 86.
- Tossell JA, Vaughan DJ (1992) Theoretical geochemistry: application of quantum mechanics in the earth and mineral sciences. Oxford University Press, USA.
- Cox PA (1995) The elements on earth: inorganic chemistry in the environment. Oxford University Press, USA.
- Grätzel M (2001) Photoelectrochemical cells. Nature 414: 338-344.
- Masel RI (1996) Principles of adsorption and reaction on solid surfaces. Wiley, New York.
- Stumm W (1992) Chemistry of the solid-water interface. Wiley-Interscience.
- West AR (2000) Basic solid state chemistry, 2nd Ed. ed, Wiley, New York.
- Brown Jr GE, Henrich VE, Casey WH, Clark DL, Eggleston C, et al (1999) Metal oxide surfaces and their interactions with aqueous solutions and microbial organisms. Chemical Reviews 99: 77-174.
- Stumm W, Morgan JJ (1996) Aquatic chemistry. New York: Wiley 679.
- Wehrli B, Sulzberger B, Stumm W (1989) Redox processes catalyzed by hydrous oxide surfaces. Chemical Geology 78: 167-179.
- Junta JL, Hochella Jr MF (1994) Manganese (II) oxidation at mineral surfaces: A microscopic and spectroscopic study. Geochimica et Cosmochimica Acta 58: 4985.
- Leland JK, Bard AJ (1987) Photochemistry of colloidal semiconducting iron oxide polymorphs. Journal of Physical Chemistry 91: 5076.
- Pehkonen SO, Siefert RL, Hoffmann MR (1995) Photoreduction of iron oxyhydroxides and the photooxidation of halogenated acetic acids. Environmental Science and Technology 29: 1215.
- Chernyshova IV (2003) An in situ FTIR study of galena and pyrite oxidation in aqueous solution. Journal of Electroanalytical Chemistry 558: 83-98.
- Chernyshova IV (2001) Anodic processes on a galena (PbS) electrode in the presence of n-Butyl xanthate studied FTIR-spectroelectrochemically. Journal of Physical Chemistry B 105: 8185-8191.
- Chernyshova IV (2001) Anodic oxidation of galena (PbS) studied FTIR-spectroelectrochemically. Journal of Physical Chemistry B 105: 8178-8184.
- Chernyshova IV (2002) In situ FTIR-spectroelectrochemical study of the anodic processes on a galena (PbS) electrode under open-air conditions in the absence and presence of n-butyl xanthate. Langmuir 18: 6962-6968.
- Morrison R (1990) The chemical physics of surfaces. New York: Plenum Press.
- Tolstoy VP, Chernyshova IV, Skryshevsky VA (2003) Handbook of infrared spectra of ultrathin films. Hoboken: Wiley.
- Chernyshova IV, Tolstoy VP (1995) Applied spectra 49: 665.
- Woods R, Bockris JO, Conway BE, White RE (1996) Modern aspects of electrochemistry Eds. Plenum Press: New York 29: 401-453.
- Richadson PE, O’Dell CSJ (1985) Semiconducting characteristics of galena electrodes - relationship to mineral flotation. Electrochemical Society 132: 1350.
- Woods R, Jones MH, Woodcock JT (1984) Principles of Mineral Flotation. The Wark Symposium. Eds Ausralasian Institute of Mining and Metallurgy (AIMM): Parkville, Victoria, Australia.
- Newman DK, Kolter R (2000) A role for excreted quinones in extracellular electron transfer. Nature 405: 94-97.
- Gül A (2007) The role of Na2S2O5 and activated carbon on the selective flotation of chalcopyrite from a copper ore using a dithiophosphine-type collector. Mineral Processing and Extractive Metallurgy Review 28: 235-245.
- Yamamoto T (1980) Complex sulfide ores. ed. MJ Jones. London: IMM 71–78.
- Khmeleva TN, Beattie DA, Georgiev TV, Skinner WM (2003) Surface study of the effect of sulfite ions on copper-activated pyrite pre-treated with xanthate. Minerals Engineering 16: 601-608.
- Forssberg KSE ed (1991) Flotation of sulfide minerals: Elsevier.
- Shen WZ, Fornasiero D, Ralston J (2001) Flotation of sphalerite and pyrite in the presence of sodium sulfite. International Journal of Mineral Processing 63: 17-28.
- Grano SR, Cnossen H, Skinner W (1997) Surface modifications in the chalcopyrite-sulfite ion system, II. Dithiophosphate collector adsorption study. International Journal of Mineral Processing 50: 27.
- Houot R, Duhamet D (1993) Floatability of chalcopyrite in the presence of dialkyl-thionocarbamate and sodium sulfite. International Journal of Mineral Processing 37: 273-282.
- Misra H, Miller D, Song QY (1985) Developments in mineral processing, flotation of sulfide minerals. ed. KSE Forssberg Amsterdam Elsevier 175-196.
- Arai Y, Sparks DL, Davis JA (2004) Effects of dissolved carbonate on arsenate adsorption and surface speciation at the hematite-water interface. Environmental Science and Technology 38: 817-824.
- Borda M, Elsetinow A, Strongin D, Schoonen M (2003) A mechanism for the production of hydroxyl radical at surface defect sites on pyrite. Geochimica et Cosmochimica Acta 67: 935-939.
- Lehmann MN, Stichnoth M, Walton D, Bailey SI (2000) Effect of chloride ions on the ambient electrochemistry of pyrite oxidation in acid media. Journal of the Electrochemical Society 147: 3263-3271.
- Javadi A, Larsson AC, Hanumantha Rao K (2013) Formation of hydrogen peroxide by pyrite and its influence on flotation. Journal of Minerals Engineering 49: 128–134.
- Javadi A, Hanumantha Rao K (accepted) Formation of Hydrogen peroxide by sulfide minerals. Journal of Hydrometallurgy.
- Maira AJ, Yeung KL, Lee CY, Yue PL, Chan CK (2000) Size effects in gas-phase photo-oxidation of trichloroethylene using nanometer-sized TiO2 catalysts. Journal of Catalysis 192: 185-196.
- Vilinska A, Hanumantha Rao K (2008) Leptosririllum ferrooxidans-sulfide mineral interactions with reference to bioflotation nad bioflocculation. Transactions of Nonferrous Metals Society of China (English Edition) 18: 1403.
- Das A, Rao KH, Sharma PK, Natarajan KA, Forssberg KSE (1999) Biohydrometallurgy and the Environment toward the Mining of the 21st Century. ed. R Amils, A Ballester Elsevier 697-707.
- Sharma PK, Rao KH, Forssberg KSE, Natarajan KA (2001) Surface chemical characterisation of Paenibacillus polymyxa before and after adaptation to sulfide minerals. International Journal of Mineral Processing 62: 3-25.
- Sharma PK, Hanumantha Rao K (1999) Role of a heterotrophic Paenibacillus polymyxa bacteria in the bioflotation of some sulfide minerals. Minerals and Metallurgical Processing 16: 35-41.
- Poortinga AT, Bos R, Norde W, Busscher HJ (2002) Electric double layer interactions in bacterial adhesion to surfaces. Surface Science Reports 47: 1-32.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 15090
- [From(publication date):
November-2013 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 10426
- PDF downloads : 4664